General Chemistry Principles and Modern Applications Petrucci Harwood








- Slides: 19

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 5: Introduction to Reactions in Aqueous Solutions Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi) 1 General Chemistry: Chapter 5 Prentice-Hall © 2002

Contents 5 -1 5 -2 5 -3 5 -4 5 -5 5 -6 5 -7 2 The Nature of Aqueous Solutions Precipitation Reactions Acid-Base Reactions Oxidation-Reduction: Some General Principles Balancing Oxidation-Reduction Equations Oxidizing and Reducing Agents Stoichiometry of Reactions in Aqueous Solutions: Titrations Focus on Water Treatment General Chemistry: Chapter 5 Prentice-Hall © 2002

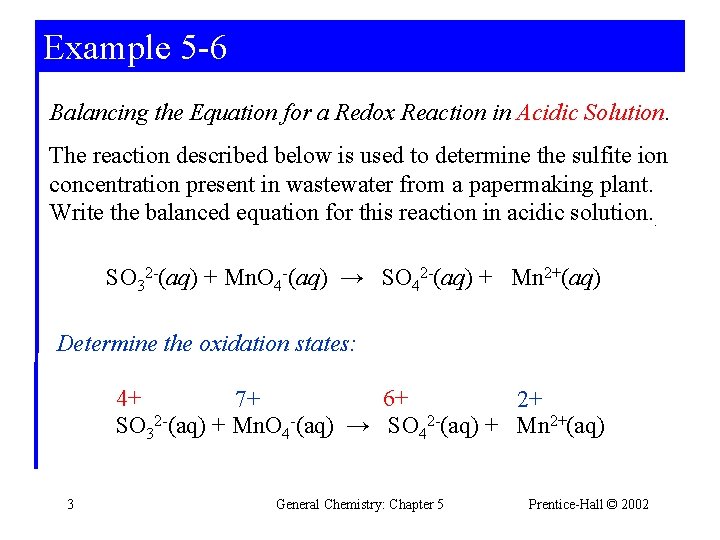
Example 5 -6 Balancing the Equation for a Redox Reaction in Acidic Solution. The reaction described below is used to determine the sulfite ion concentration present in wastewater from a papermaking plant. Write the balanced equation for this reaction in acidic solution. . SO 32 -(aq) + Mn. O 4 -(aq) → SO 42 -(aq) + Mn 2+(aq) Determine the oxidation states: 4+ 6+ 7+ 2+ SO 32 -(aq) + Mn. O 4 -(aq) → SO 42 -(aq) + Mn 2+(aq) 3 General Chemistry: Chapter 5 Prentice-Hall © 2002

Example 5 -6 Write the half-reactions: SO 32 -(aq) → SO 42 -(aq) Mn. O 4 -(aq) → Mn 2+(aq) Balance atoms other than H and O: Already balanced for elements. SO 32 -(aq) → SO 42 -(aq) Mn. O 4 -(aq) → Mn 2+(aq) 4 General Chemistry: Chapter 5 Prentice-Hall © 2002

Example 5 -6 Balance O by adding H 2 O: H 2 O(l) + SO 32 -(aq) → SO 42 -(aq) Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) Balance hydrogen by adding H+: H 2 O(l) + SO 32 -(aq) → SO 42 -(aq) + 2 H+(aq) 8 H+(aq) +Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) Check that the charges are balanced: Add e- if necessary. H 2 O(l) + SO 32 -(aq) → SO 42 -(aq) + 2 H+(aq) + 2 e 5 e- + 8 H+(aq) +Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) 5 General Chemistry: Chapter 5 Prentice-Hall © 2002

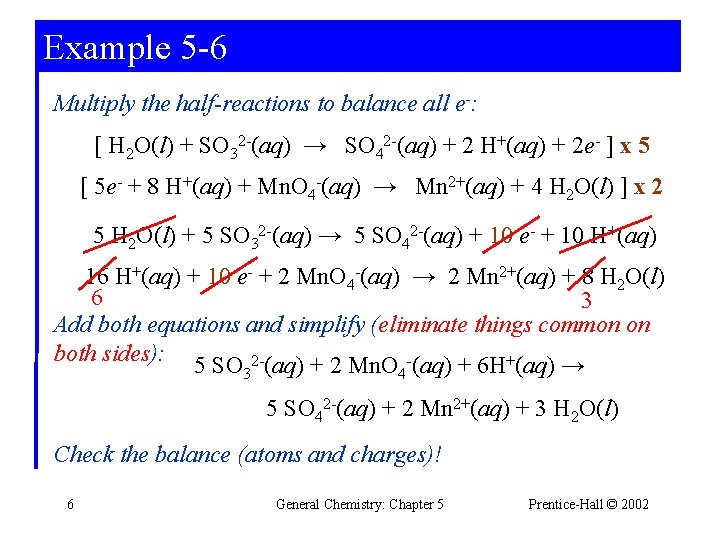
Example 5 -6 Multiply the half-reactions to balance all e-: [ H 2 O(l) + SO 32 -(aq) → SO 42 -(aq) + 2 H+(aq) + 2 e- ] x 5 [ 5 e- + 8 H+(aq) + Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) ] x 2 5 H 2 O(l) + 5 SO 32 -(aq) → 5 SO 42 -(aq) + 10 e- + 10 H+(aq) 16 H+(aq) + 10 e- + 2 Mn. O 4 -(aq) → 2 Mn 2+(aq) + 8 H 2 O(l) 6 3 Add both equations and simplify (eliminate things common on both sides): 5 SO 32 -(aq) + 2 Mn. O 4 -(aq) + 6 H+(aq) → 5 SO 42 -(aq) + 2 Mn 2+(aq) + 3 H 2 O(l) Check the balance (atoms and charges)! 6 General Chemistry: Chapter 5 Prentice-Hall © 2002

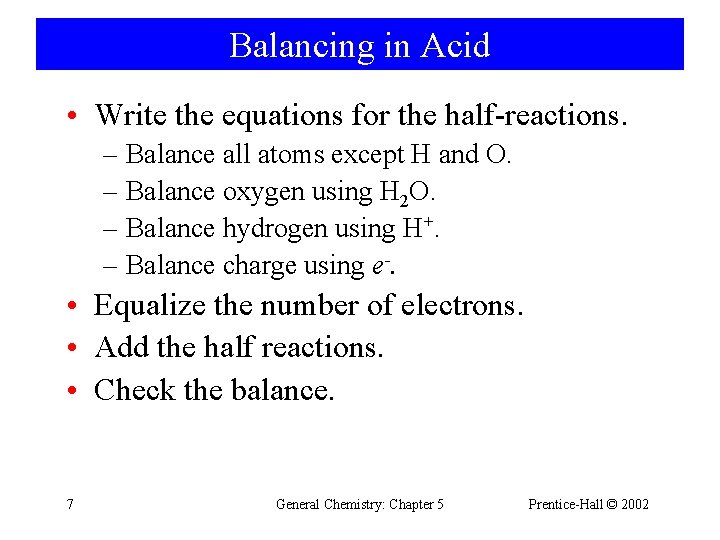
Balancing in Acid • Write the equations for the half-reactions. – Balance all atoms except H and O. – Balance oxygen using H 2 O. – Balance hydrogen using H+. – Balance charge using e-. • Equalize the number of electrons. • Add the half reactions. • Check the balance. 7 General Chemistry: Chapter 5 Prentice-Hall © 2002

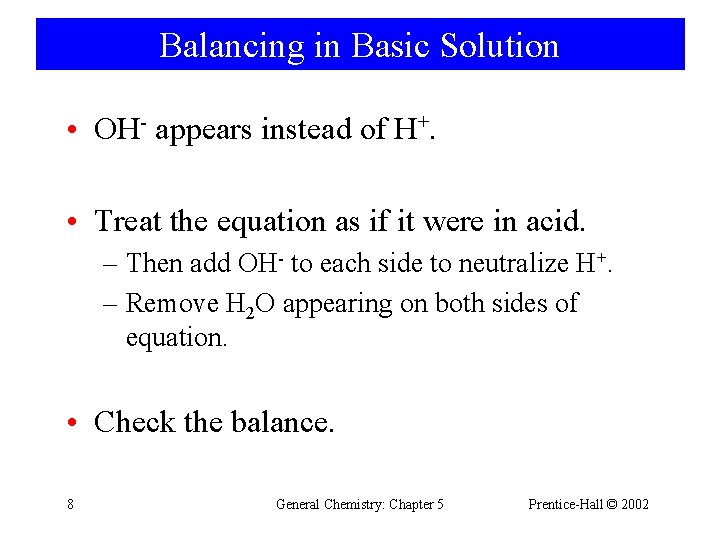
Balancing in Basic Solution • OH- appears instead of H+. • Treat the equation as if it were in acid. – Then add OH- to each side to neutralize H+. – Remove H 2 O appearing on both sides of equation. • Check the balance. 8 General Chemistry: Chapter 5 Prentice-Hall © 2002

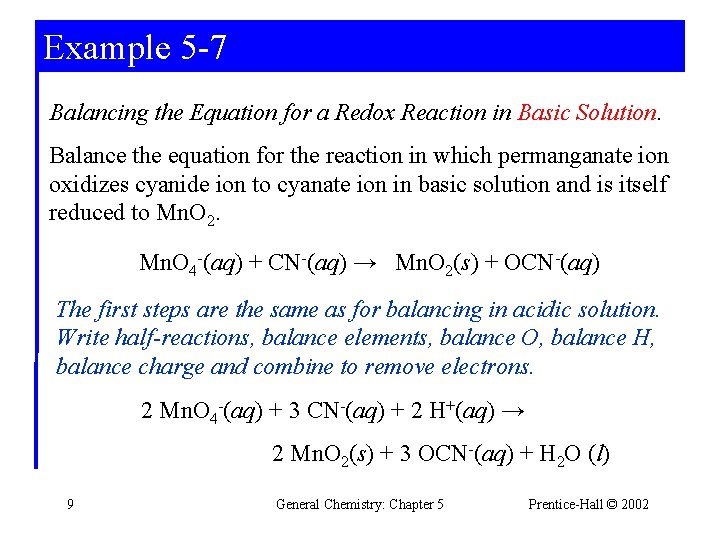
Example 5 -7 Balancing the Equation for a Redox Reaction in Basic Solution. Balance the equation for the reaction in which permanganate ion oxidizes cyanide ion to cyanate ion in basic solution and is itself reduced to Mn. O 2. Mn. O 4 -(aq) + CN-(aq) → Mn. O 2(s) + OCN-(aq) The first steps are the same as for balancing in acidic solution. Write half-reactions, balance elements, balance O, balance H, balance charge and combine to remove electrons. 2 Mn. O 4 -(aq) + 3 CN-(aq) + 2 H+(aq) → 2 Mn. O 2(s) + 3 OCN-(aq) + H 2 O (l) 9 General Chemistry: Chapter 5 Prentice-Hall © 2002

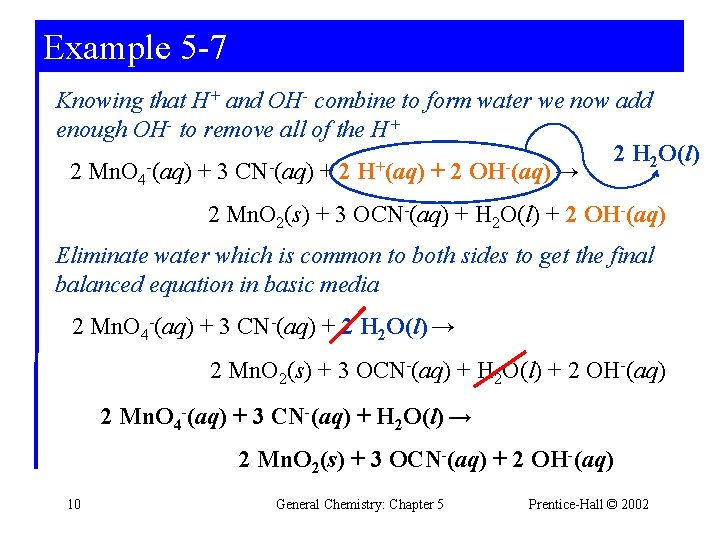
Example 5 -7 Knowing that H+ and OH- combine to form water we now add enough OH- to remove all of the H+ 2 H 2 O(l) + 2 Mn. O 4 (aq) + 3 CN (aq) + 2 H (aq) + 2 OH (aq) → 2 Mn. O 2(s) + 3 OCN-(aq) + H 2 O(l) + 2 OH-(aq) Eliminate water which is common to both sides to get the final balanced equation in basic media 2 Mn. O 4 -(aq) + 3 CN-(aq) + 2 H 2 O(l) → 2 Mn. O 2(s) + 3 OCN-(aq) + H 2 O(l) + 2 OH-(aq) 2 Mn. O 4 -(aq) + 3 CN-(aq) + H 2 O(l) → 2 Mn. O 2(s) + 3 OCN-(aq) + 2 OH-(aq) 10 General Chemistry: Chapter 5 Prentice-Hall © 2002

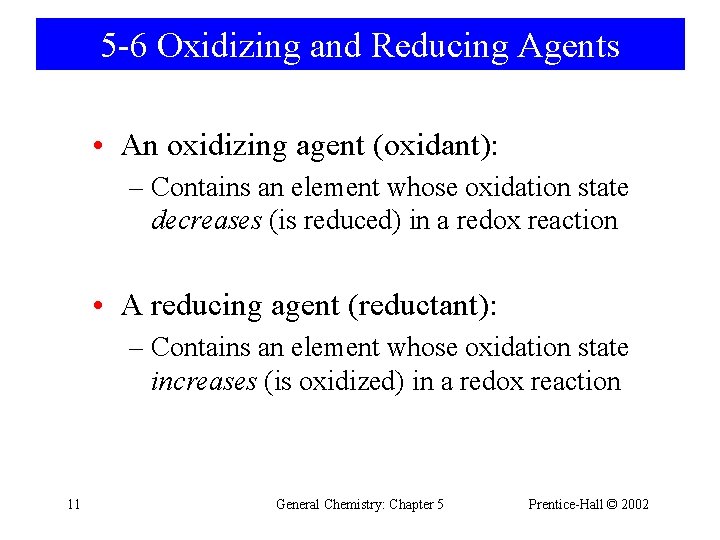
5 -6 Oxidizing and Reducing Agents • An oxidizing agent (oxidant): – Contains an element whose oxidation state decreases (is reduced) in a redox reaction • A reducing agent (reductant): – Contains an element whose oxidation state increases (is oxidized) in a redox reaction 11 General Chemistry: Chapter 5 Prentice-Hall © 2002

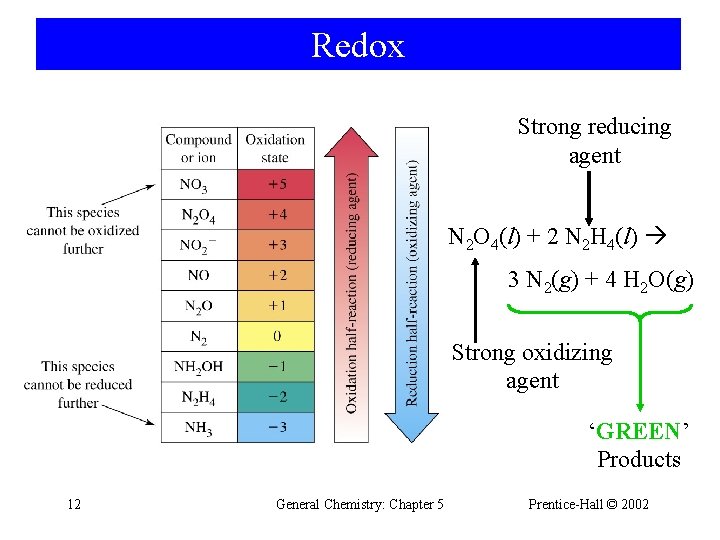
Redox Strong reducing agent N 2 O 4(l) + 2 N 2 H 4(l) 3 N 2(g) + 4 H 2 O(g) Strong oxidizing agent ‘GREEN’ Products 12 General Chemistry: Chapter 5 Prentice-Hall © 2002

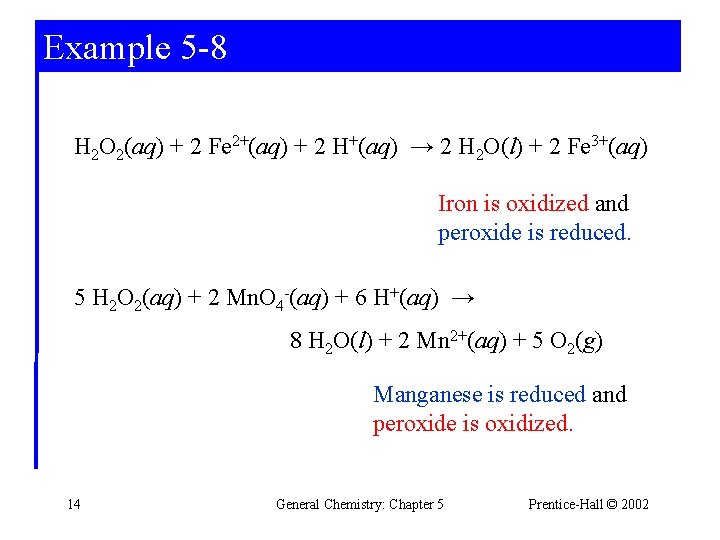
Example 5 -8 Identifying Oxidizing and Reducing Agents. Hydrogen peroxide, H 2 O 2, is a versatile chemical. Its uses include bleaching wood pulp and fabrics and substituting for chlorine in water purification. One reason for its versatility is that it can be either an oxidizing or a reducing agent. For the following reactions, identify whether hydrogen peroxide is an oxidizing or reducing agent. 13 General Chemistry: Chapter 5 Prentice-Hall © 2002

Example 5 -8 H 2 O 2(aq) + 2 Fe 2+(aq) + 2 H+(aq) → 2 H 2 O(l) + 2 Fe 3+(aq) Iron is oxidized and peroxide is reduced. 5 H 2 O 2(aq) + 2 Mn. O 4 -(aq) + 6 H+(aq) → 8 H 2 O(l) + 2 Mn 2+(aq) + 5 O 2(g) Manganese is reduced and peroxide is oxidized. 14 General Chemistry: Chapter 5 Prentice-Hall © 2002

5 -7 Stoichiometry of Reactions in Aqueous Solutions: Titrations. • Titration – Carefully controlled addition of one solution to another. • Equivalence Point – Both reactants have reacted completely. • Indicators – Substances which change color near an equivalence point. 15 General Chemistry: Chapter 5 Prentice-Hall © 2002

Indicators 16 General Chemistry: Chapter 5 Prentice-Hall © 2002

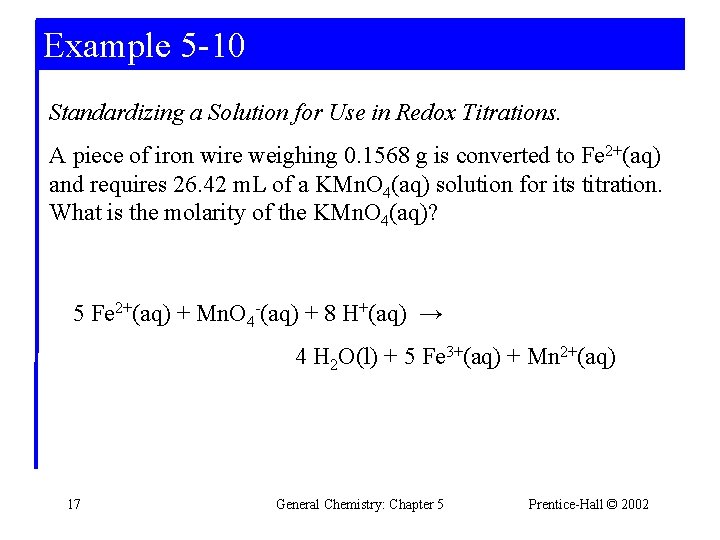
Example 5 -10 Standardizing a Solution for Use in Redox Titrations. A piece of iron wire weighing 0. 1568 g is converted to Fe 2+(aq) and requires 26. 42 m. L of a KMn. O 4(aq) solution for its titration. What is the molarity of the KMn. O 4(aq)? 5 Fe 2+(aq) + Mn. O 4 -(aq) + 8 H+(aq) → 4 H 2 O(l) + 5 Fe 3+(aq) + Mn 2+(aq) 17 General Chemistry: Chapter 5 Prentice-Hall © 2002

Example 5 -10 5 Fe 2+(aq) + Mn. O 4 -(aq) + 8 H+(aq) → 4 H 2 O(l) + 5 Fe 3+(aq) + Mn 2+(aq) Determine KMn. O 4 consumed in the reaction: 4 Determine the concentration: 18 General Chemistry: Chapter 5 Prentice-Hall © 2002

Chapter 5 Questions 1, 3, 5, 6, 8, 14, 16, 17, 19, 24, 25, 27, 33, 37, 41, 43, 51, 53, 59, 68, 71, 82, 96. 19 General Chemistry: Chapter 5 Prentice-Hall © 2002
 Genel kimya petrucci harwood herring
Genel kimya petrucci harwood herring Klorpentan
Klorpentan Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Insan ilişkileri yaklaşımı nedir
Insan ilişkileri yaklaşımı nedir Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen nuttall
Gwen nuttall Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Allan
Allan Learning principles and applications
Learning principles and applications 25 m/s
25 m/s