General Chemistry Principles and Modern Applications Petrucci Harwood


- Slides: 18

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 11: Chemical Bonding I: Basic Concepts Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi) Prentice-Hall © 2002 General Chemistry: Chapter 11

Contents 11 -1 11 -2 11 -3 11 -4 11 -5 11 -6 11 -7 11 -8 11 -9 Prentice-Hall © 2002 Lewis Theory: An Overview Covalent Bonding: An Introduction Polar Covalent Bonds Writing Lewis Structures Resonance Exceptions to the Octet Rule The Shapes of Molecules Bond Order and Bond Lengths Bond Energies Focus on Polymers— Macromolecular Substances General Chemistry: Chapter 11

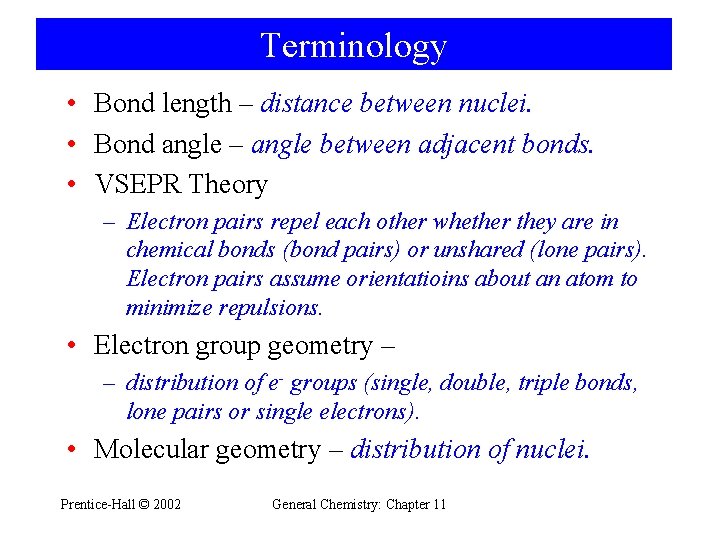
Terminology • Bond length – distance between nuclei. • Bond angle – angle between adjacent bonds. • VSEPR Theory – Electron pairs repel each other whether they are in chemical bonds (bond pairs) or unshared (lone pairs). Electron pairs assume orientatioins about an atom to minimize repulsions. • Electron group geometry – – distribution of e- groups (single, double, triple bonds, lone pairs or single electrons). • Molecular geometry – distribution of nuclei. Prentice-Hall © 2002 General Chemistry: Chapter 11

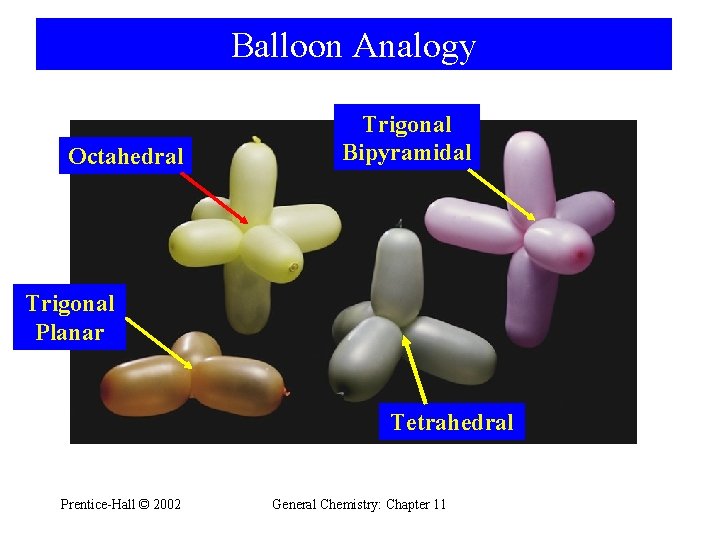
Balloon Analogy Octahedral Trigonal Bipyramidal Trigonal Planar Tetrahedral Prentice-Hall © 2002 General Chemistry: Chapter 11

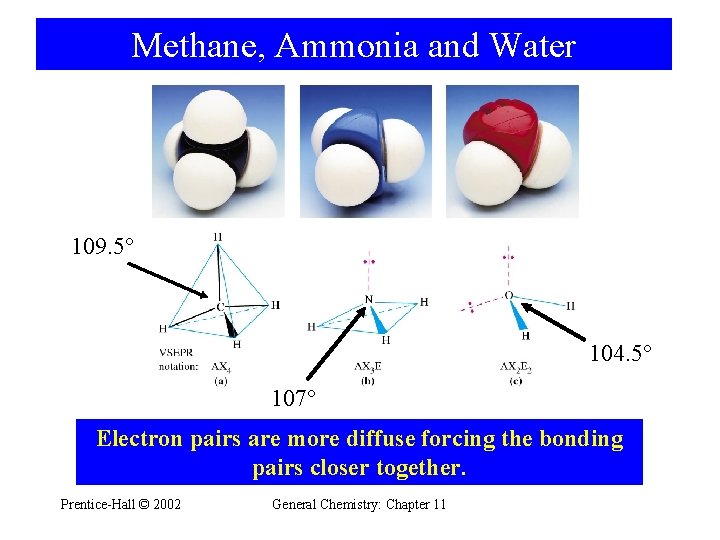
Methane, Ammonia and Water 109. 5° 104. 5° 107° Electron pairs are more diffuse forcing the bonding pairs closer together. Prentice-Hall © 2002 General Chemistry: Chapter 11

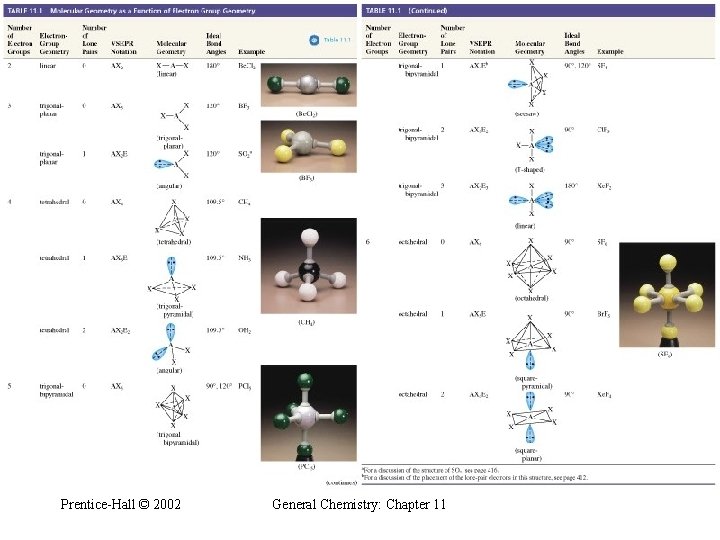
Table 11. 1 Molecular Geometry as a Function of Electron Group Geometry Prentice-Hall © 2002 General Chemistry: Chapter 11

Applying VSEPR Theory • Draw a plausible Lewis structure. • Determine the number of e- groups and identify them as bond or lone pairs. • Establish the e- group geometry. • Determine the molecular geometry. ü Multiple bonds count as one group of electrons. ü More than one central atom can be handled individually. Prentice-Hall © 2002 General Chemistry: Chapter 11

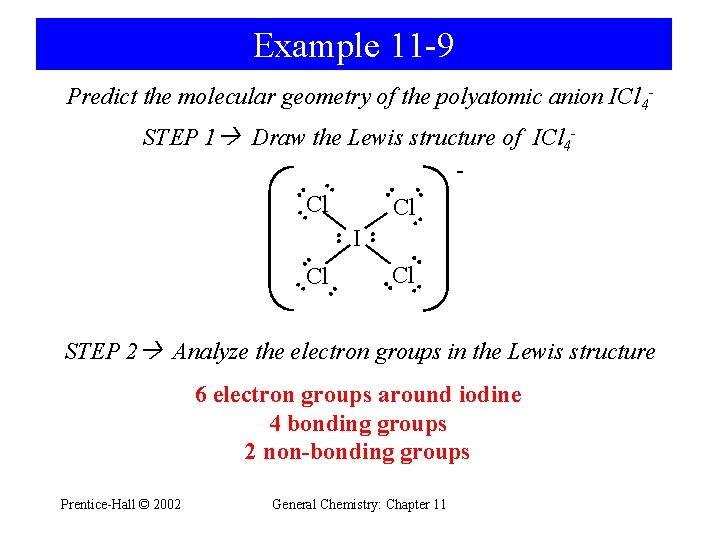
Example 11 -9 Predict the molecular geometry of the polyatomic anion ICl 4 - • • STEP 1 Draw the Lewis structure of ICl 4 • • Cl • I Cl • • • • • Cl • STEP 2 Analyze the electron groups in the Lewis structure 6 electron groups around iodine 4 bonding groups 2 non-bonding groups Prentice-Hall © 2002 General Chemistry: Chapter 11

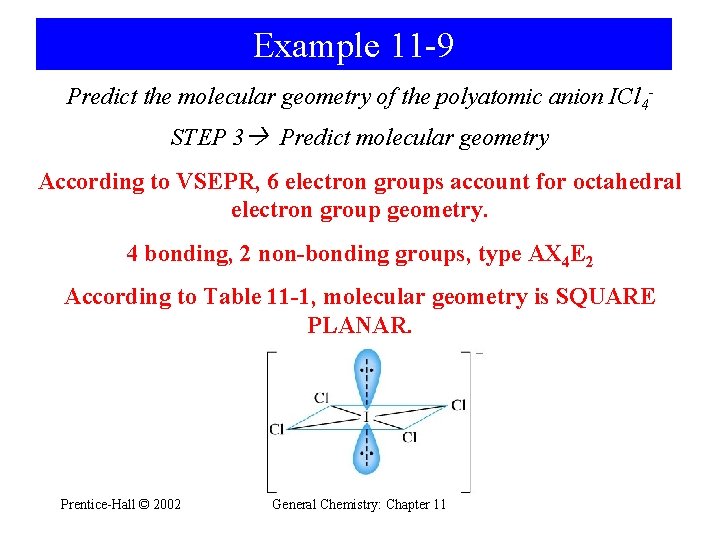
Example 11 -9 Predict the molecular geometry of the polyatomic anion ICl 4 STEP 3 Predict molecular geometry According to VSEPR, 6 electron groups account for octahedral electron group geometry. 4 bonding, 2 non-bonding groups, type AX 4 E 2 According to Table 11 -1, molecular geometry is SQUARE PLANAR. Prentice-Hall © 2002 General Chemistry: Chapter 11

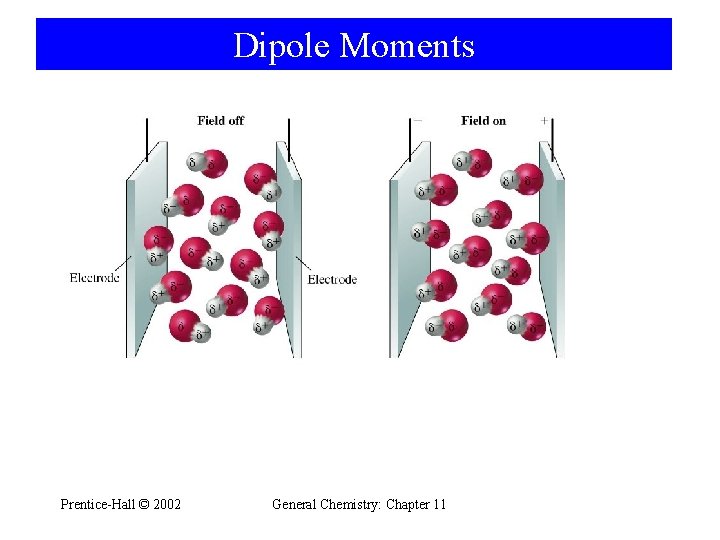
Dipole Moments Prentice-Hall © 2002 General Chemistry: Chapter 11

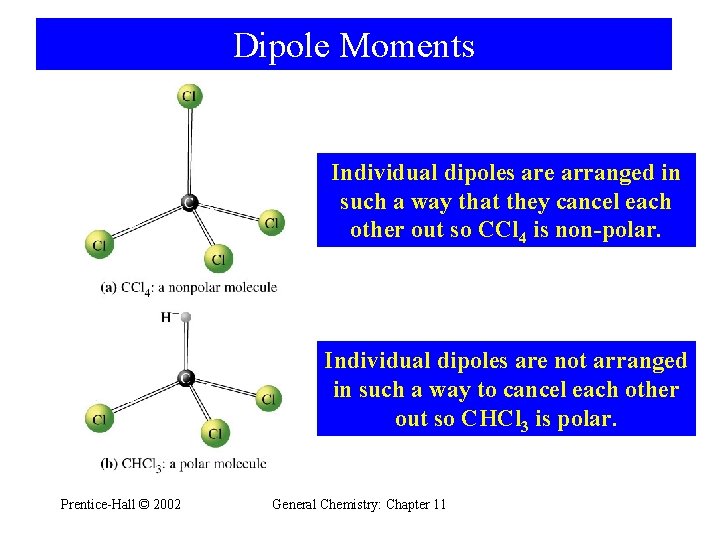
Dipole Moments Individual dipoles are arranged in such a way that they cancel each other out so CCl 4 is non-polar. Individual dipoles are not arranged in such a way to cancel each other out so CHCl 3 is polar. Prentice-Hall © 2002 General Chemistry: Chapter 11

Bond Order and Bond Length • Bond Order –Single bond, order = 1 –Double bond, order = 2 –Triple bond, order = 3 • Bond Length –Distance between two nuclei • Higher bond order –Shorter bond –Stronger bond Prentice-Hall © 2002 General Chemistry: Chapter 11

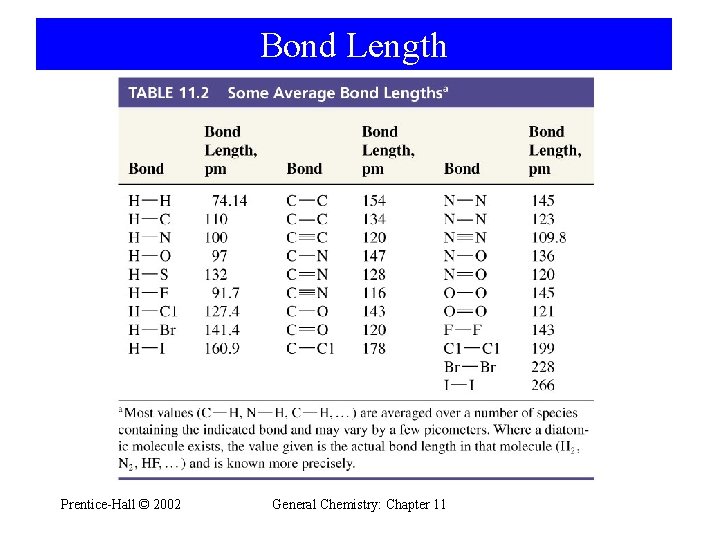
Bond Length Prentice-Hall © 2002 General Chemistry: Chapter 11

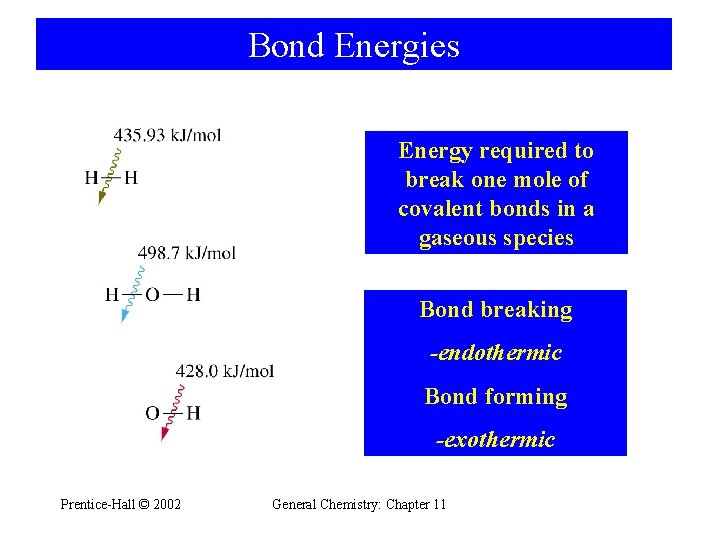
Bond Energies Energy required to break one mole of covalent bonds in a gaseous species Bond breaking -endothermic Bond forming -exothermic Prentice-Hall © 2002 General Chemistry: Chapter 11

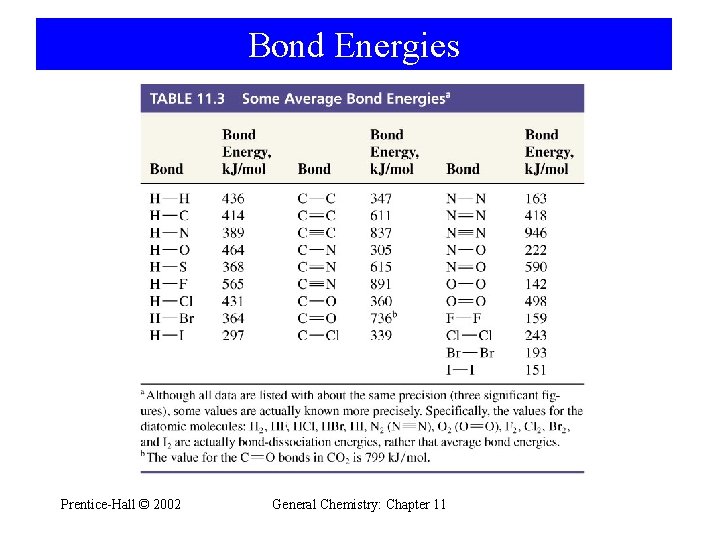
Bond Energies Prentice-Hall © 2002 General Chemistry: Chapter 11

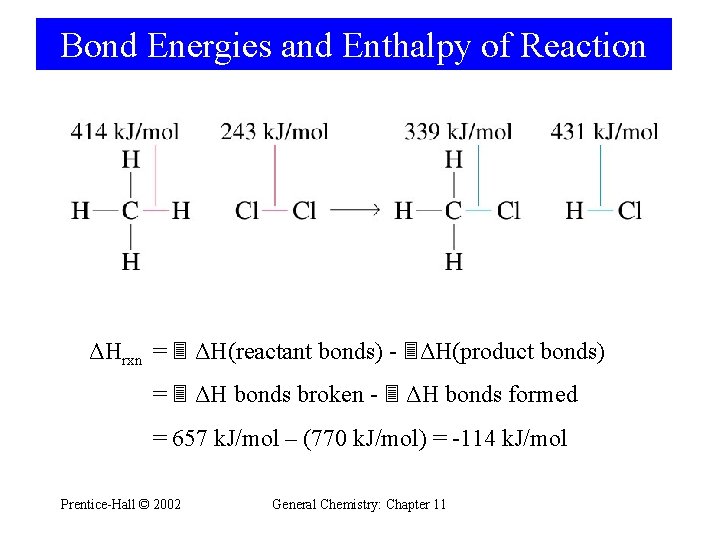
Bond Energies and Enthalpy of Reaction ΔHrxn = ΔH(reactant bonds) - ΔH(product bonds) = ΔH bonds broken - ΔH bonds formed = 657 k. J/mol – (770 k. J/mol) = -114 k. J/mol Prentice-Hall © 2002 General Chemistry: Chapter 11

Focus on Polymers – Macromolecular Substances Polymers are made up of simple molecules with low molecular weights joined together into extremely large molecules. Examples include plastic, cellulose, rubber, latex, DNA and nylon. Prentice-Hall © 2002 General Chemistry: Chapter 11

Chapter 11 Questions 1, 4, 6, 8, 10, 11, 15, 27, 33, 37, 53, 57, 65 (also calculate formal charges), 71, 86, 94 Prentice-Hall © 2002 General Chemistry: Chapter 11
 Gazlarda yoğunluk formülü
Gazlarda yoğunluk formülü Organik kimya adlandırma öncelik sırası
Organik kimya adlandırma öncelik sırası Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Insan ilişkileri yaklaşımı nedir
Insan ilişkileri yaklaşımı nedir Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen harwood: selected poems
Gwen harwood: selected poems Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications