General Chemistry Principles and Modern Applications Petrucci Harwood



- Slides: 37

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 9: The Periodic Table and Some Atomic Properties Philip Dutton University of Windsor, Canada Prentice-Hall © 2002

Contents 9 -1 9 -2 9 -3 9 -4 9 -5 9 -6 9 -7 Classifying the Elements: The Periodic Law and the Periodic Table Metals and Nonmetals and Their Ions The Sizes of Atoms and Ions Ionization Energy Electron Affinity Magnetic Properties Periodic Properties of the Elements Focus on The Periodic Law and Mercury Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 2 of 35

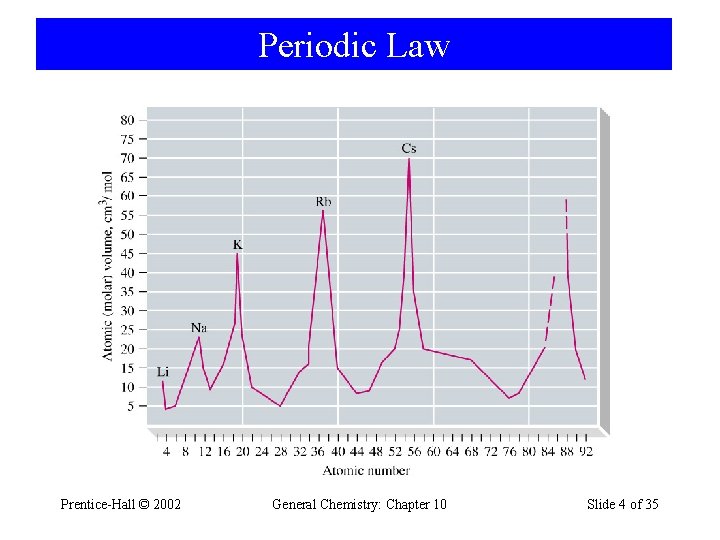
9 -1 Classifying the Elements: The Periodic Law and the Periodic Table • 1869, Dimitri Mendeleev Lothar Meyer When the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 3 of 35

Periodic Law Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 4 of 35

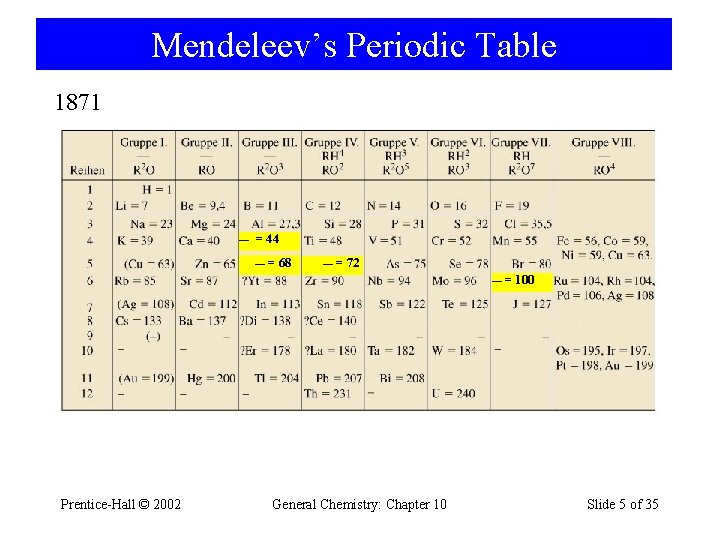
Mendeleev’s Periodic Table 1871 — = 44 — = 68 — = 72 —= Prentice-Hall © 2002 General Chemistry: Chapter 10 100 Slide 5 of 35

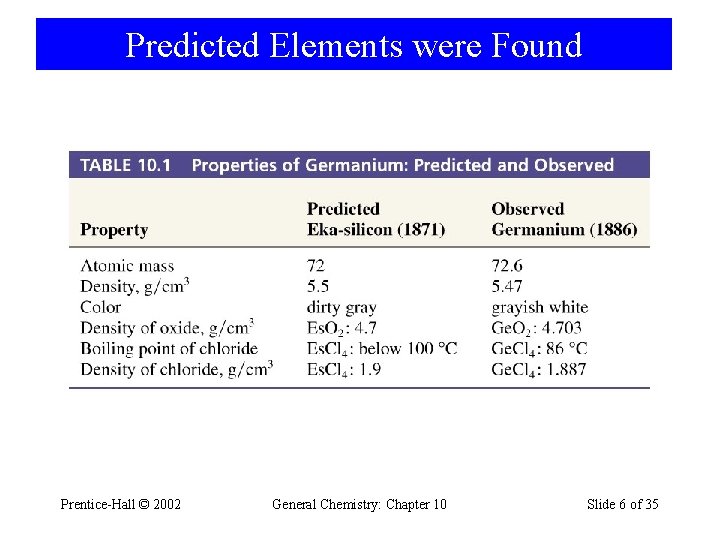
Predicted Elements were Found Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 6 of 35

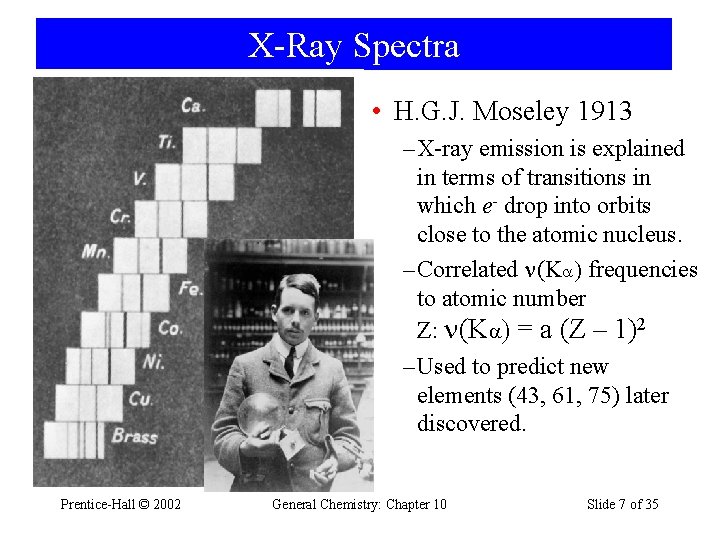
X-Ray Spectra • H. G. J. Moseley 1913 – X-ray emission is explained in terms of transitions in which e- drop into orbits close to the atomic nucleus. – Correlated (Ka) frequencies to atomic number Z: (Ka) = a (Z – 1)2 – Used to predict new elements (43, 61, 75) later discovered. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 7 of 35

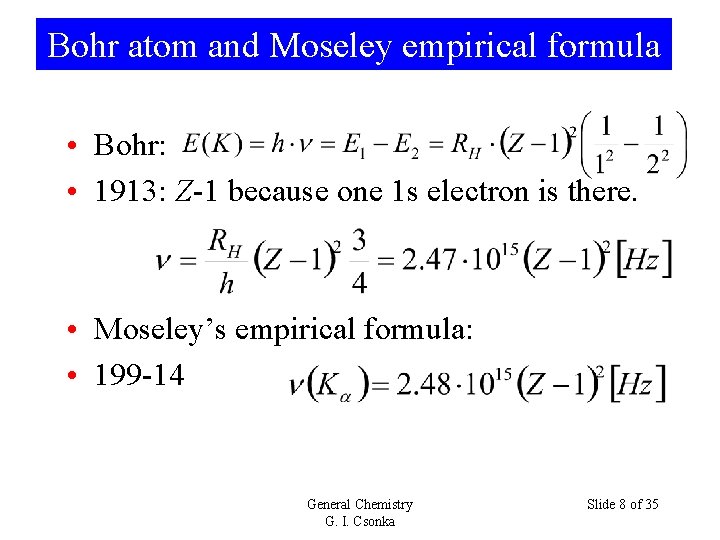
Bohr atom and Moseley empirical formula • Bohr: • 1913: Z-1 because one 1 s electron is there. • Moseley’s empirical formula: • 199 -14 General Chemistry G. I. Csonka Slide 8 of 35

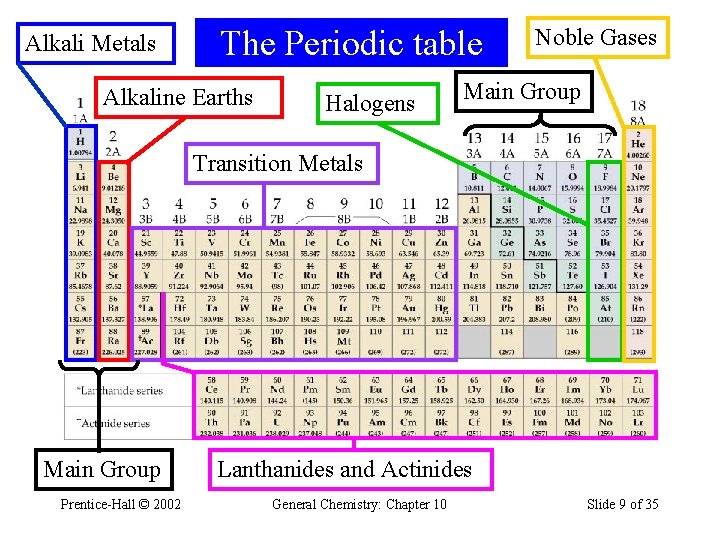
Alkali Metals The Periodic table Alkaline Earths Halogens Noble Gases Main Group Transition Metals Main Group Prentice-Hall © 2002 Lanthanides and Actinides General Chemistry: Chapter 10 Slide 9 of 35

9 -2 Metals and Nonmetals and Their Ions • Metals – Good conductors of heat and electricity. – Malleable and ductile. – Moderate to high melting points. • Nonmetals – Nonconductors of heat and electricity. – Brittle solids. – Some are gases at room temperature. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 10 of 35

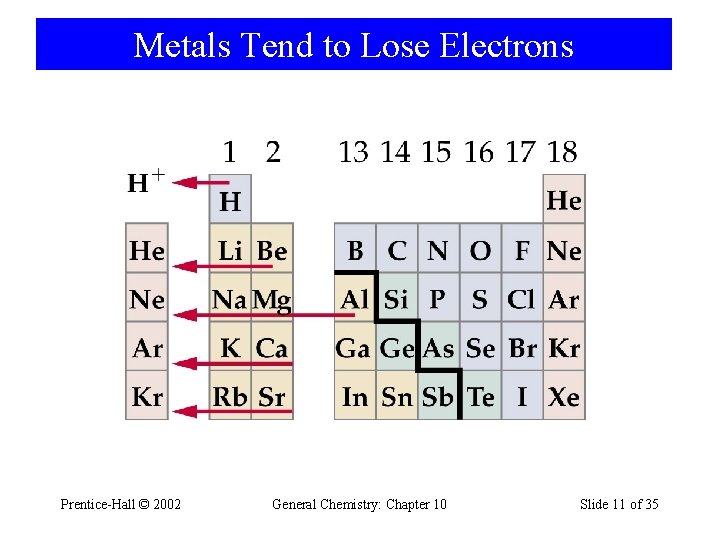
Metals Tend to Lose Electrons Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 11 of 35

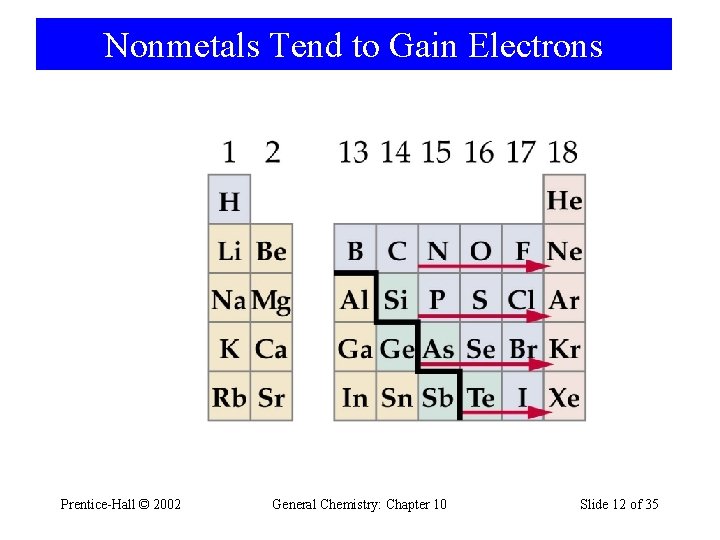
Nonmetals Tend to Gain Electrons Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 12 of 35

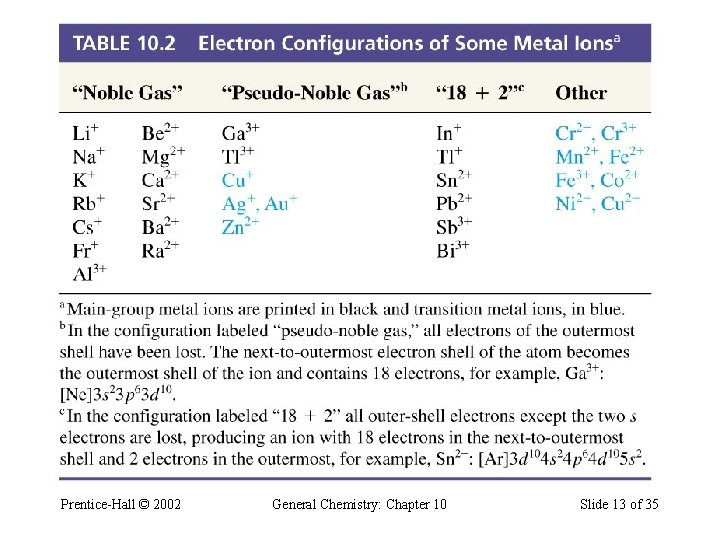
Electron Configuration of Some Ions Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 13 of 35

9 -3 The Sizes of Atoms and Ions Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 14 of 35

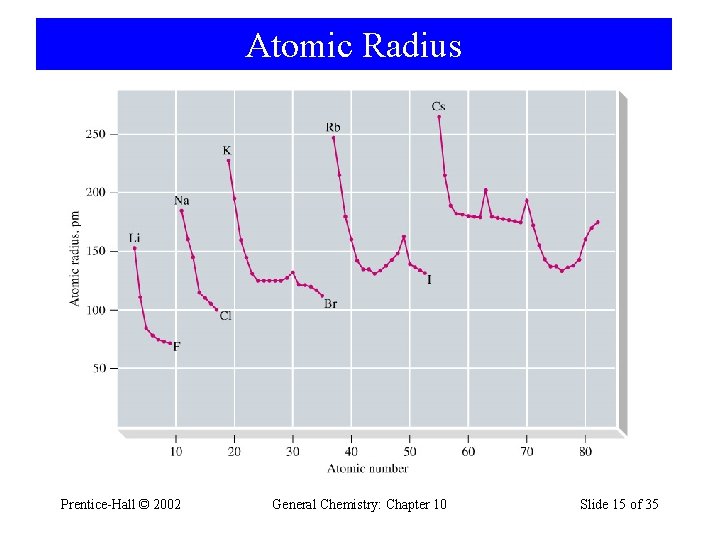
Atomic Radius Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 15 of 35

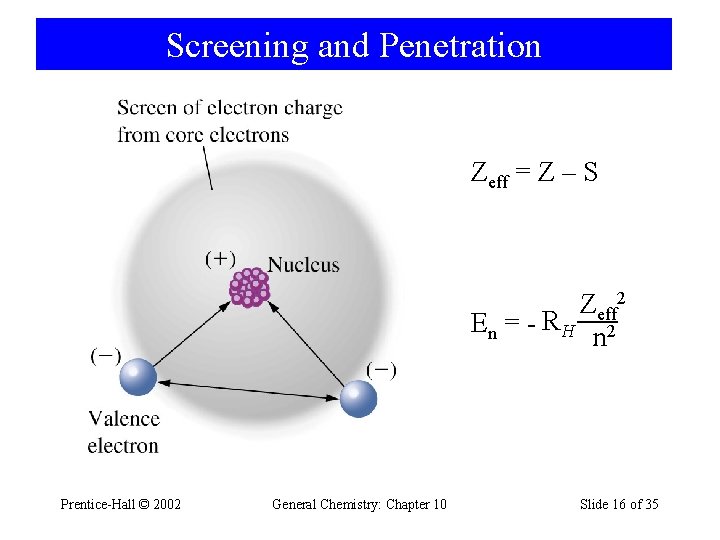
Screening and Penetration Zeff = Z – S Zeff 2 En = - RH 2 n Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 16 of 35

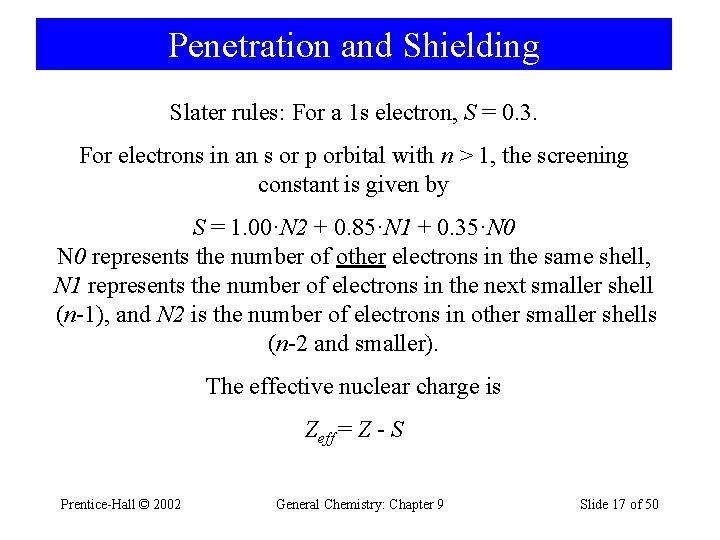
Penetration and Shielding Slater rules: For a 1 s electron, S = 0. 3. For electrons in an s or p orbital with n > 1, the screening constant is given by S = 1. 00·N 2 + 0. 85·N 1 + 0. 35·N 0 represents the number of other electrons in the same shell, N 1 represents the number of electrons in the next smaller shell (n-1), and N 2 is the number of electrons in other smaller shells (n-2 and smaller). The effective nuclear charge is Zeff = Z - S Prentice-Hall © 2002 General Chemistry: Chapter 9 Slide 17 of 50

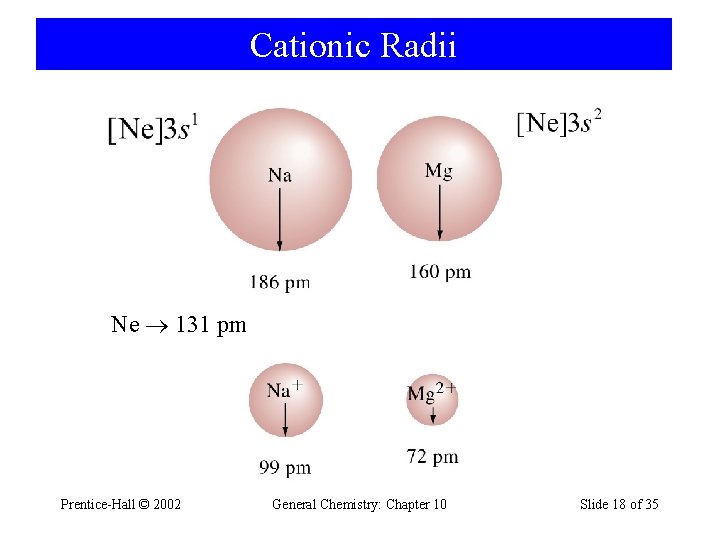
Cationic Radii Ne 131 pm Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 18 of 35

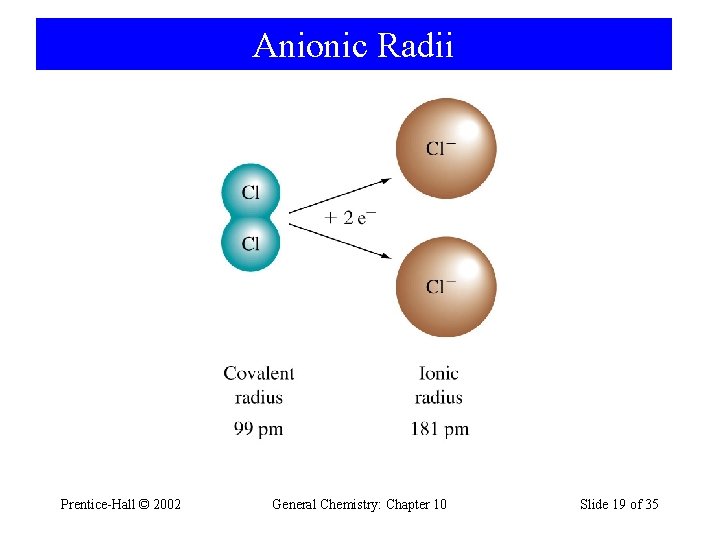
Anionic Radii Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 19 of 35

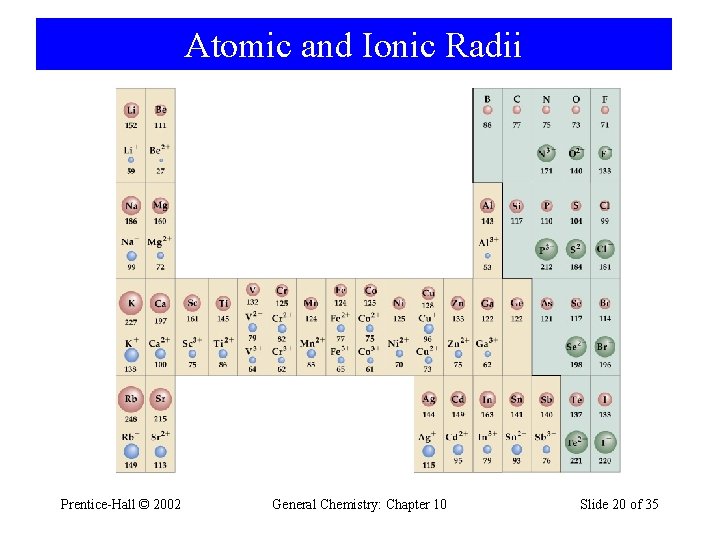
Atomic and Ionic Radii Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 20 of 35

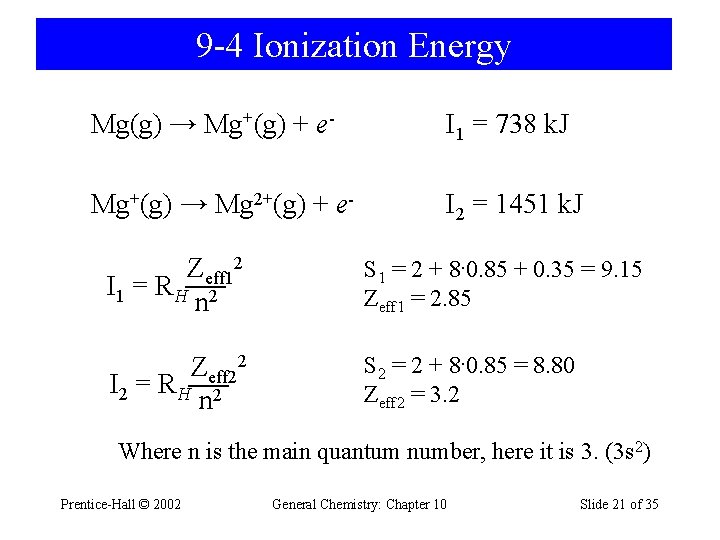
9 -4 Ionization Energy Mg(g) → Mg+(g) + e- I 1 = 738 k. J Mg+(g) → Mg 2+(g) + e- I 2 = 1451 k. J Zeff 12 I 1 = R H 2 n S 1 = 2 + 8· 0. 85 + 0. 35 = 9. 15 Zeff 1 = 2. 85 Zeff 22 I 2 = R H 2 n S 2 = 2 + 8· 0. 85 = 8. 80 Zeff 2 = 3. 2 Where n is the main quantum number, here it is 3. (3 s 2) Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 21 of 35

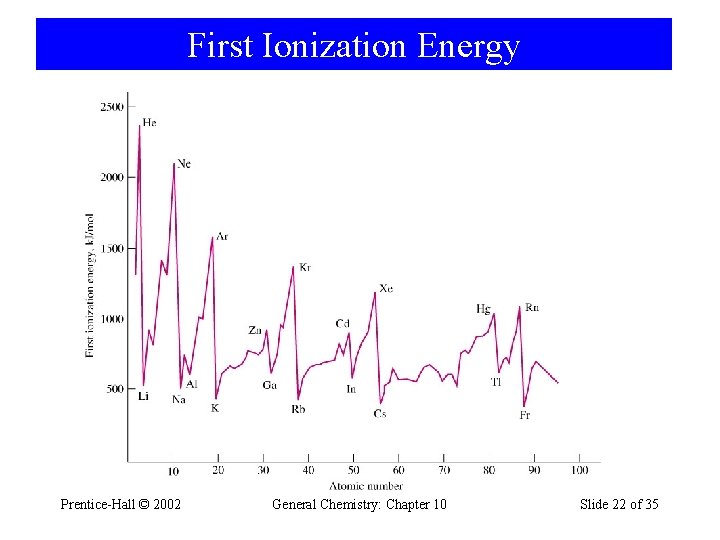
First Ionization Energy Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 22 of 35

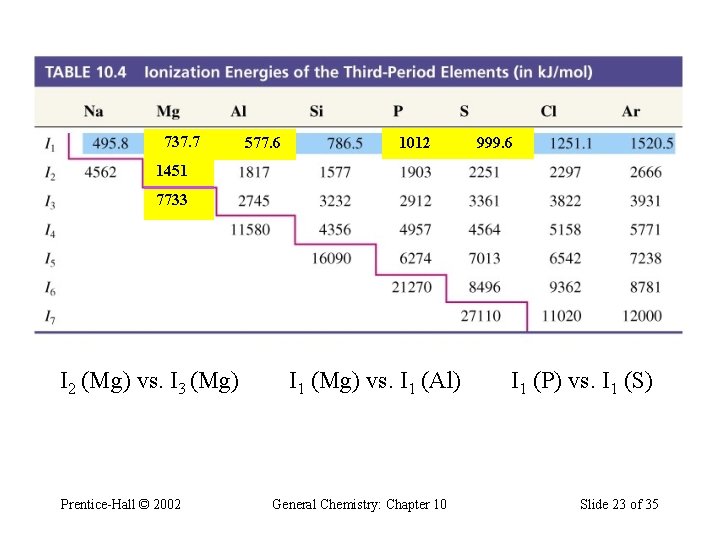
Table 10. 4 Ionization Energies of the Third-Period Elements (in k. J/mol) 737. 7 577. 6 1012 999. 6 1451 7733 I 2 (Mg) vs. I 3 (Mg) Prentice-Hall © 2002 I 1 (Mg) vs. I 1 (Al) General Chemistry: Chapter 10 I 1 (P) vs. I 1 (S) Slide 23 of 35

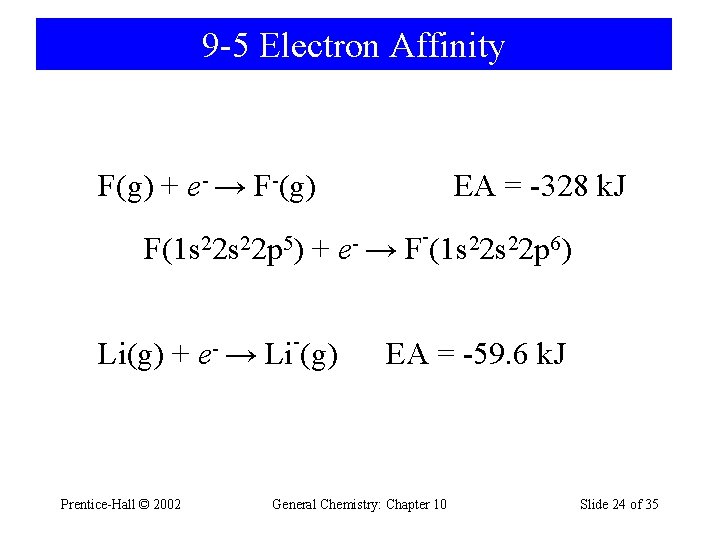
9 -5 Electron Affinity F(g) + e- → F-(g) F(1 s 22 p 5) Li(g) + Prentice-Hall © 2002 e- → - + Li (g) EA = -328 k. J e- → - F (1 s 22 p 6) EA = -59. 6 k. J General Chemistry: Chapter 10 Slide 24 of 35

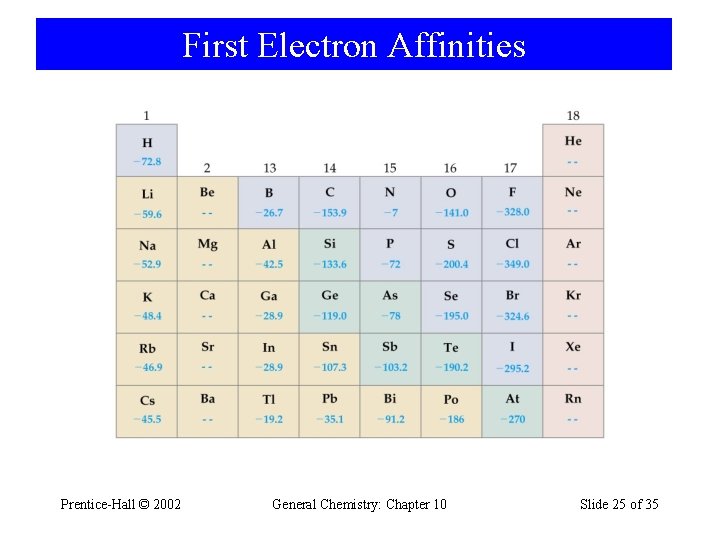
First Electron Affinities Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 25 of 35

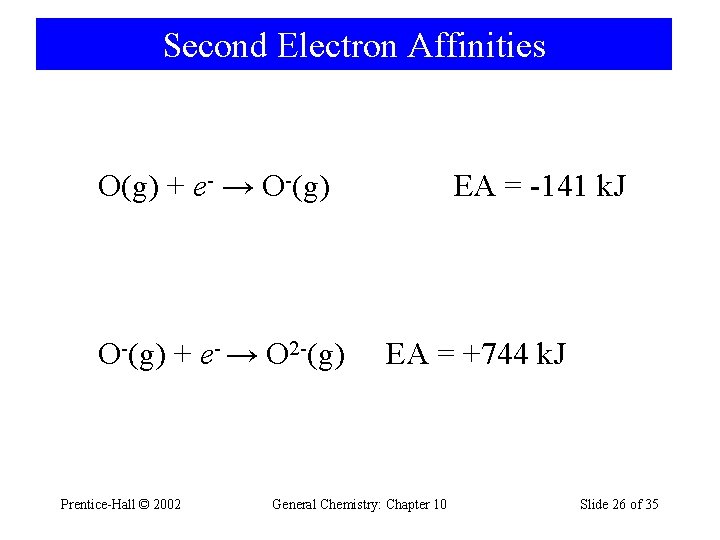
Second Electron Affinities O(g) + e- → O-(g) + e- → O 2 -(g) Prentice-Hall © 2002 EA = -141 k. J EA = +744 k. J General Chemistry: Chapter 10 Slide 26 of 35

9 -6 Magnetic Properties • Diamagnetic atoms or ions: – All e- are paired. – Weakly repelled by a magnetic field. • Paramagnetic atoms or ions: – Unpaired e-. – Attracted to an external magnetic field. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 27 of 35

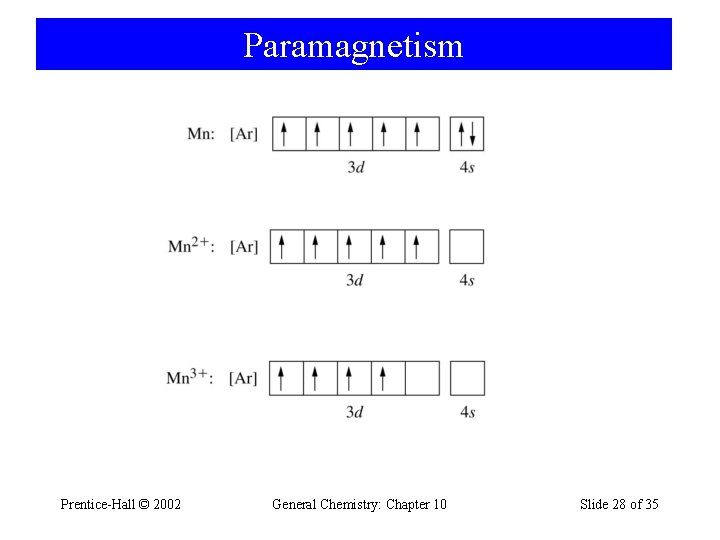
Paramagnetism Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 28 of 35

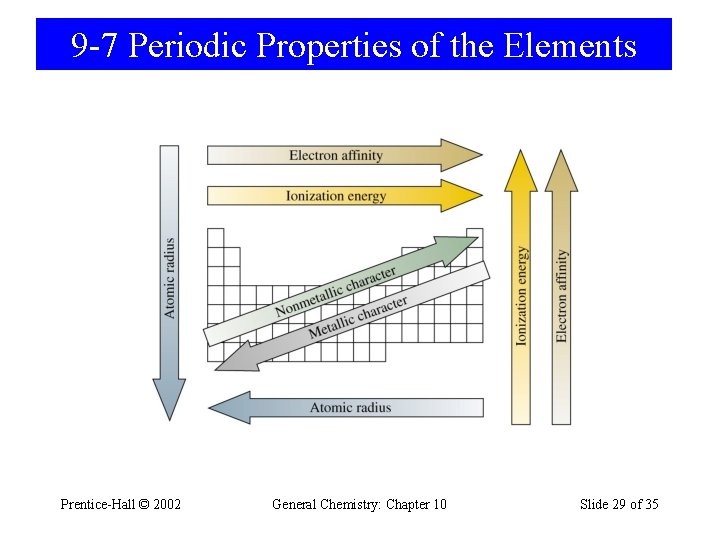
9 -7 Periodic Properties of the Elements Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 29 of 35

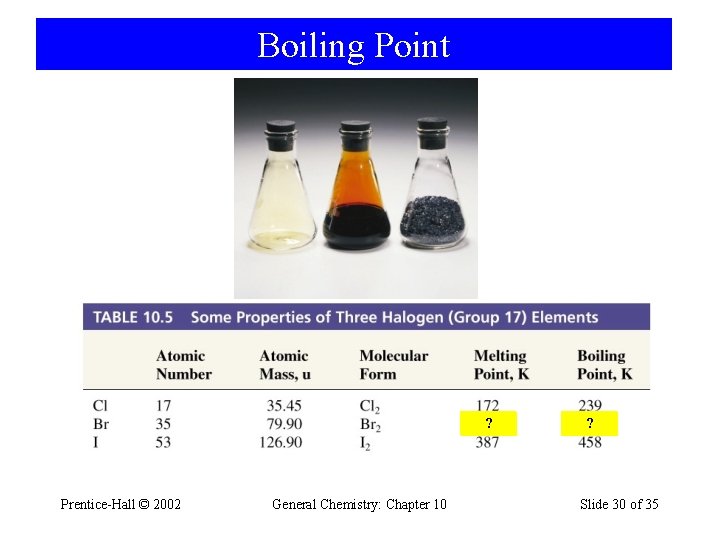
Boiling Point ? 266 Prentice-Hall © 2002 General Chemistry: Chapter 10 ? 332 Slide 30 of 35

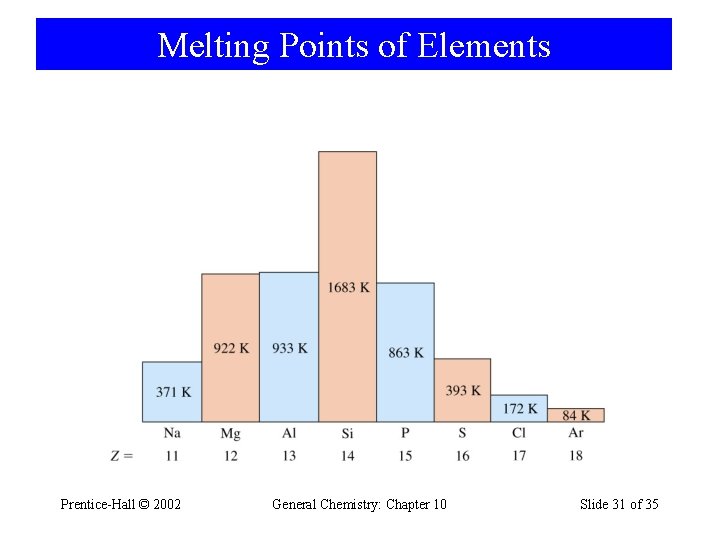
Melting Points of Elements Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 31 of 35

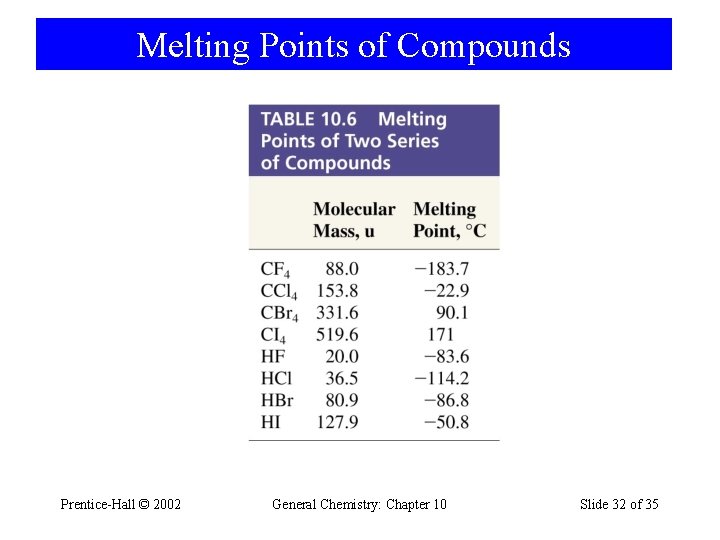
Melting Points of Compounds Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 32 of 35

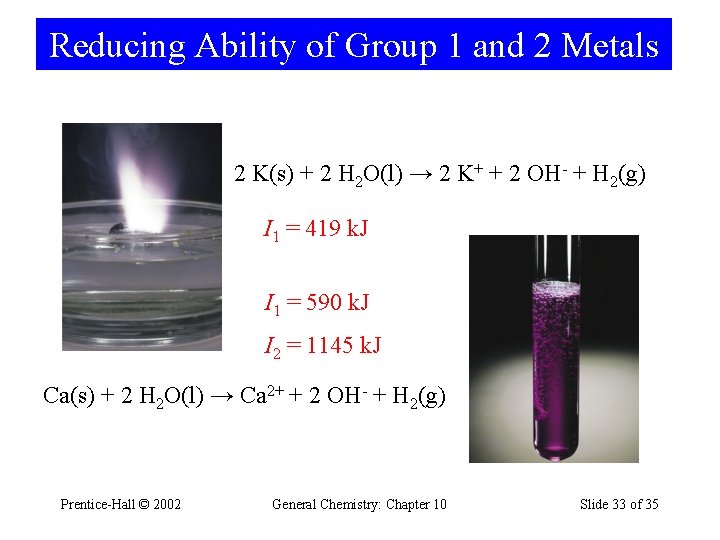
Reducing Ability of Group 1 and 2 Metals 2 K(s) + 2 H 2 O(l) → 2 K+ + 2 OH- + H 2(g) I 1 = 419 k. J I 1 = 590 k. J I 2 = 1145 k. J Ca(s) + 2 H 2 O(l) → Ca 2+ + 2 OH- + H 2(g) Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 33 of 35

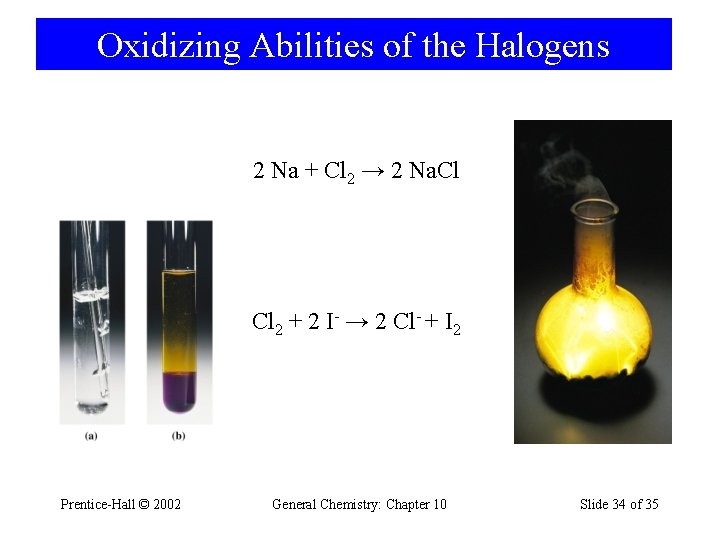
Oxidizing Abilities of the Halogens 2 Na + Cl 2 → 2 Na. Cl Cl 2 + 2 I- → 2 Cl- + I 2 Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 34 of 35

Acid Base Nature of Element Oxides • Basic oxides or base anhydrides: Li 2 O(s) + H 2 O(l) → 2 Li+(aq) + 2 OH-(aq) • Acidic oxides or acid anhydrides: SO 2 (g) + H 2 O(l) → H 2 SO 3(aq) • Na 2 O and Mg. O yield basic solutions • Cl 2 O, SO 2 and P 4 O 10 yield acidic solutions • Si. O 2 dissolves in strong base, acidic oxide. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 35 of 35

Focus on The Periodic Law and Mercury • Should be a solid. • Relativistic shrinking of s-orbitals affects all heavy metals but is maximum with Hg. Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 36 of 35

Chapter 10 Questions 1, 2, 18, 21, 27, 33, 39, 43, 51, 55 Prentice-Hall © 2002 General Chemistry: Chapter 10 Slide 37 of 35
 Sodyum metalinin su ile tepkimesi
Sodyum metalinin su ile tepkimesi General chemistry ders notları
General chemistry ders notları Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Yankee city araştırması nedir
Yankee city araştırması nedir Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen harwood selected poems
Gwen harwood selected poems Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Allan
Allan Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Irradiated food
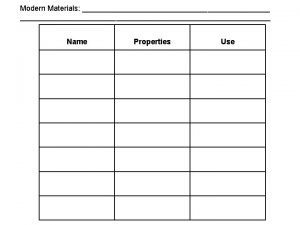
Irradiated food Properties of smart and modern materials
Properties of smart and modern materials Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Chemical formula of love
Chemical formula of love Modern chemistry chapter 15
Modern chemistry chapter 15 Modern chemistry chapter 14 review answers
Modern chemistry chapter 14 review answers Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Chapter 12 solutions chemistry
Chapter 12 solutions chemistry Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
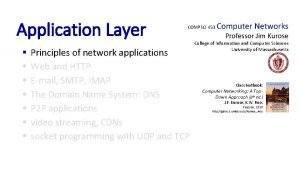
Organic vs inorganic chemistry Principles of network applications in computer networks
Principles of network applications in computer networks Principles of network applications
Principles of network applications General receiving principles
General receiving principles General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature