General Chemistry Principles and Modern Applications Petrucci Harwood

- Slides: 23

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 6: Gases Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi)

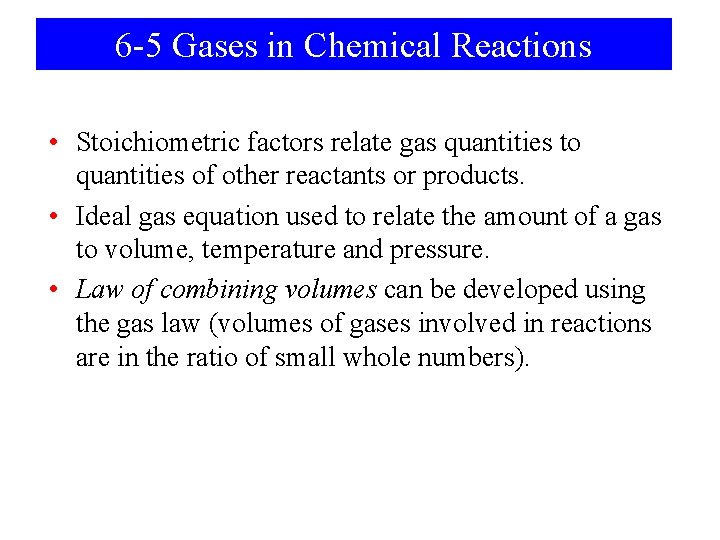
Contents 6 -1 Properties of Gases: Gas Pressure 6 -2 The Simple Gas Laws 6 -3 Combining the Gas Laws: The Ideal Gas Equation and The General Gas Equation 6 -4 Applications of the Ideal Gas Equation 6 -5 Gases in Chemical Reactions 6 -6 Mixtures of Gases

Contents 6 -6 Mixtures of Gases 6 -7 Kinetic—Molecular Theory of Gases 6 -8 Gas Properties Relating to the Kinetic—Molecular Theory 6 -9 Non-ideal (real) Gases Focus on The Chemistry of Air-Bag Systems

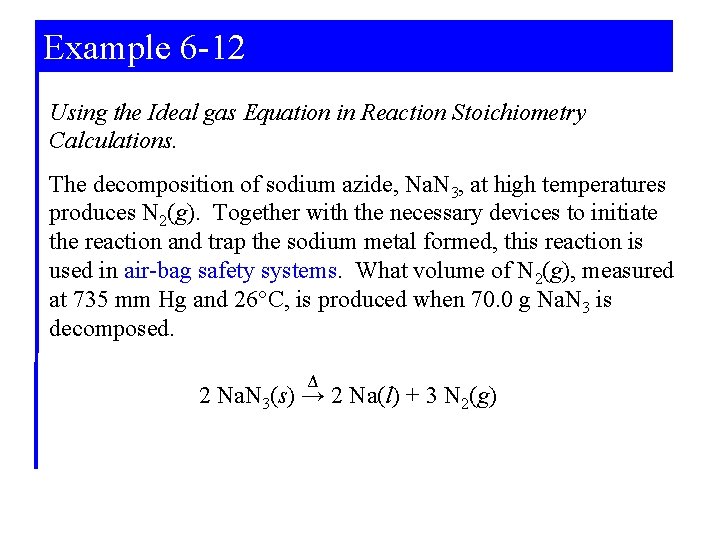
6 -5 Gases in Chemical Reactions • Stoichiometric factors relate gas quantities to quantities of other reactants or products. • Ideal gas equation used to relate the amount of a gas to volume, temperature and pressure. • Law of combining volumes can be developed using the gas law (volumes of gases involved in reactions are in the ratio of small whole numbers).

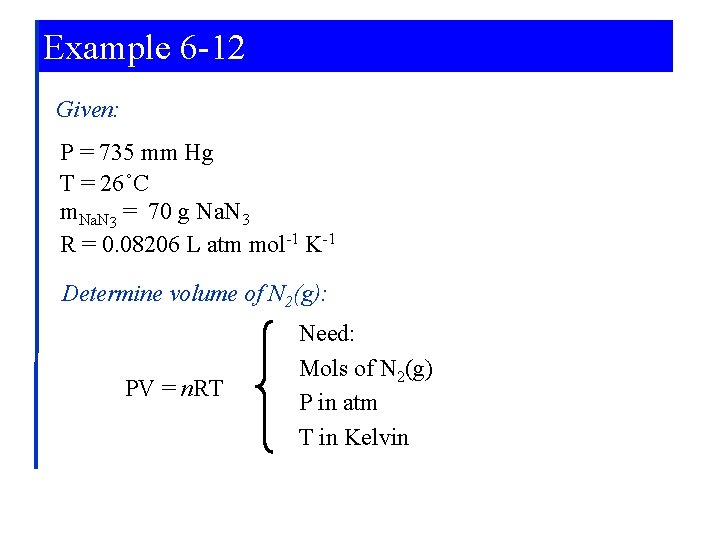
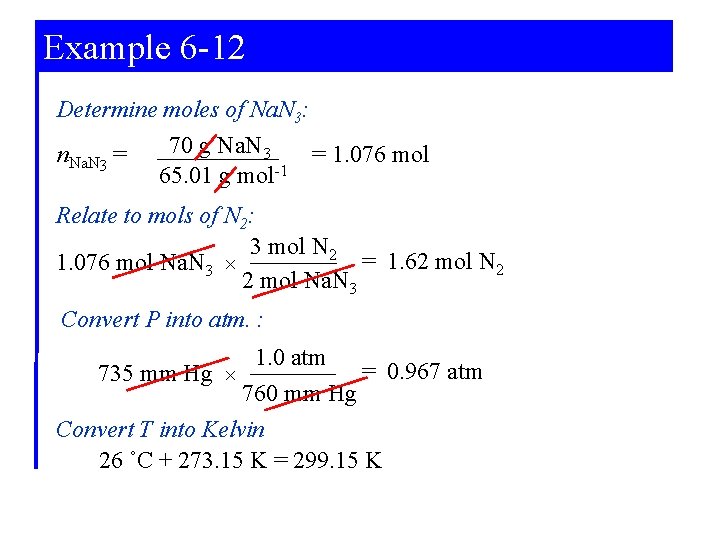
Example 6 -12 Using the Ideal gas Equation in Reaction Stoichiometry Calculations. The decomposition of sodium azide, Na. N 3, at high temperatures produces N 2(g). Together with the necessary devices to initiate the reaction and trap the sodium metal formed, this reaction is used in air-bag safety systems. What volume of N 2(g), measured at 735 mm Hg and 26°C, is produced when 70. 0 g Na. N 3 is decomposed. Δ 2 Na. N 3(s) → 2 Na(l) + 3 N 2(g)

Example 6 -12 Given: P = 735 mm Hg T = 26˚C m. Na. N 3 = 70 g Na. N 3 R = 0. 08206 L atm mol-1 K-1 Determine volume of N 2(g): PV = n. RT Need: Mols of N 2(g) P in atm T in Kelvin

Example 6 -12 Determine moles of Na. N 3: n. Na. N 3 = 70 g Na. N 3 65. 01 g mol-1 = 1. 076 mol Relate to mols of N 2: 3 mol N 2 = 1. 62 mol N 2 1. 076 mol Na. N 3 × 2 mol Na. N 3 Convert P into atm. : 1. 0 atm = 0. 967 atm 735 mm Hg × 760 mm Hg Convert T into Kelvin 26 ˚C + 273. 15 K = 299. 15 K

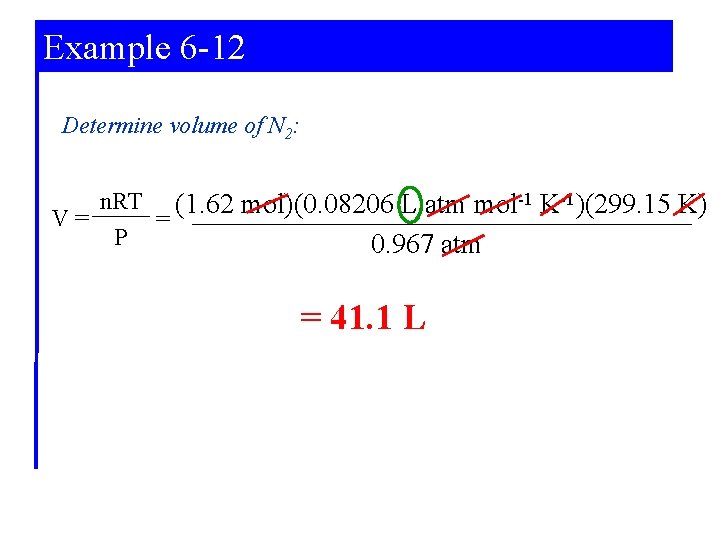
Example 6 -12 Determine volume of N 2: n. RT (1. 62 mol)(0. 08206 L atm mol-1 K-1)(299. 15 K) V= = P 0. 967 atm = 41. 1 L

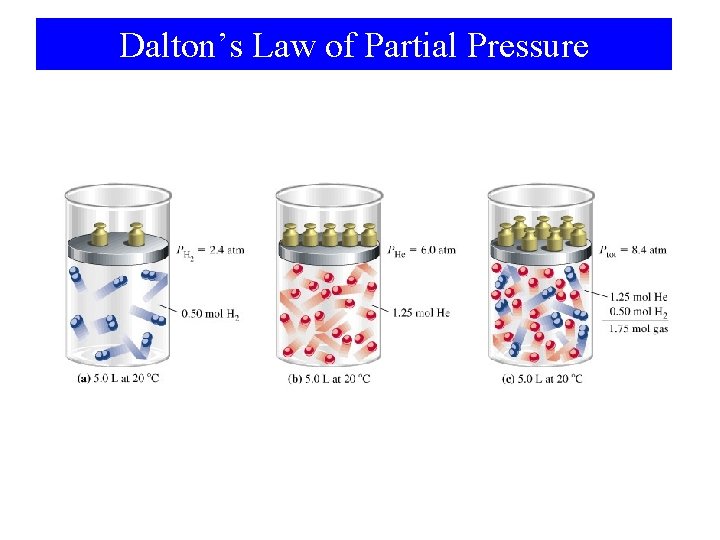
6 -6 Mixtures of Gases • Gas laws apply to mixtures of gases. • Simplest approach is to use ntotal, but. . • Partial pressure – Each component of a gas mixture exerts a pressure that it would exert if it were in the container alone.

Dalton’s Law of Partial Pressure

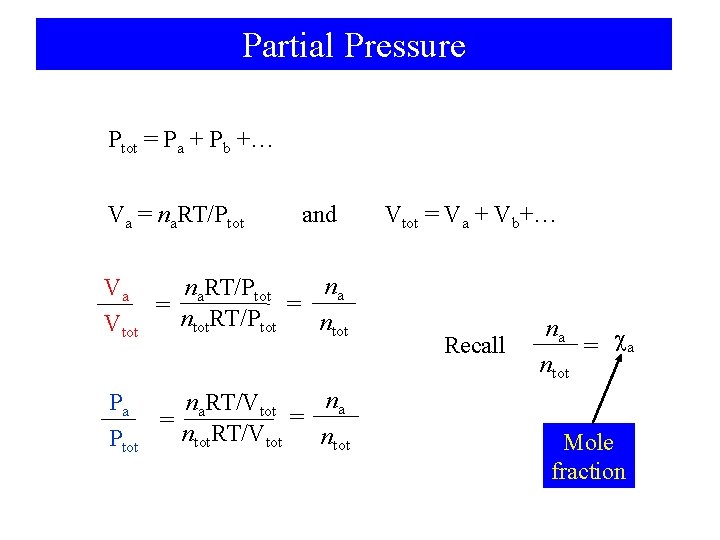
Partial Pressure Ptot = Pa + Pb +… Va = na. RT/Ptot and na na. RT/Ptot Va = = ntot. RT/Ptot ntot Vtot na na. RT/Vtot Pa = = ntot. RT/Vtot ntot Ptot Vtot = Va + Vb+… Recall na = a ntot Mole fraction

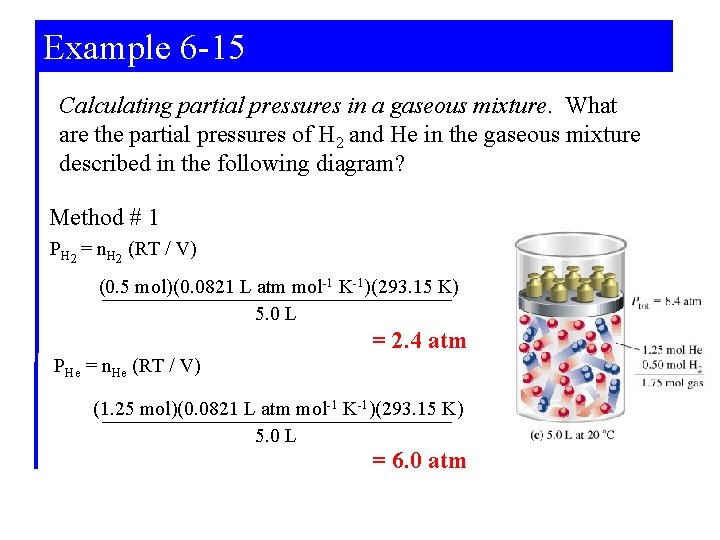
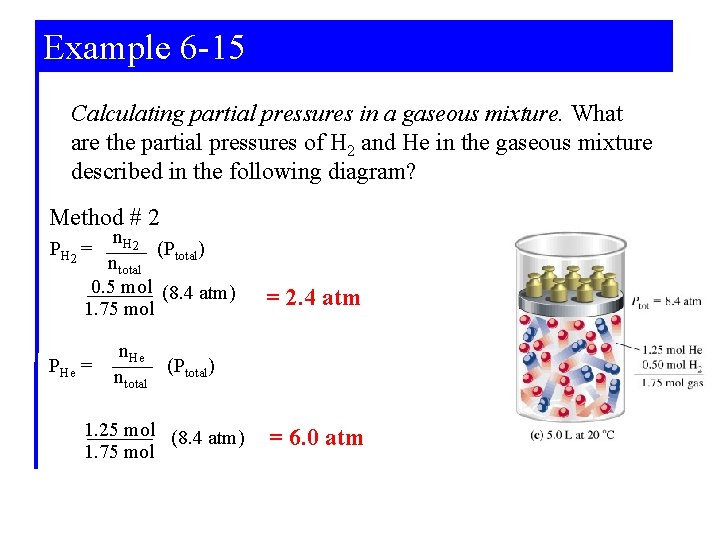
Example 6 -15 Calculating partial pressures in a gaseous mixture. What are the partial pressures of H 2 and He in the gaseous mixture described in the following diagram? Method # 1 PH 2 = n. H 2 (RT / V) (0. 5 mol)(0. 0821 L atm mol-1 K-1)(293. 15 K) 5. 0 L = 2. 4 atm PHe = n. He (RT / V) (1. 25 mol)(0. 0821 L atm mol-1 K-1)(293. 15 K) 5. 0 L = 6. 0 atm

Example 6 -15 Calculating partial pressures in a gaseous mixture. What are the partial pressures of H 2 and He in the gaseous mixture described in the following diagram? Method # 2 n. H 2 (Ptotal) ntotal 0. 5 mol (8. 4 atm) 1. 75 mol PH 2 = PHe = = 2. 4 atm n. He (Ptotal) ntotal 1. 25 mol (8. 4 atm) 1. 75 mol = 6. 0 atm

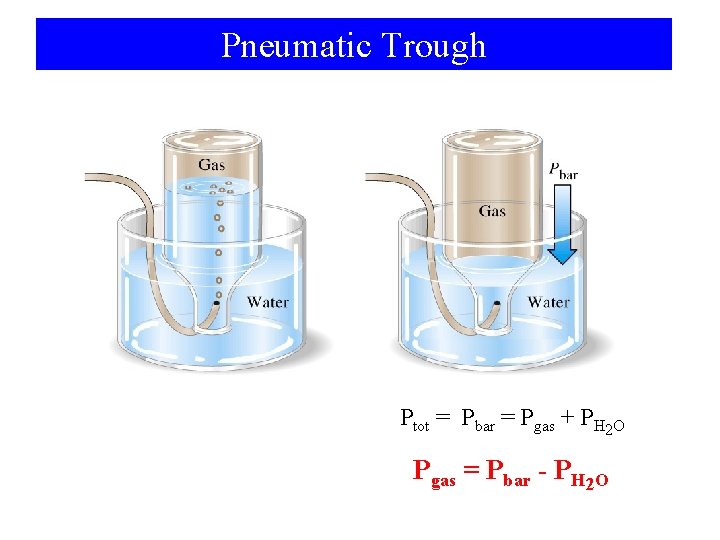
Pneumatic Trough Ptot = Pbar = Pgas + PH 2 O Pgas = Pbar - PH 2 O

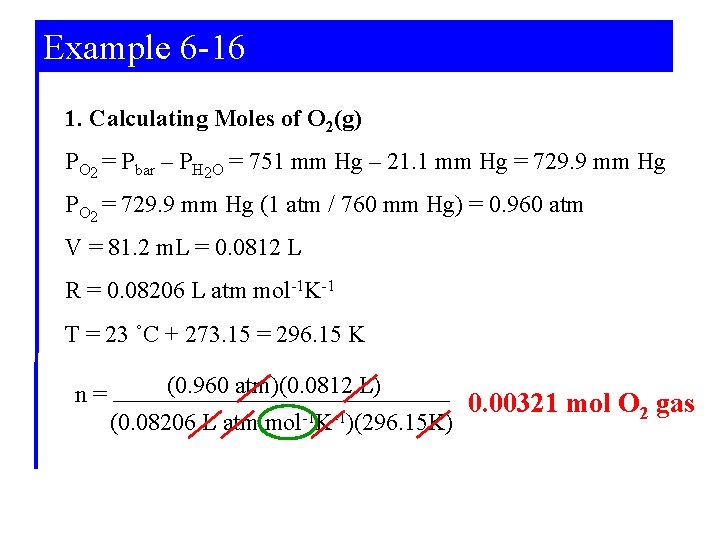
Example 6 -16 Collecting gas over a liquid (water). In the following reaction, 81. 2 m. L of O 2(g) is collected over water at 23 ˚C and barometric pressure 751 mm Hg. What must have been the mass of Ag 2 O(s) decomposed? (the vapour pressure of water at 23 ˚C is 21. 1 mm Hg) 2 Ag 2 O(s) 4 Ag(s) + O 2(g) Strategy: 1. Calculate moles of O 2(g) 2. Relate moles of O 2(g) to moles of Ag 2 O 3. Convert moles of Ag 2 O to mass

Example 6 -16 1. Calculating Moles of O 2(g) PO 2 = Pbar – PH 2 O = 751 mm Hg – 21. 1 mm Hg = 729. 9 mm Hg PO 2 = 729. 9 mm Hg (1 atm / 760 mm Hg) = 0. 960 atm V = 81. 2 m. L = 0. 0812 L R = 0. 08206 L atm mol-1 K-1 T = 23 ˚C + 273. 15 = 296. 15 K n= (0. 960 atm)(0. 0812 L) (0. 08206 L atm mol-1 K-1)(296. 15 K) 0. 00321 mol O 2 gas

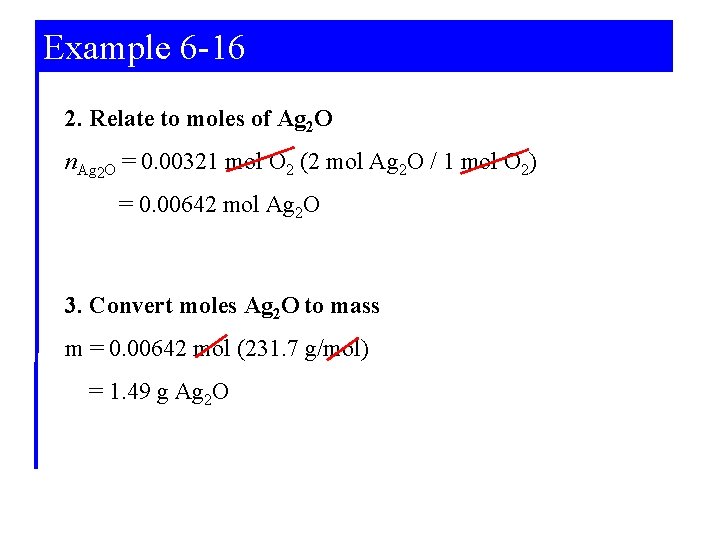
Example 6 -16 2. Relate to moles of Ag 2 O n. Ag 2 O = 0. 00321 mol O 2 (2 mol Ag 2 O / 1 mol O 2) = 0. 00642 mol Ag 2 O 3. Convert moles Ag 2 O to mass m = 0. 00642 mol (231. 7 g/mol) = 1. 49 g Ag 2 O

6 -7 Kinetic Molecular Theory of Gases • Gas particles are point masses in constant, random, straight line motion. • Gas particles are separated by great distances. • Collisions are rapid and elastic. • No force between particles. • Total energy remains constant.

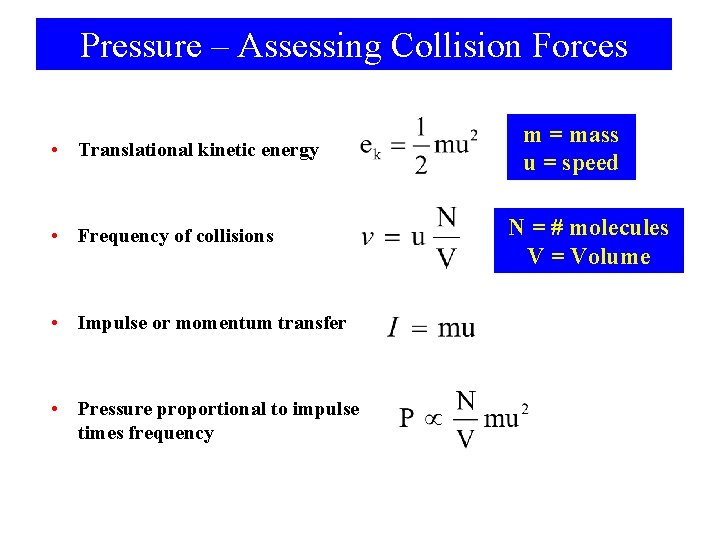
Pressure – Assessing Collision Forces • Translational kinetic energy • Frequency of collisions • Impulse or momentum transfer • Pressure proportional to impulse times frequency m = mass u = speed N = # molecules V = Volume

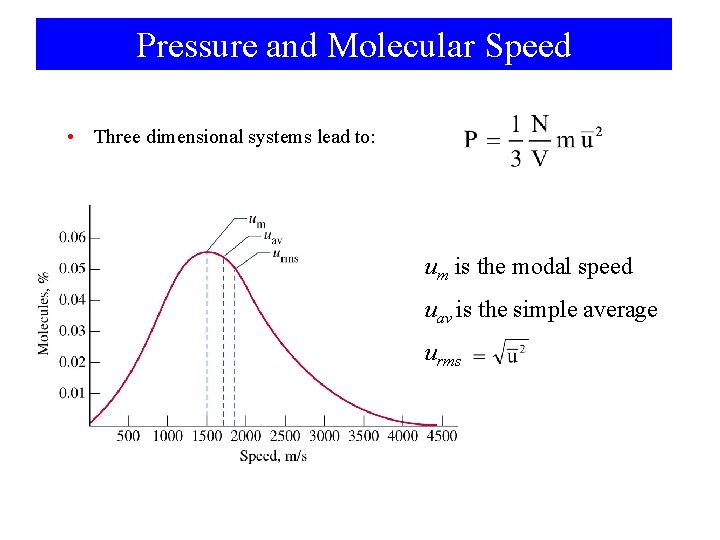
Pressure and Molecular Speed • Three dimensional systems lead to: um is the modal speed uav is the simple average urms

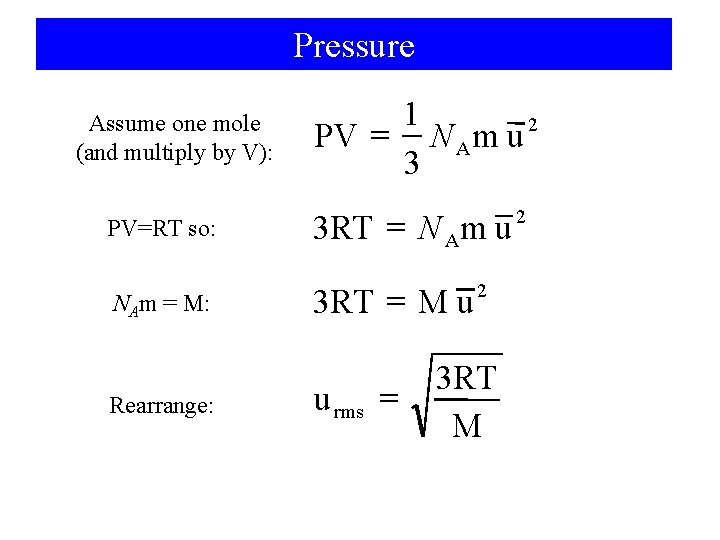
Pressure Assume one mole (and multiply by V): 1 2 = PV NAm u 3 PV=RT so: 2 = 3 RT N A m u NAm = M: 2 = 3 RT M u Rearrange: u rms = 3 RT M

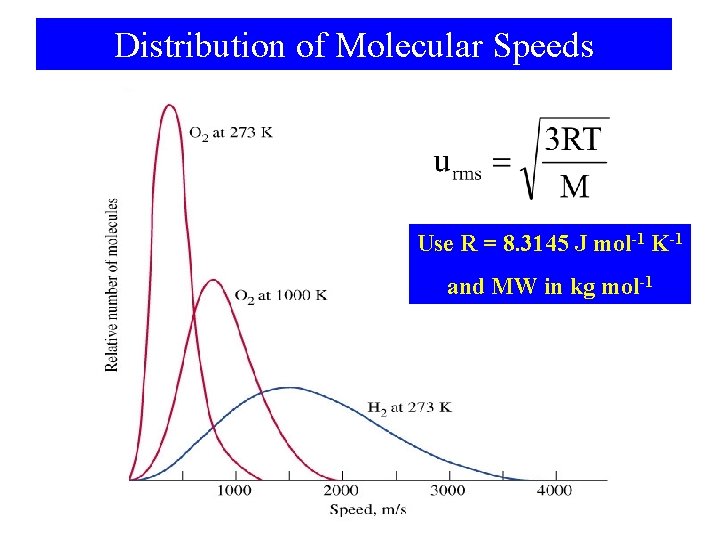
Distribution of Molecular Speeds Use R = 8. 3145 J mol-1 K-1 and MW in kg mol-1

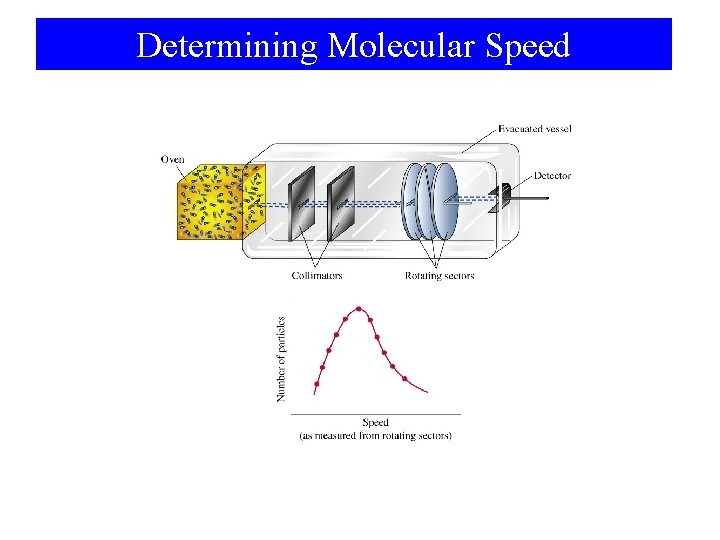
Determining Molecular Speed
 Genel kimya 1 gazlar
Genel kimya 1 gazlar Klorpentan
Klorpentan Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Harwood imalat işletmesi araştırması
Harwood imalat işletmesi araştırması Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen harwood selected poems
Gwen harwood selected poems Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Principles and applications of electrical engineering
Principles and applications of electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Applications of nuclear chemistry
Applications of nuclear chemistry Stomatex fabric
Stomatex fabric Modern chemistry chapter 9 review answers
Modern chemistry chapter 9 review answers Chemical formula of love
Chemical formula of love