General Chemistry Principles and Modern Applications Petrucci Harwood

![Example 15 -2 What is the concentration at 100 s? [H 2 O 2]i Example 15 -2 What is the concentration at 100 s? [H 2 O 2]i](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-9.jpg)
![Example 15 -3 R 3 = k [Hg. Cl 2]3 m [C 2 O Example 15 -3 R 3 = k [Hg. Cl 2]3 m [C 2 O](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-13.jpg)
![Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42 Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-14.jpg)
![Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42 Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-15.jpg)
![15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k 15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-16.jpg)
![Integrated Rate Law -Δ[A] Δt = k Move to the -d[A] infinitesimal dt = Integrated Rate Law -Δ[A] Δt = k Move to the -d[A] infinitesimal dt =](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-17.jpg)
![Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-26.jpg)

![The Steady State Approximation d[N 2 O 2] dt = k 1[NO]2 – k The Steady State Approximation d[N 2 O 2] dt = k 1[NO]2 – k](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-47.jpg)
![Kinetic Consequences of Assumptions 2 NO(g) d[NO 2] dt = k 1 k 3[NO]2[O Kinetic Consequences of Assumptions 2 NO(g) d[NO 2] dt = k 1 k 3[NO]2[O](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-48.jpg)
![Saturation Kinetics k 1 d[P] k 2 E + S ES → E + Saturation Kinetics k 1 d[P] k 2 E + S ES → E +](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-53.jpg)
![Michaelis-Menten d[P] dt d[P] = k 2[E]0 dt d[P] dt = = k 1 Michaelis-Menten d[P] dt d[P] = k 2[E]0 dt d[P] dt = = k 1](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-54.jpg)

- Slides: 55

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 15: Chemical Kinetics Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002

Contents 15 -1 15 -2 15 -3 15 -4 15 -5 15 -6 15 -7 Prentice-Hall © 2002 The Rate of a Chemical Reaction Measuring Reaction Rates Effect of Concentration on Reaction Rates: The Rate Law Zero-Order Reactions First-Order Reactions Second-Order Reactions Reaction Kinetics: A Summary General Chemistry: Chapter 15 Slide 2 of 55

Contents 15 -8 15 -9 15 -10 15 -11 Theoretical Models for Chemical Kinetics The Effect of Temperature on Reaction Rates Reaction Mechanisms Catalysis Focus On Combustion and Explosions Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 3 of 55

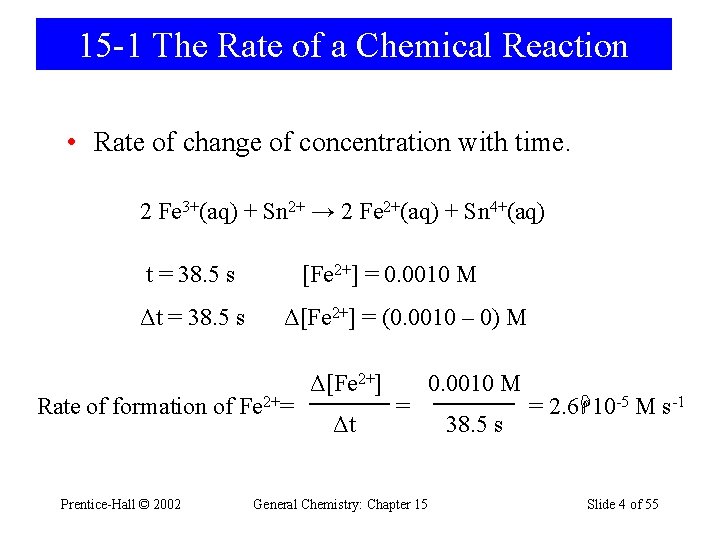
15 -1 The Rate of a Chemical Reaction • Rate of change of concentration with time. 2 Fe 3+(aq) + Sn 2+ → 2 Fe 2+(aq) + Sn 4+(aq) t = 38. 5 s Δt = 38. 5 s [Fe 2+] = 0. 0010 M Δ[Fe 2+] = (0. 0010 – 0) M Rate of formation of Fe 2+= Prentice-Hall © 2002 Δ[Fe 2+] Δt = 0. 0010 M General Chemistry: Chapter 15 38. 5 s = 2. 6 10 -5 M s-1 Slide 4 of 55

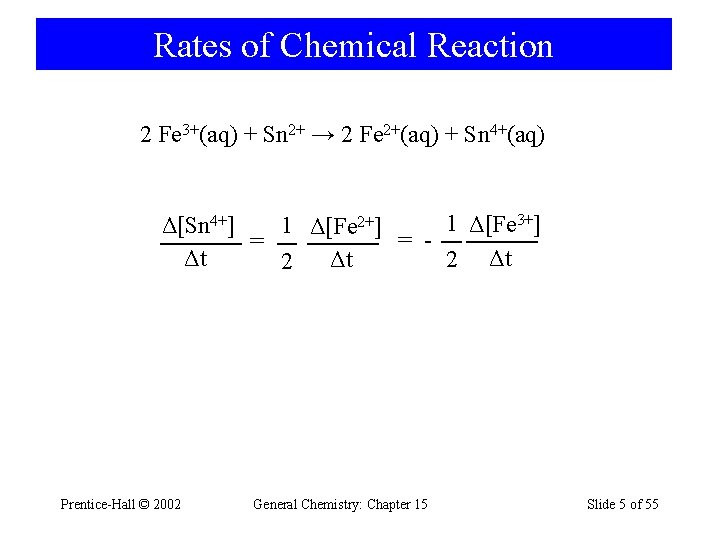
Rates of Chemical Reaction 2 Fe 3+(aq) + Sn 2+ → 2 Fe 2+(aq) + Sn 4+(aq) 1 Δ[Fe 3+] Δ[Sn 4+] 1 Δ[Fe 2+] = = Δt Δt 2 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 5 of 55

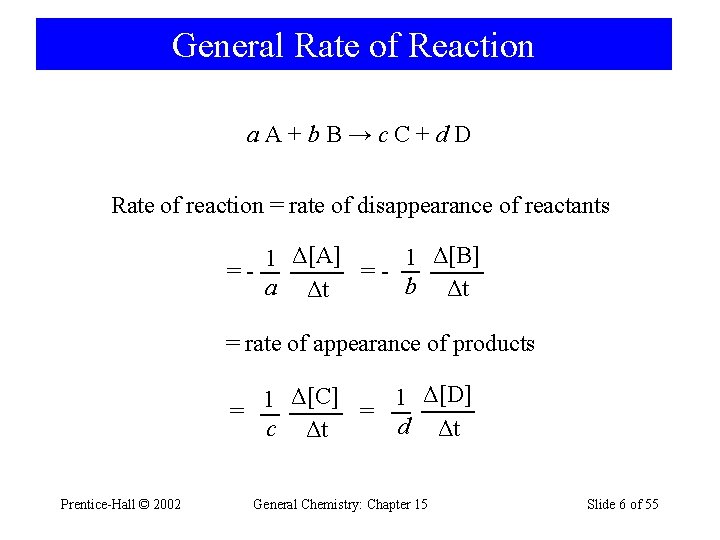
General Rate of Reaction a. A+b. B→c. C+d. D Rate of reaction = rate of disappearance of reactants 1 Δ[B] 1 Δ[A] ==b Δt a Δt = rate of appearance of products 1 Δ[D] 1 Δ[C] = = d Δt c Δt Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 6 of 55

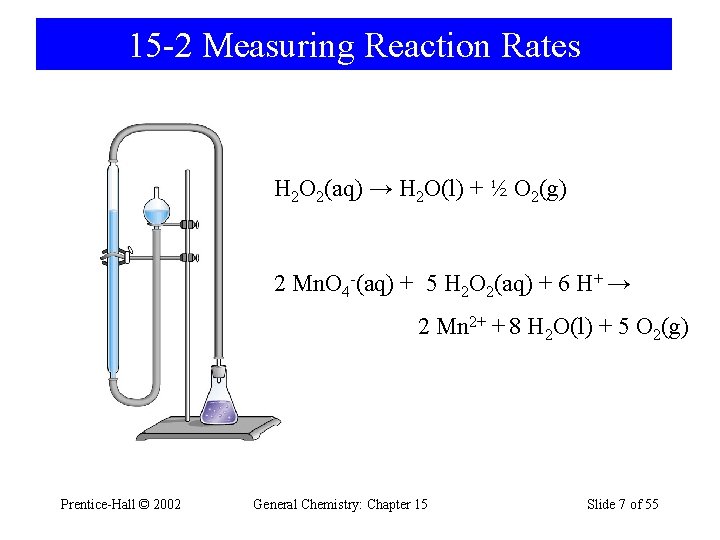
15 -2 Measuring Reaction Rates H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) 2 Mn. O 4 -(aq) + 5 H 2 O 2(aq) + 6 H+ → 2 Mn 2+ + 8 H 2 O(l) + 5 O 2(g) Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 7 of 55

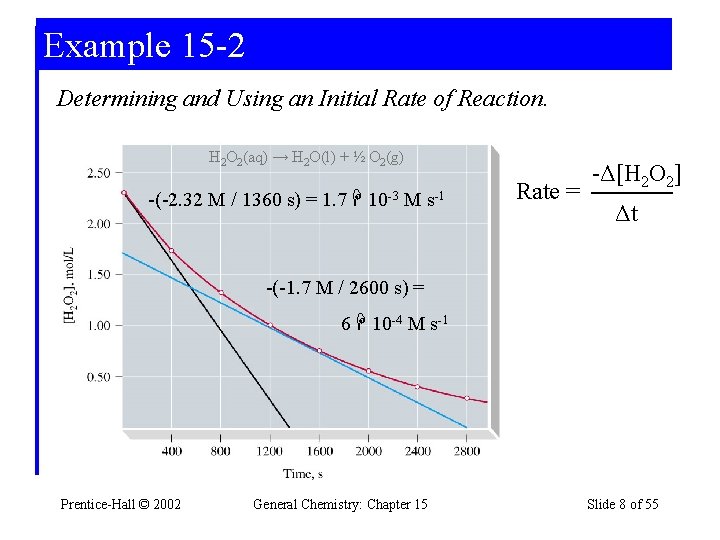
Example 15 -2 Determining and Using an Initial Rate of Reaction. H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) -(-2. 32 M / 1360 s) = 1. 7 10 -3 M s-1 Rate = -Δ[H 2 O 2] Δt -(-1. 7 M / 2600 s) = 6 10 -4 M s-1 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 8 of 55
![Example 15 2 What is the concentration at 100 s H 2 O 2i Example 15 -2 What is the concentration at 100 s? [H 2 O 2]i](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-9.jpg)
Example 15 -2 What is the concentration at 100 s? [H 2 O 2]i = 2. 32 M Rate = 1. 7 10 -3 M s-1 = - Δ[H 2 O 2] Δt -Δ[H 2 O 2] = -([H 2 O 2]f - [H 2 O 2]i) = 1. 7 10 -3 M s-1 Δt [H 2 O 2]100 s – 2. 32 M = -1. 7 10 -3 M s-1 100 s [H 2 O 2]100 s = 2. 32 M - 0. 17 M = 2. 17 M Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 9 of 55

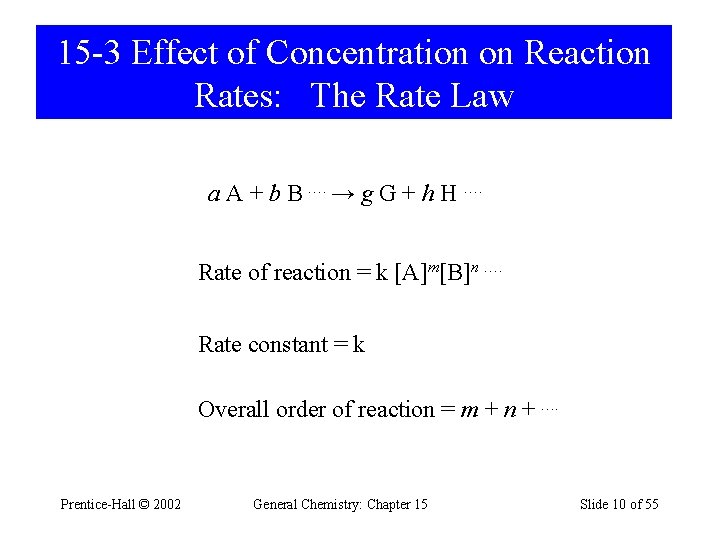
15 -3 Effect of Concentration on Reaction Rates: The Rate Law a A + b B …. → g G + h H …. Rate of reaction = k [A]m[B]n …. Rate constant = k Overall order of reaction = m + n + …. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 10 of 55

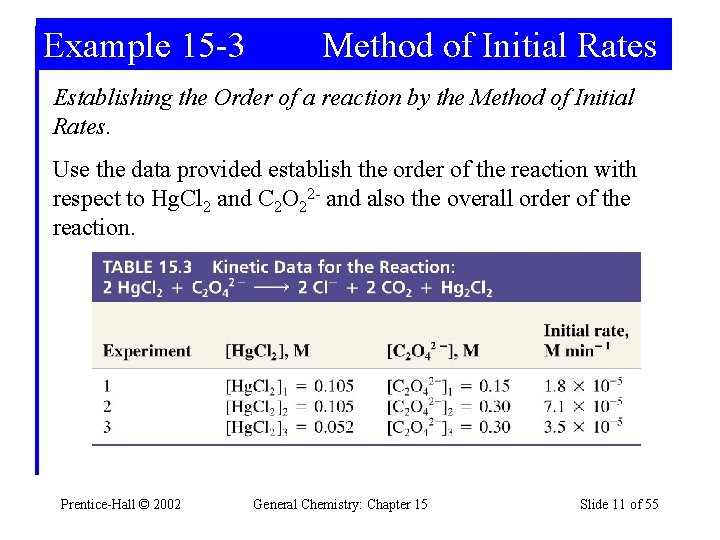
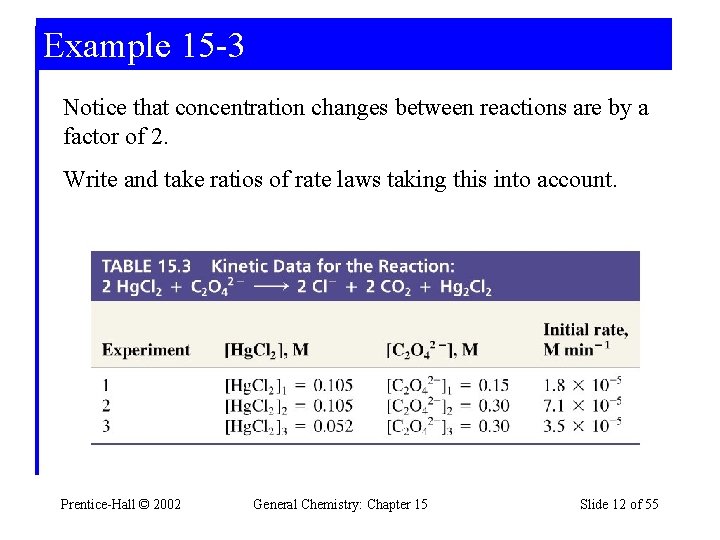
Example 15 -3 Method of Initial Rates Establishing the Order of a reaction by the Method of Initial Rates. Use the data provided establish the order of the reaction with respect to Hg. Cl 2 and C 2 O 22 - and also the overall order of the reaction. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 11 of 55

Example 15 -3 Notice that concentration changes between reactions are by a factor of 2. Write and take ratios of rate laws taking this into account. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 12 of 55
![Example 15 3 R 3 k Hg Cl 23 m C 2 O Example 15 -3 R 3 = k [Hg. Cl 2]3 m [C 2 O](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-13.jpg)
Example 15 -3 R 3 = k [Hg. Cl 2]3 m [C 2 O 42 -]3 n R 2 = k [Hg. Cl 2]2 m [C 2 O 42 -]2 n = k (2[Hg. Cl 2]3)m [C 2 O 42 -]3 n R 2 = R 3 k [Hg. Cl 2]3 m [C 2 O 42 -]3 n k 2 m [Hg. Cl 2]3 m [C 2 O 42 -]3 n R 2 2 m. R 3 = = = 2. 0 m 2 n R 3 k [Hg. Cl 2]3 [C 2 O 4 ]3 R 3 2 m = 2. 0 therefore m = 1. 0 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 13 of 55
![Example 15 3 R 2 k Hg Cl 221 C 2 O 42 Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-14.jpg)
Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42 -]2 n = k (0. 105) (0. 30)n R 1 = k [Hg. Cl 2]11 [C 2 O 42 -]1 n = k (0. 105) (0. 15)n k (0. 105) (0. 30)n R 2 = R 1 k (0. 105) (0. 15)n R 2 = R 1 (0. 30)n (0. 15)n = 2 n 7. 1 10 -5 = = 3. 94 -5 1. 8 10 2 n = 3. 98 therefore n = 2. 0 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 14 of 55
![Example 15 3 R 2 k Hg Cl 221 C 2 O 42 Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-15.jpg)
Example 15 -3 R 2 = k [Hg. Cl 2]21 [C 2 O 42 -]22 First order + Prentice-Hall © 2002 Second order General Chemistry: Chapter 15 = Third Order Slide 15 of 55
![15 4 ZeroOrder Reactions A products Rrxn k A0 Rrxn k 15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-16.jpg)
15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k [k] = mol L-1 s-1 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 16 of 55
![Integrated Rate Law ΔA Δt k Move to the dA infinitesimal dt Integrated Rate Law -Δ[A] Δt = k Move to the -d[A] infinitesimal dt =](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-17.jpg)
Integrated Rate Law -Δ[A] Δt = k Move to the -d[A] infinitesimal dt = k And integrate from 0 to time t [A]t - d[A] [A]0 t = k dt 0 -[A]t + [A]0 = kt [A]t = [A]0 - kt Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 17 of 55

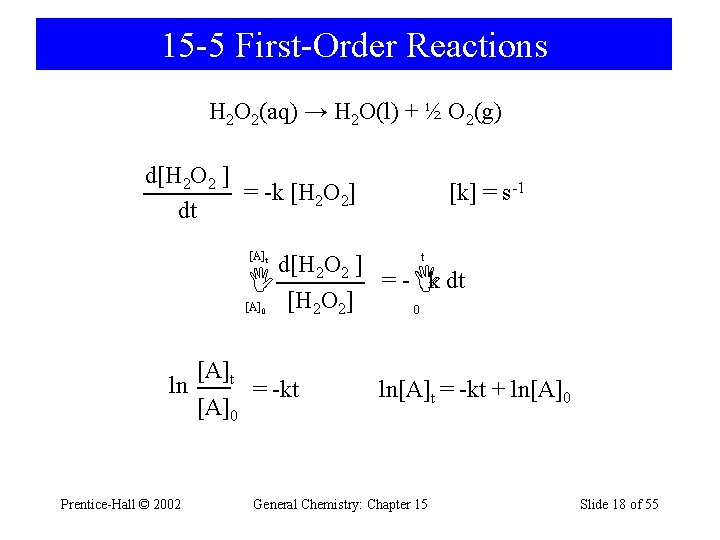
15 -5 First-Order Reactions H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) d[H 2 O 2 ] = -k [H 2 O 2] dt [k] = s-1 [A]t t d[H 2 O 2 ] [H O ] = - k dt [A] 0 2 2 0 ln Prentice-Hall © 2002 [A]t [A]0 = -kt ln[A]t = -kt + ln[A]0 General Chemistry: Chapter 15 Slide 18 of 55

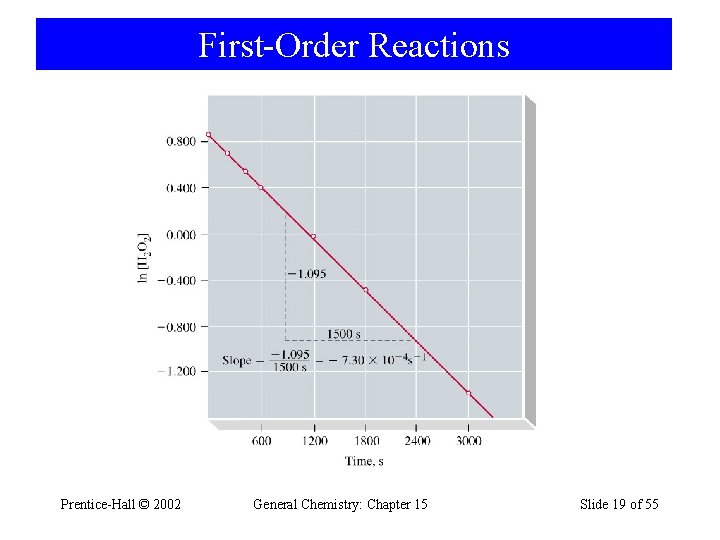
First-Order Reactions Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 19 of 55

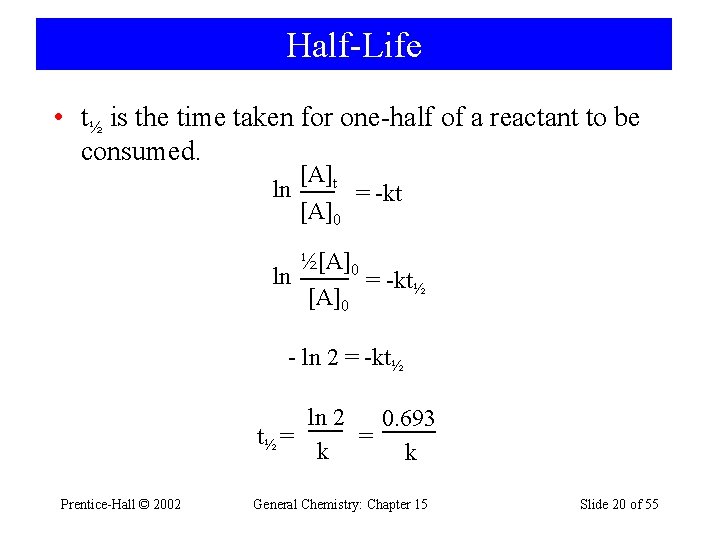
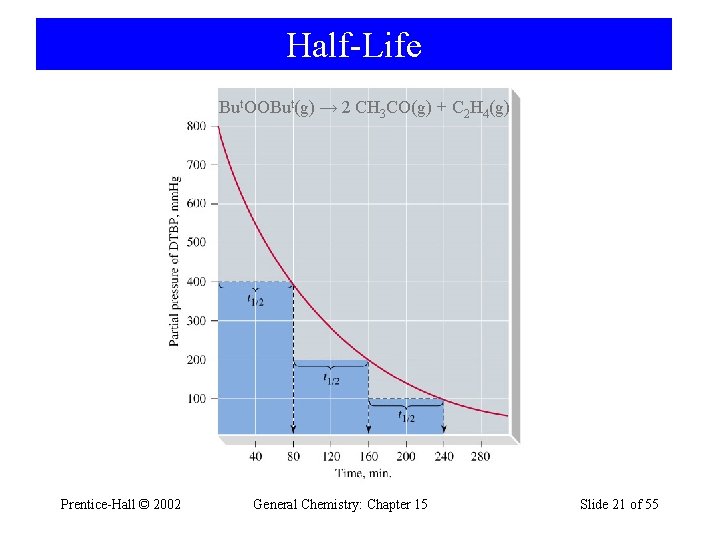
Half-Life • t½ is the time taken for one-half of a reactant to be consumed. ln ln [A]t [A]0 = -kt ½[A]0 = -kt½ [A]0 - ln 2 = -kt½ ln 2 0. 693 t½ = = k k Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 20 of 55

Half-Life But. OOBut(g) → 2 CH 3 CO(g) + C 2 H 4(g) Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 21 of 55

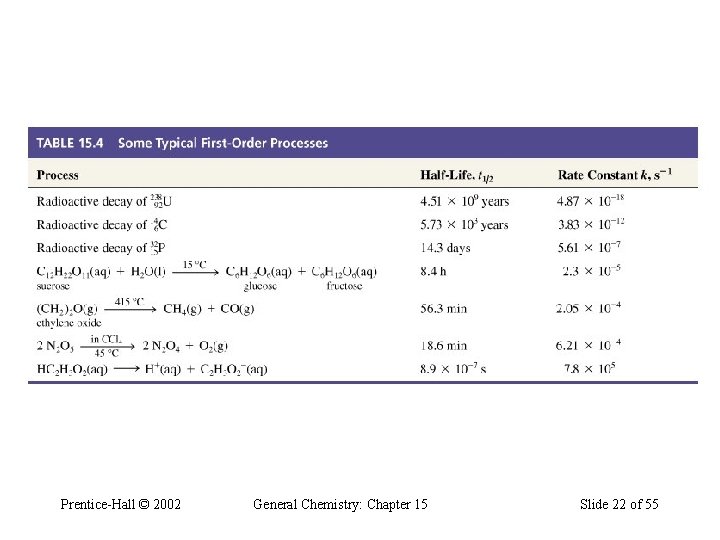
Some Typical First-Order Processes Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 22 of 55

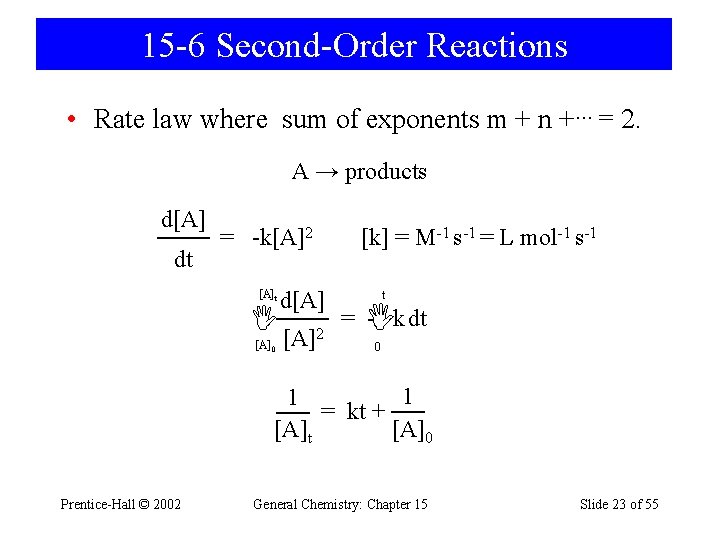
15 -6 Second-Order Reactions • Rate law where sum of exponents m + n +… = 2. A → products d[A] dt = -k[A]2 [A]t d[A] [A]2 [A]0 [k] = M-1 s-1 = L mol-1 s-1 t = - k dt 0 1 1 = kt + [A]t [A]0 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 23 of 55

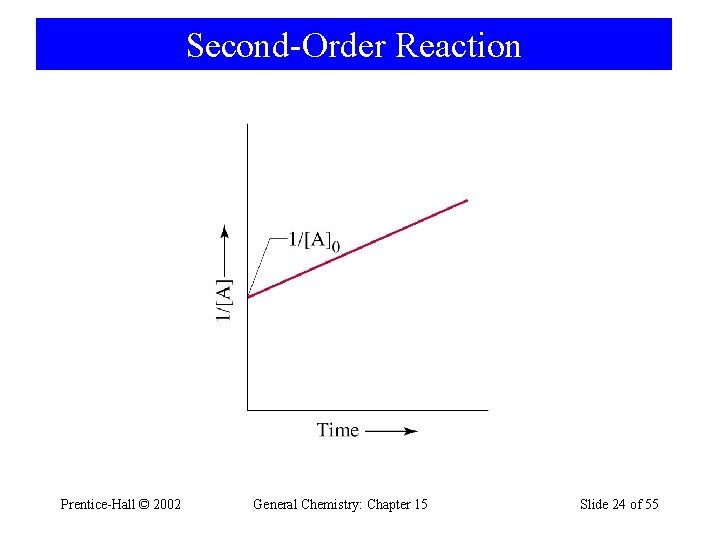
Second-Order Reaction Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 24 of 55

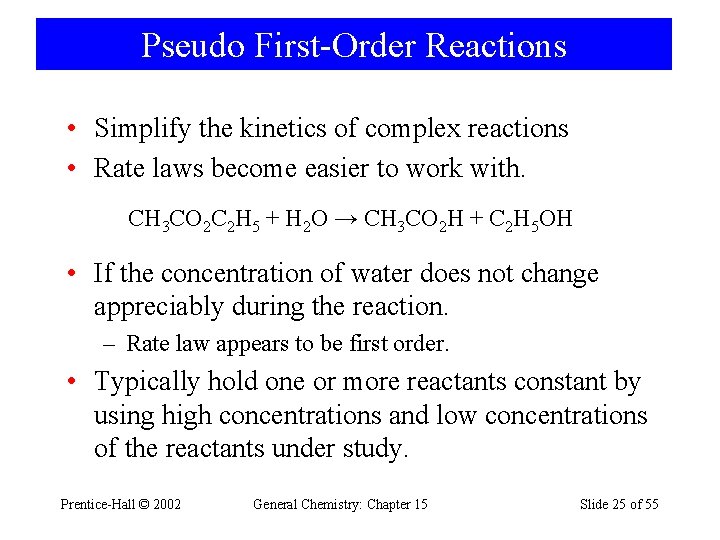
Pseudo First-Order Reactions • Simplify the kinetics of complex reactions • Rate laws become easier to work with. CH 3 CO 2 C 2 H 5 + H 2 O → CH 3 CO 2 H + C 2 H 5 OH • If the concentration of water does not change appreciably during the reaction. – Rate law appears to be first order. • Typically hold one or more reactants constant by using high concentrations and low concentrations of the reactants under study. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 25 of 55
![Testing for a Rate Law Plot A vs t Plot lnA vs t Plot Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-26.jpg)
Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot 1/[A] vs t. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 26 of 55

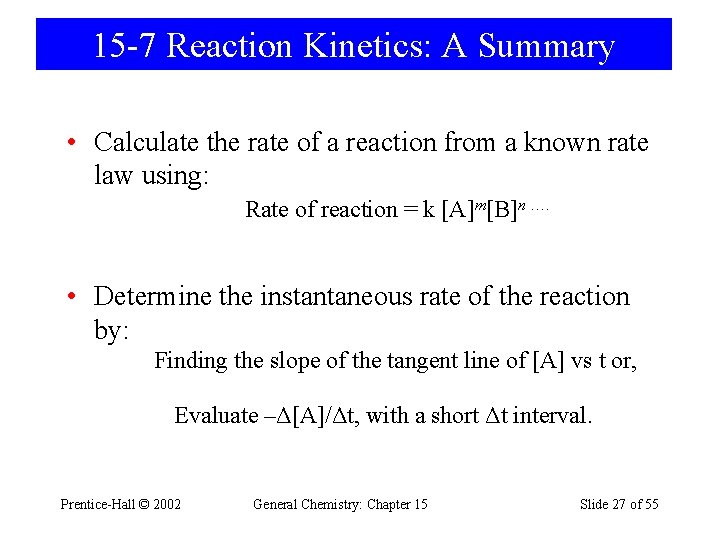
15 -7 Reaction Kinetics: A Summary • Calculate the rate of a reaction from a known rate law using: Rate of reaction = k [A]m[B]n …. • Determine the instantaneous rate of the reaction by: Finding the slope of the tangent line of [A] vs t or, Evaluate –Δ[A]/Δt, with a short Δt interval. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 27 of 55

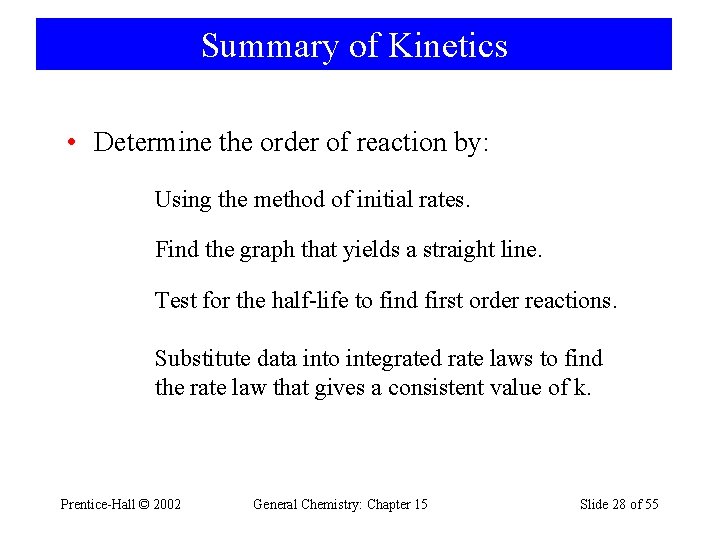
Summary of Kinetics • Determine the order of reaction by: Using the method of initial rates. Find the graph that yields a straight line. Test for the half-life to find first order reactions. Substitute data into integrated rate laws to find the rate law that gives a consistent value of k. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 28 of 55

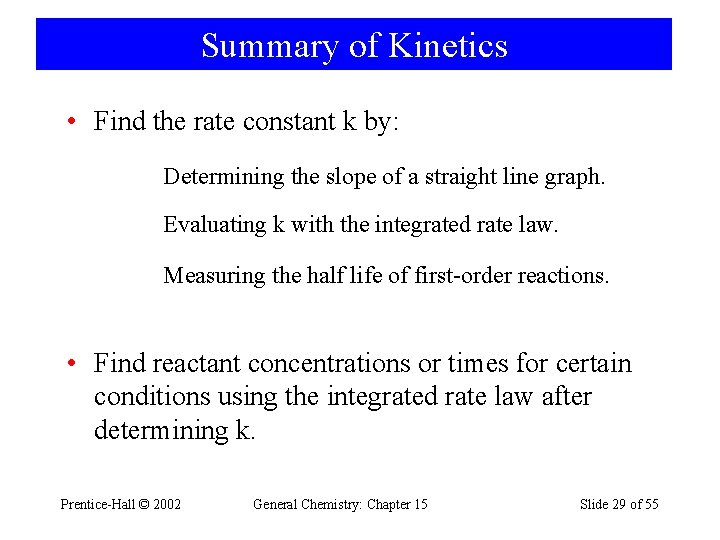
Summary of Kinetics • Find the rate constant k by: Determining the slope of a straight line graph. Evaluating k with the integrated rate law. Measuring the half life of first-order reactions. • Find reactant concentrations or times for certain conditions using the integrated rate law after determining k. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 29 of 55

15 -8 Theoretical Models for Chemical Kinetics Collision Theory • Kinetic-Molecular theory can be used to calculate the collision frequency. – In gases 1030 collisions per second. – If each collision produced a reaction, the rate would be about 106 M s-1. – Actual rates are on the order of 104 M s-1. • Still a very rapid rate. – Only a fraction of collisions yield a reaction. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 30 of 55

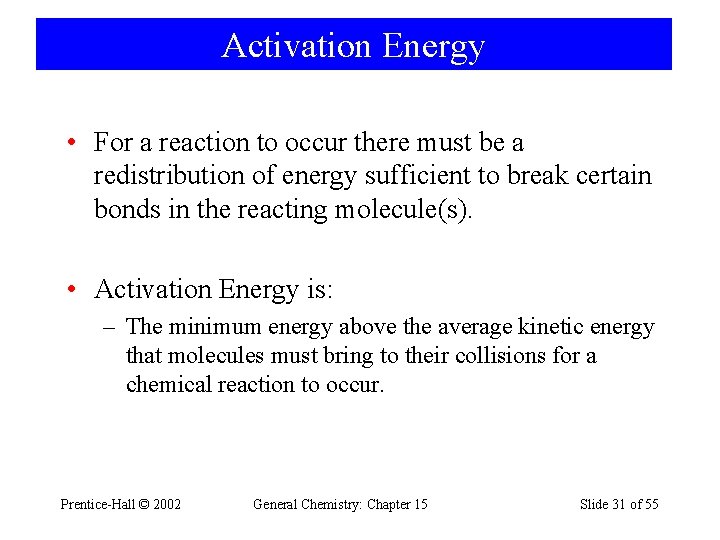
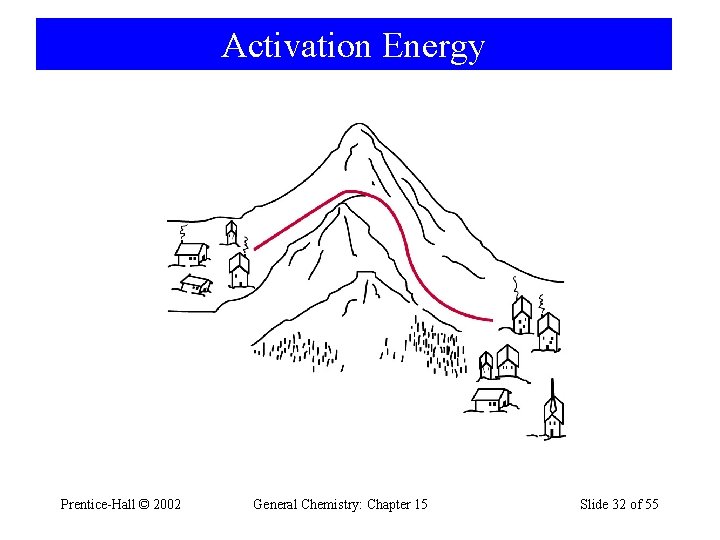
Activation Energy • For a reaction to occur there must be a redistribution of energy sufficient to break certain bonds in the reacting molecule(s). • Activation Energy is: – The minimum energy above the average kinetic energy that molecules must bring to their collisions for a chemical reaction to occur. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 31 of 55

Activation Energy Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 32 of 55

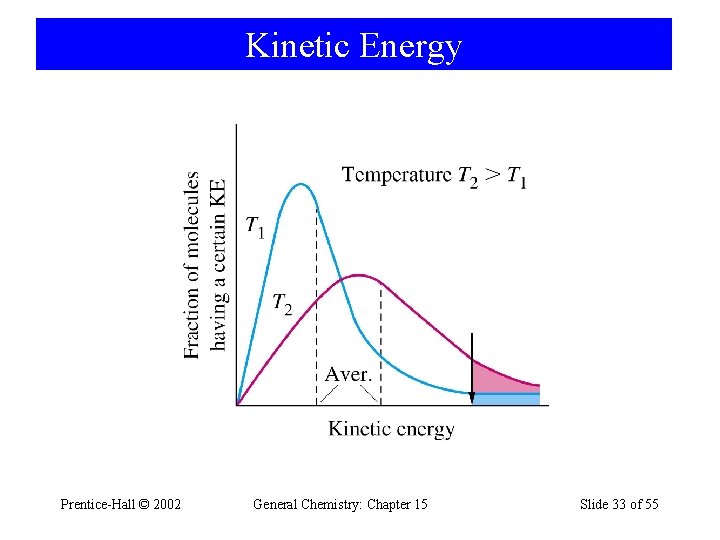
Kinetic Energy Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 33 of 55

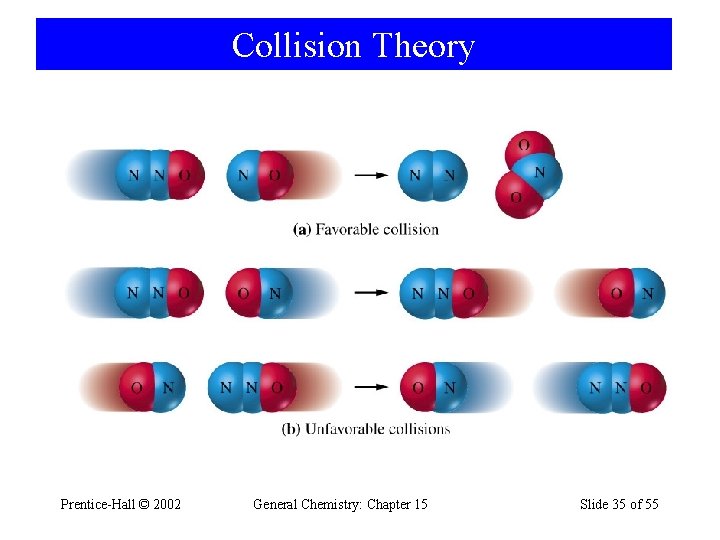
Collision Theory • If activation barrier is high, only a few molecules have sufficient kinetic energy and the reaction is slower. • As temperature increases, reaction rate increases. • Orientation of molecules may be important. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 34 of 55

Collision Theory Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 35 of 55

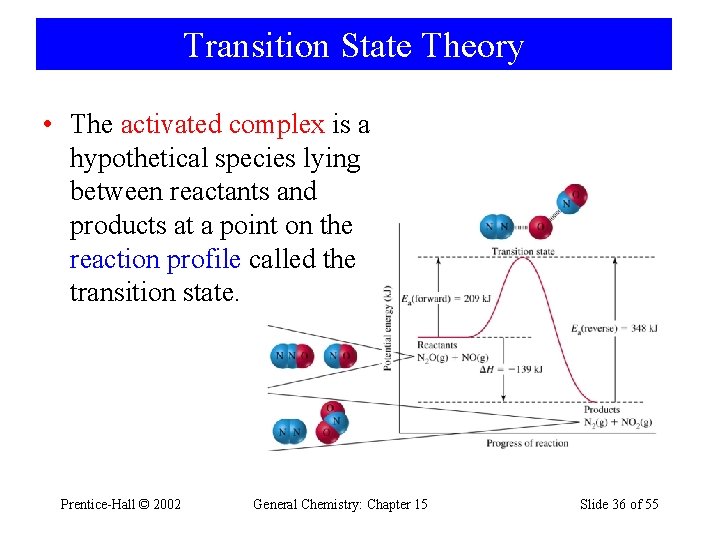
Transition State Theory • The activated complex is a hypothetical species lying between reactants and products at a point on the reaction profile called the transition state. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 36 of 55

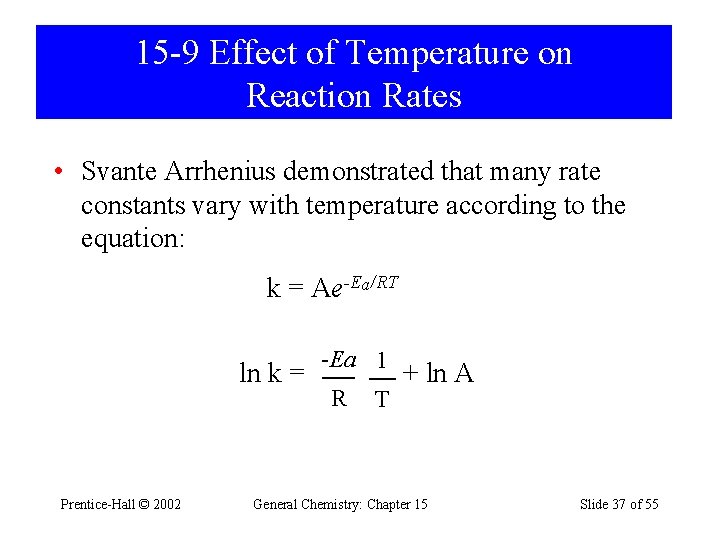
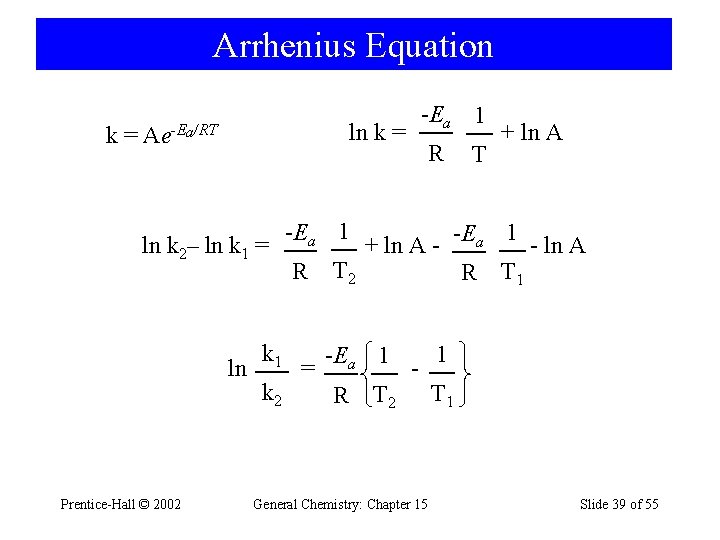
15 -9 Effect of Temperature on Reaction Rates • Svante Arrhenius demonstrated that many rate constants vary with temperature according to the equation: k = Ae-Ea/RT ln k = Prentice-Hall © 2002 -Ea 1 R T + ln A General Chemistry: Chapter 15 Slide 37 of 55

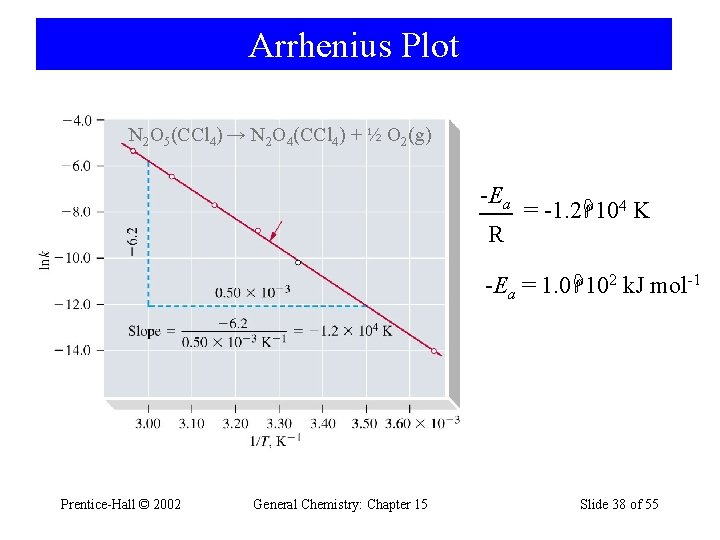
Arrhenius Plot N 2 O 5(CCl 4) → N 2 O 4(CCl 4) + ½ O 2(g) -Ea R = -1. 2 104 K -Ea = 1. 0 102 k. J mol-1 Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 38 of 55

Arrhenius Equation k= ln k 2– ln k 1 = ln Prentice-Hall © 2002 -Ea 1 ln k = Ae-Ea/RT k 1 k 2 R T + ln A -Ea 1 R = 1 -E a + ln A - ln A T 2 R T 1 -Ea 1 R T 2 - General Chemistry: Chapter 15 1 T 1 Slide 39 of 55

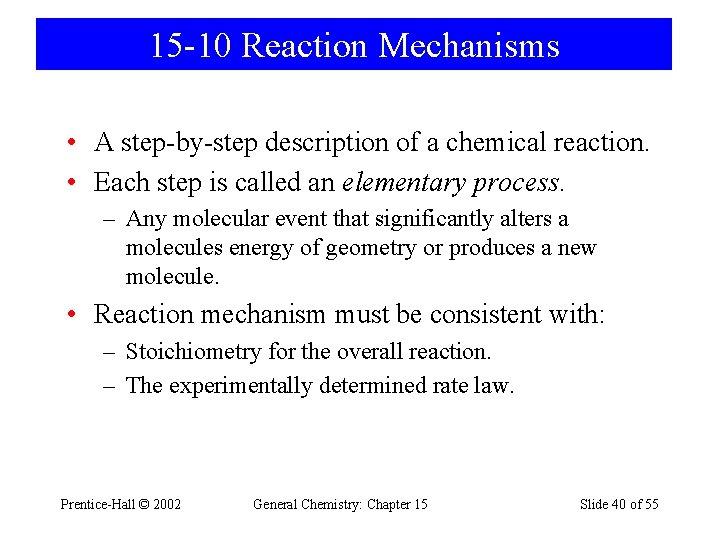
15 -10 Reaction Mechanisms • A step-by-step description of a chemical reaction. • Each step is called an elementary process. – Any molecular event that significantly alters a molecules energy of geometry or produces a new molecule. • Reaction mechanism must be consistent with: – Stoichiometry for the overall reaction. – The experimentally determined rate law. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 40 of 55

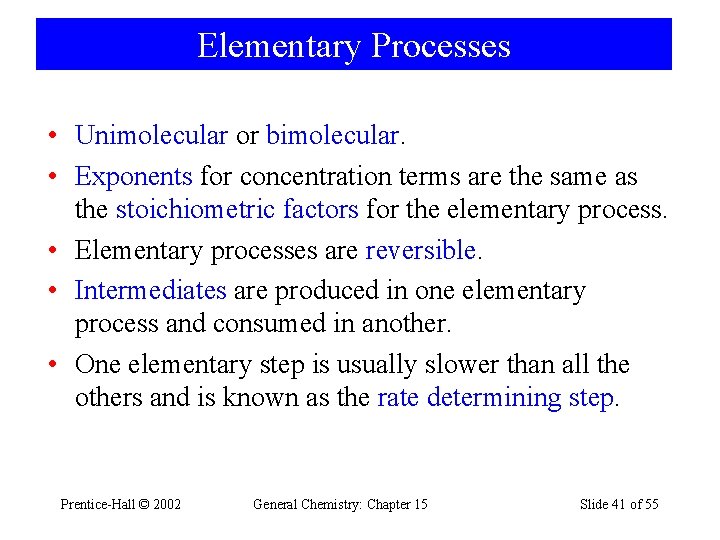
Elementary Processes • Unimolecular or bimolecular. • Exponents for concentration terms are the same as the stoichiometric factors for the elementary process. • Elementary processes are reversible. • Intermediates are produced in one elementary process and consumed in another. • One elementary step is usually slower than all the others and is known as the rate determining step. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 41 of 55

A Rate Determining Step Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 42 of 55

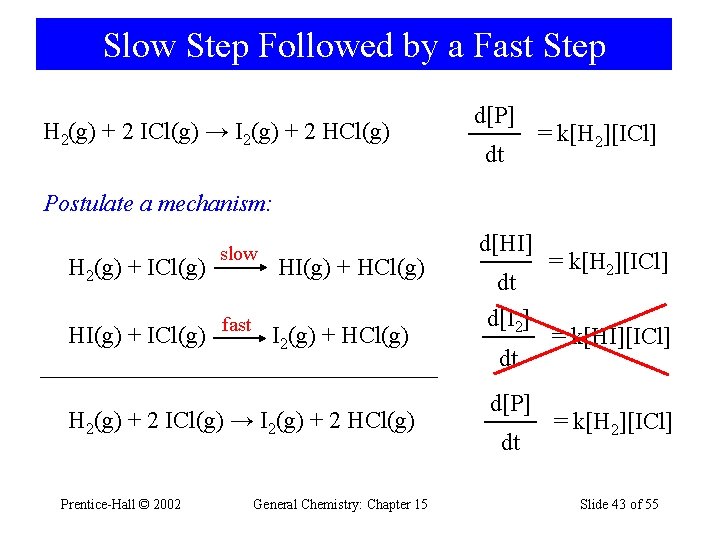
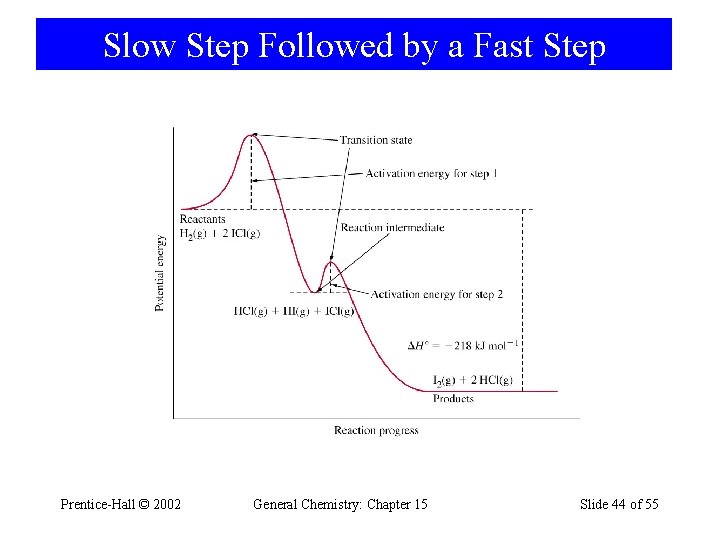
Slow Step Followed by a Fast Step H 2(g) + 2 ICl(g) → I 2(g) + 2 HCl(g) d[P] dt = k[H 2][ICl] Postulate a mechanism: H 2(g) + ICl(g) slow HI(g) + HCl(g) HI(g) + ICl(g) fast I 2(g) + HCl(g) H 2(g) + 2 ICl(g) → I 2(g) + 2 HCl(g) Prentice-Hall © 2002 General Chemistry: Chapter 15 d[HI] dt d[I 2] dt d[P] dt = k[H 2][ICl] = k[HI][ICl] = k[H 2][ICl] Slide 43 of 55

Slow Step Followed by a Fast Step Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 44 of 55

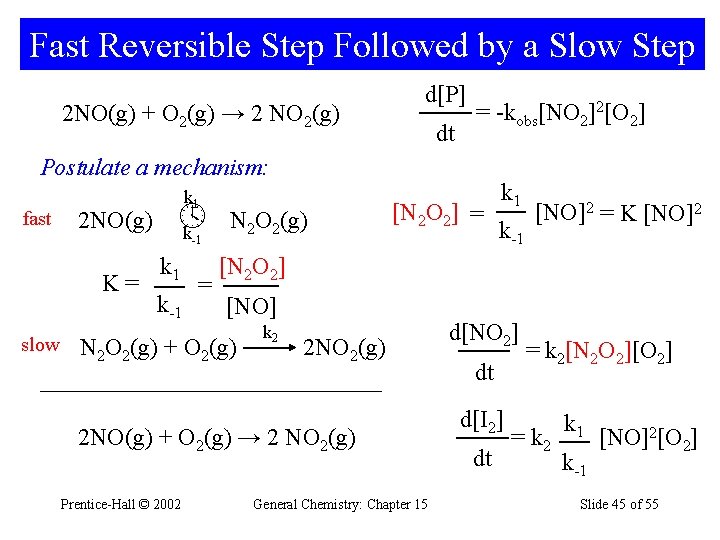
Fast Reversible Step Followed by a Slow Step 2 NO(g) + O 2(g) → 2 NO 2(g) d[P] dt = -kobs[NO 2]2[O 2] Postulate a mechanism: fast 2 NO(g) k 1 k -1 K= slow k 1 k-1 = N 2 O 2(g) [N 2 O 2] = k-1 [NO]2 = K [NO]2 [N 2 O 2] [NO] N 2 O 2(g) + O 2(g) k 2 2 NO 2(g) 2 NO(g) + O 2(g) → 2 NO 2(g) Prentice-Hall © 2002 k 1 General Chemistry: Chapter 15 d[NO 2] dt d[I 2] dt = k 2[N 2 O 2][O 2] = k 2 k 1 k-1 [NO]2[O 2] Slide 45 of 55

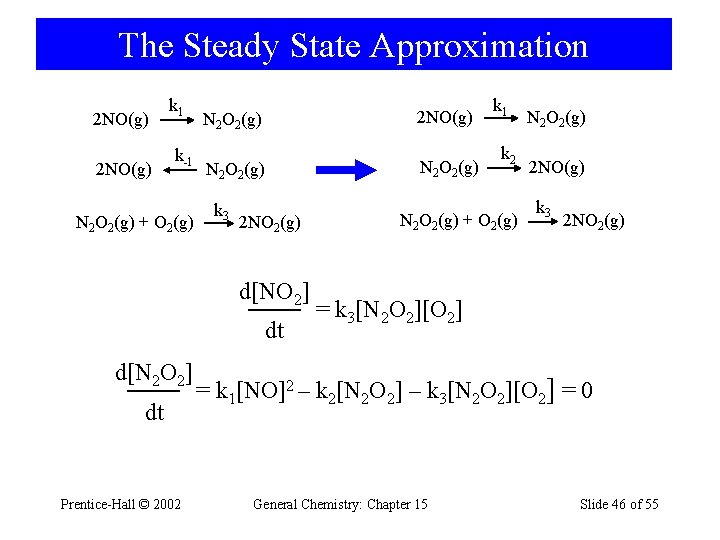
The Steady State Approximation 2 NO(g) k 1 k-1 N 2 O 2(g) + O 2(g) 2 NO(g) N 2 O 2(g) k 3 2 NO 2(g) d[NO 2] dt d[N 2 O 2] dt Prentice-Hall © 2002 N 2 O 2(g) k 1 k 2 N 2 O 2(g) + O 2(g) N 2 O 2(g) 2 NO(g) k 3 2 NO 2(g) = k 3[N 2 O 2][O 2] = k 1[NO]2 – k 2[N 2 O 2] – k 3[N 2 O 2][O 2] = 0 General Chemistry: Chapter 15 Slide 46 of 55
![The Steady State Approximation dN 2 O 2 dt k 1NO2 k The Steady State Approximation d[N 2 O 2] dt = k 1[NO]2 – k](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-47.jpg)
The Steady State Approximation d[N 2 O 2] dt = k 1[NO]2 – k 2[N 2 O 2] – k 3[N 2 O 2][O 2] = 0 k 1[NO]2 = [N 2 O 2](k 2 + k 3[O 2]) [N 2 O 2] = d[NO 2] dt Prentice-Hall © 2002 k 1[NO]2 (k 2 + k 3[O 2]) = k 3[N 2 O 2][O 2] = k 1 k 3[NO]2[O 2] General Chemistry: Chapter 15 (k 2 + k 3[O 2]) Slide 47 of 55
![Kinetic Consequences of Assumptions 2 NOg dNO 2 dt k 1 k 3NO2O Kinetic Consequences of Assumptions 2 NO(g) d[NO 2] dt = k 1 k 3[NO]2[O](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-48.jpg)
Kinetic Consequences of Assumptions 2 NO(g) d[NO 2] dt = k 1 k 3[NO]2[O 2] Let k 2 << k 3 (k 2 + k 3[O 2]) d[NO 2] dt Let k 2 >> k 3 Prentice-Hall © 2002 dt N 2 O 2(g) k 2 N 2 O 2(g) + O 2(g) = Or d[NO 2] k 1 = k 1 k 3[NO]2[O 2] ( k 3[O 2]) k 1 k 3[NO]2[O 2] ( k 2) General Chemistry: Chapter 15 2 NO(g) k 3 2 NO 2(g) = k 1[NO]2 = k 1 k 3 k 2 [NO]2[O 2] Slide 48 of 55

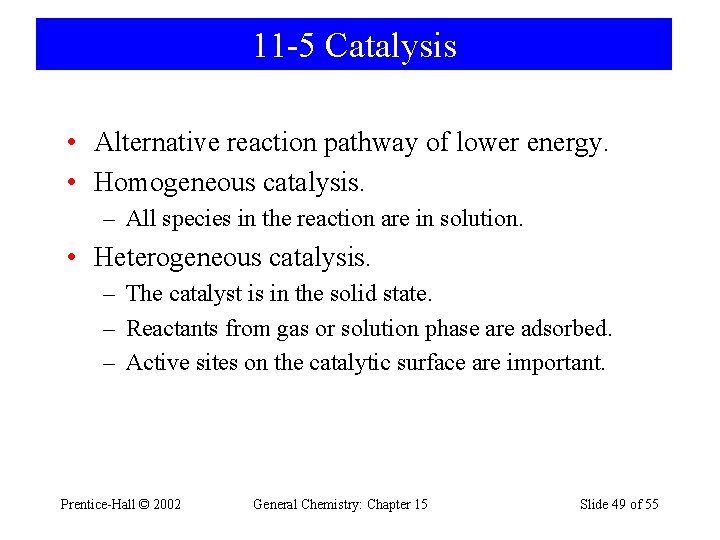
11 -5 Catalysis • Alternative reaction pathway of lower energy. • Homogeneous catalysis. – All species in the reaction are in solution. • Heterogeneous catalysis. – The catalyst is in the solid state. – Reactants from gas or solution phase are adsorbed. – Active sites on the catalytic surface are important. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 49 of 55

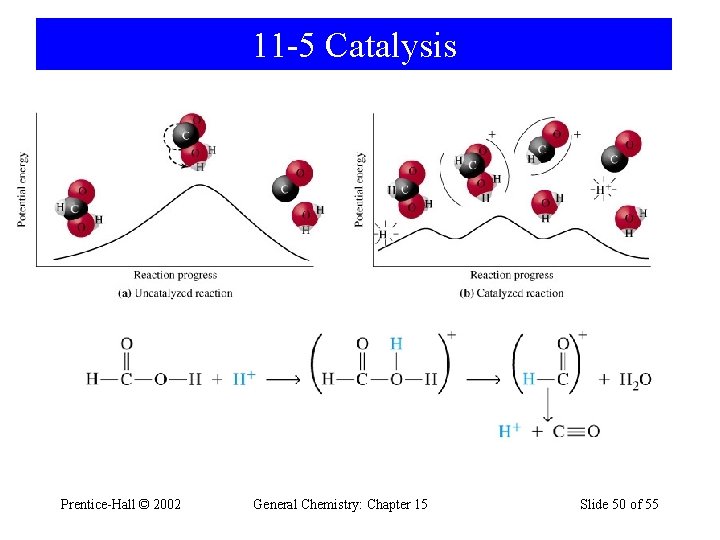
11 -5 Catalysis Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 50 of 55

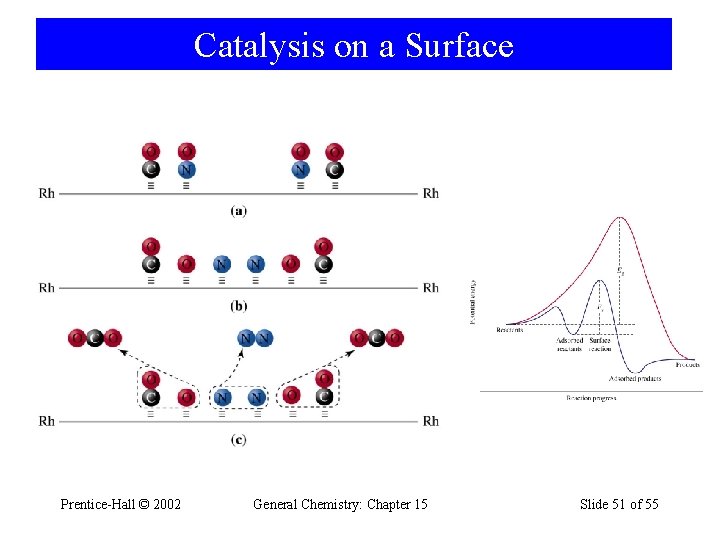
Catalysis on a Surface Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 51 of 55

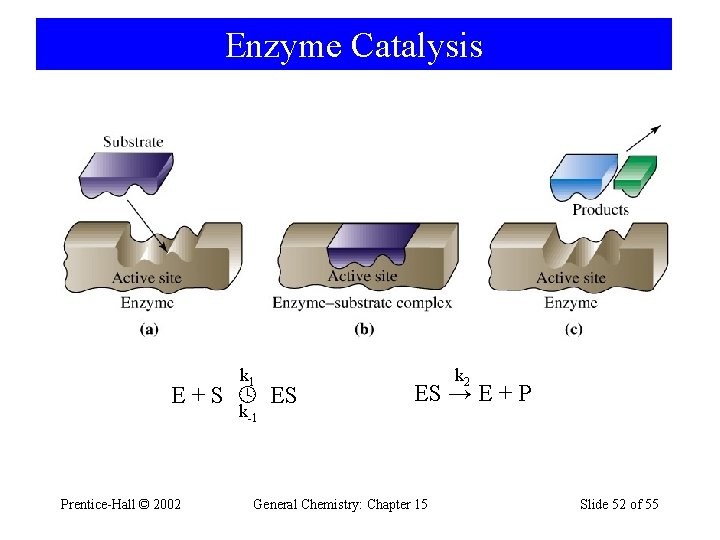
Enzyme Catalysis k 1 E + S ES k-1 Prentice-Hall © 2002 k 2 ES → E + P General Chemistry: Chapter 15 Slide 52 of 55
![Saturation Kinetics k 1 dP k 2 E S ES E Saturation Kinetics k 1 d[P] k 2 E + S ES → E +](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-53.jpg)
Saturation Kinetics k 1 d[P] k 2 E + S ES → E + P dt k-1 d[P] dt = k 2[ES] = k 1[E][S] – k-1[ES] – k 2[ES]= 0 k 1[E][S] = (k-1+k 2 )[ES] [E] = [E]0 – [ES] k 1[S]([E]0 –[ES]) = (k-1+k 2 )[ES] = Prentice-Hall © 2002 General Chemistry: Chapter 15 k 1[E]0 [S] (k-1+k 2 ) + k 1[S] Slide 53 of 55
![MichaelisMenten dP dt dP k 2E0 dt dP dt k 1 Michaelis-Menten d[P] dt d[P] = k 2[E]0 dt d[P] dt = = k 1](https://slidetodoc.com/presentation_image_h2/9b526c430bbf12d737eb1fc05ea1f355/image-54.jpg)
Michaelis-Menten d[P] dt d[P] = k 2[E]0 dt d[P] dt = = k 1 k 2[E]0 [S] (k-1+k 2 ) + k 1[S] k 2[E]0 [S] (k-1+k 2 ) + [S] k 1 d[P] dt = Prentice-Hall © 2002 k 2 [E]0 [S] KM General Chemistry: Chapter 15 d[P] dt = k 2[E]0 [S] KM + [S] Slide 54 of 55

Chapter 15 Questions Develop problem solving skills and base your strategy not on solutions to specific problems but on understanding. Choose a variety of problems from the text as examples. Practice good techniques and get coaching from people who have been here before. Prentice-Hall © 2002 General Chemistry: Chapter 15 Slide 55 of 55
 Kimya gaz kanunları
Kimya gaz kanunları General chemistry ders notları
General chemistry ders notları Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Hawthorne araştırmaları sonuçları
Hawthorne araştırmaları sonuçları Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen harwood: selected poems
Gwen harwood: selected poems Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Applications of nuclear chemistry
Applications of nuclear chemistry Properties of modern materials
Properties of modern materials Modern chemistry chapter 9 stoichiometry test b answers
Modern chemistry chapter 9 stoichiometry test b answers Modern chemistry chapter 7 test
Modern chemistry chapter 7 test Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 acids and bases
Chapter 14 acids and bases Modern chemistry chapter 13
Modern chemistry chapter 13 Chapter 12 review solutions answer key
Chapter 12 review solutions answer key Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Principles of network applications in computer networks
Principles of network applications in computer networks Principles of network applications
Principles of network applications General receiving principles
General receiving principles General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Modern marketing principles
Modern marketing principles Postmodern principles
Postmodern principles Build a modern computer from first principles
Build a modern computer from first principles Postmodern principles of art
Postmodern principles of art Principles of postmodernism
Principles of postmodernism Twelve principles of green chemistry
Twelve principles of green chemistry Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Instrument grasp
Instrument grasp General principles of congestion control
General principles of congestion control