General Chemistry Principles and Modern Applications Petrucci Harwood

- Slides: 17

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 6: Gases Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi)

Contents 6 -1 Properties of Gases: Gas Pressure 6 -2 The Simple Gas Laws 6 -3 Combining the Gas Laws: The Ideal Gas Equation and The General Gas Equation 6 -4 Applications of the Ideal Gas Equation 6 -5 Gases in Chemical Reactions 6 -6 Mixtures of Gases

Contents 6 -6 Mixtures of Gases 6 -7 Kinetic—Molecular Theory of Gases 6 -8 Gas Properties Relating to the Kinetic—Molecular Theory 6 -9 Non-ideal (real) Gases Focus on The Chemistry of Air-Bag Systems

Standard Conditions (STP) Pressure 1 atm 760 mm Hg 760 torr 101. 325 k. Pa 1. 01325 bar Temperature 0°C 273. 15 K Volume 1. 0 mol of gas occupies 22. 4 L at STP The Gas Constant 0. 082057 L atm mol-1 K-1 8. 3145 m 3 Pa mol-1 K-1 8. 3145 J mol-1 K-1 62. 364 L Torr mol-1 K-1

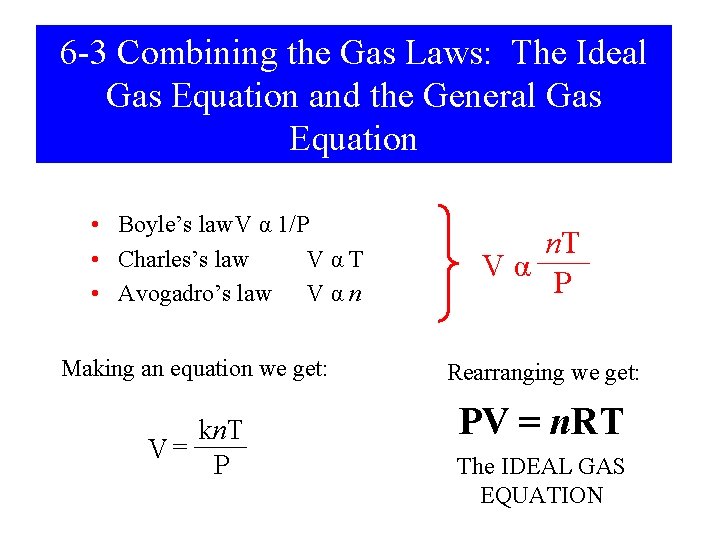
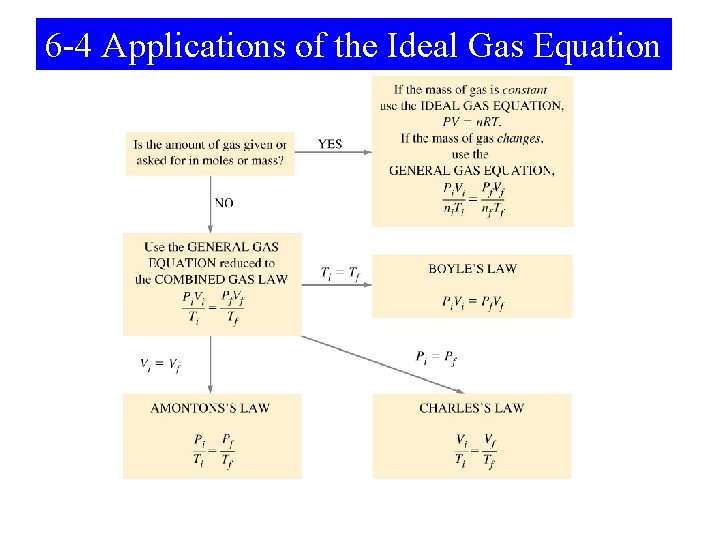
6 -3 Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation • Boyle’s law. V α 1/P • Charles’s law VαT • Avogadro’s law V α n Making an equation we get: kn. T V= P n. T Vα P Rearranging we get: PV = n. RT The IDEAL GAS EQUATION

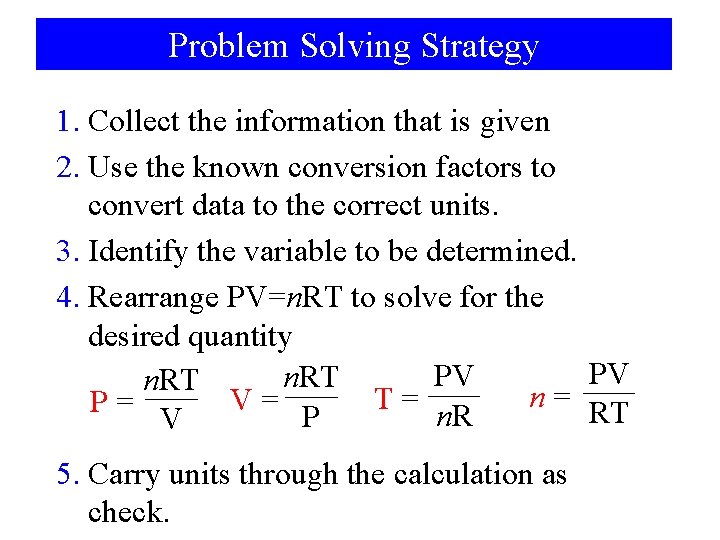
Problem Solving Strategy 1. Collect the information that is given 2. Use the known conversion factors to convert data to the correct units. 3. Identify the variable to be determined. 4. Rearrange PV=n. RT to solve for the desired quantity PV PV n. RT n= T= V= P= RT n. R P V 5. Carry units through the calculation as check.

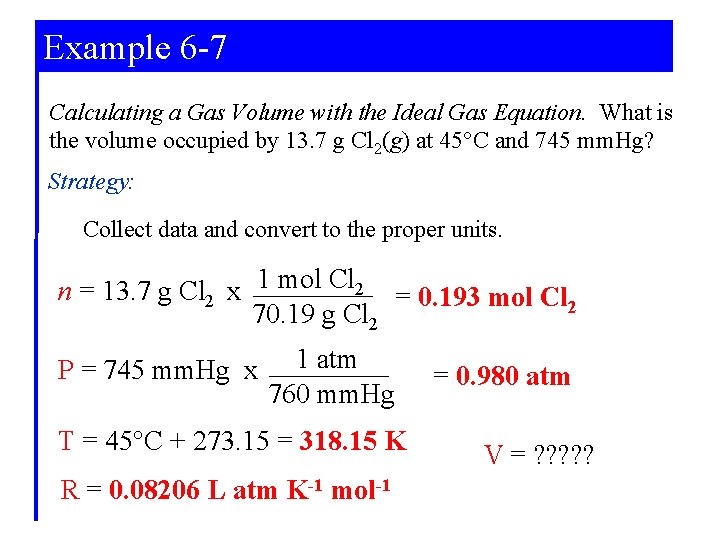
Example 6 -7 Calculating a Gas Volume with the Ideal Gas Equation. What is the volume occupied by 13. 7 g Cl 2(g) at 45°C and 745 mm. Hg? Strategy: Collect data and convert to the proper units. n = 13. 7 g Cl 2 x 1 mol Cl 2 = 0. 193 mol Cl 2 70. 19 g Cl 2 P = 745 mm. Hg x 1 atm 760 mm. Hg T = 45°C + 273. 15 = 318. 15 K R = 0. 08206 L atm K-1 mol-1 = 0. 980 atm V = ? ? ?

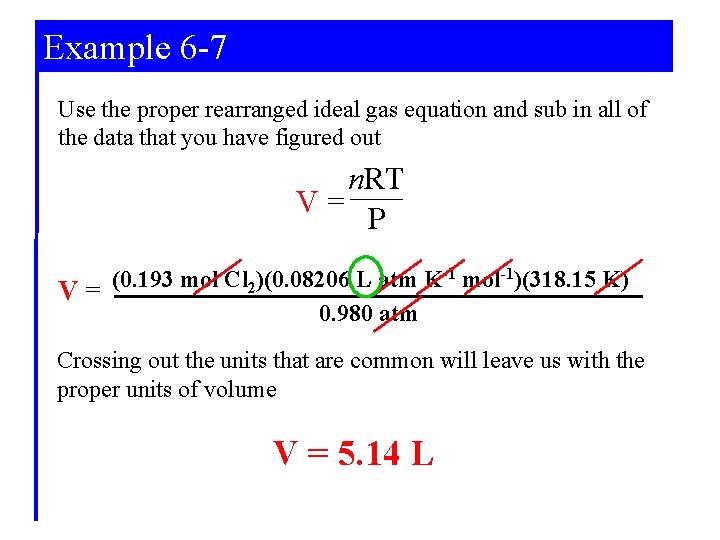
Example 6 -7 Use the proper rearranged ideal gas equation and sub in all of the data that you have figured out n. RT V= P (0. 193 mol Cl 2)(0. 08206 L atm K-1 mol-1)(318. 15 K) V= 0. 980 atm Crossing out the units that are common will leave us with the proper units of volume V = 5. 14 L

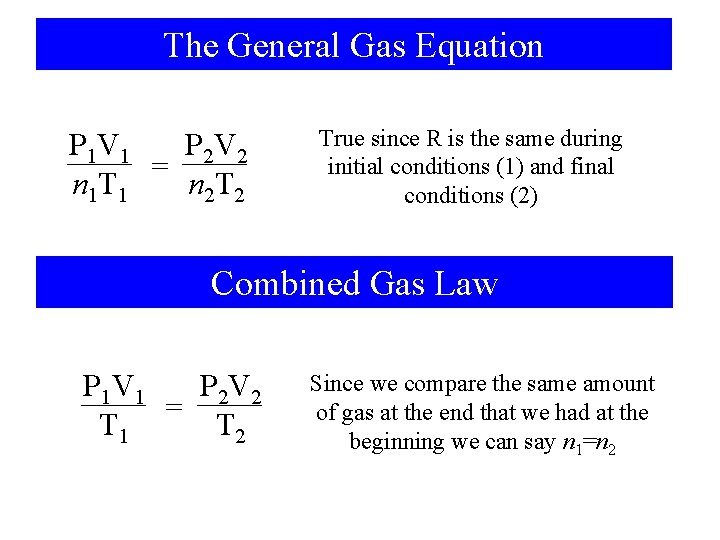
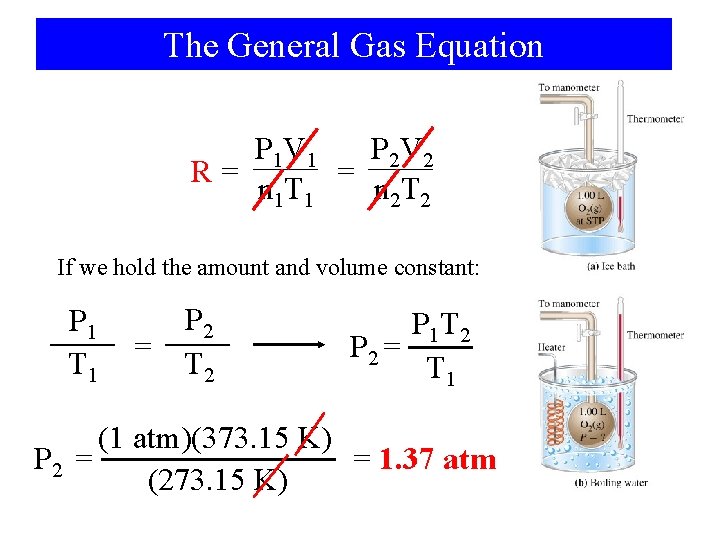
The General Gas Equation P 2 V 2 P 1 V 1 = n 1 T 1 n 2 T 2 True since R is the same during initial conditions (1) and final conditions (2) Combined Gas Law P 1 V 1 P 2 V 2 = T 1 T 2 Since we compare the same amount of gas at the end that we had at the beginning we can say n 1=n 2

The General Gas Equation P 2 V 2 P 1 V 1 R= = n 1 T 1 n 2 T 2 If we hold the amount and volume constant: P 1 T 1 = P 2 T 2 P 1 T 2 P 2 = T 1 (1 atm)(373. 15 K) P 2 = = 1. 37 atm (273. 15 K)

6 -4 Applications of the Ideal Gas Equation

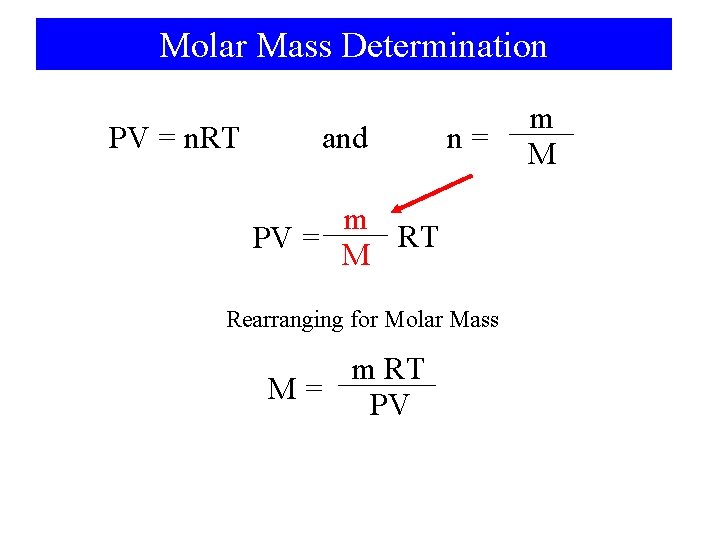
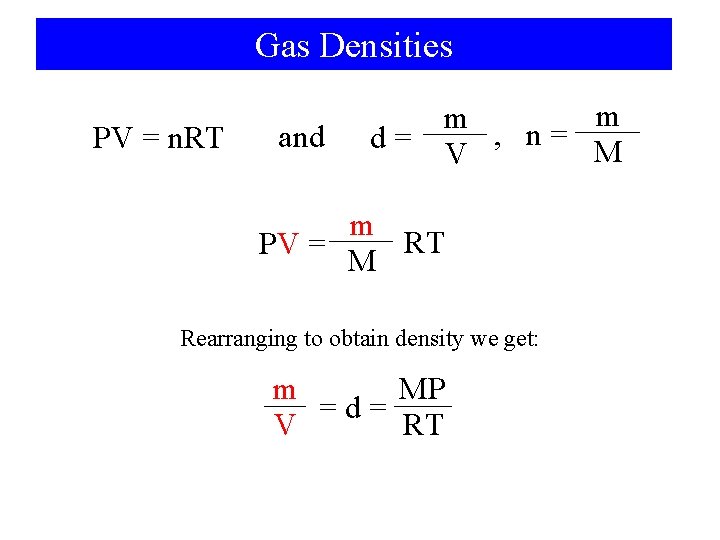
Molar Mass Determination PV = n. RT and n= m RT PV = M Rearranging for Molar Mass m RT M= PV m M

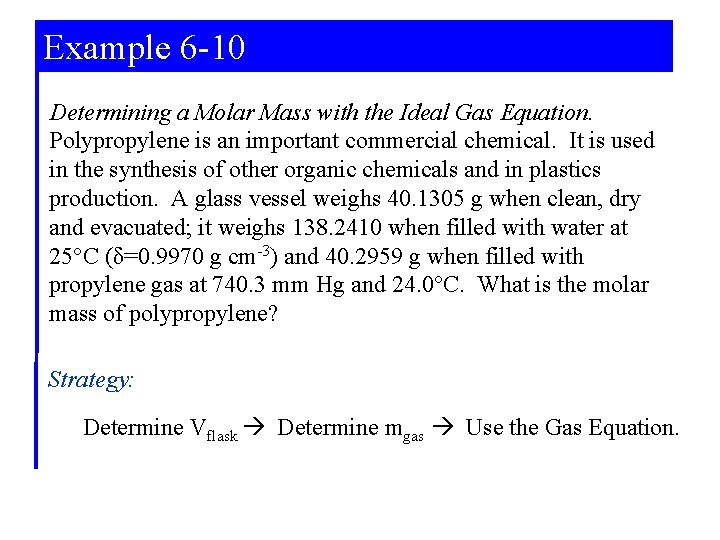
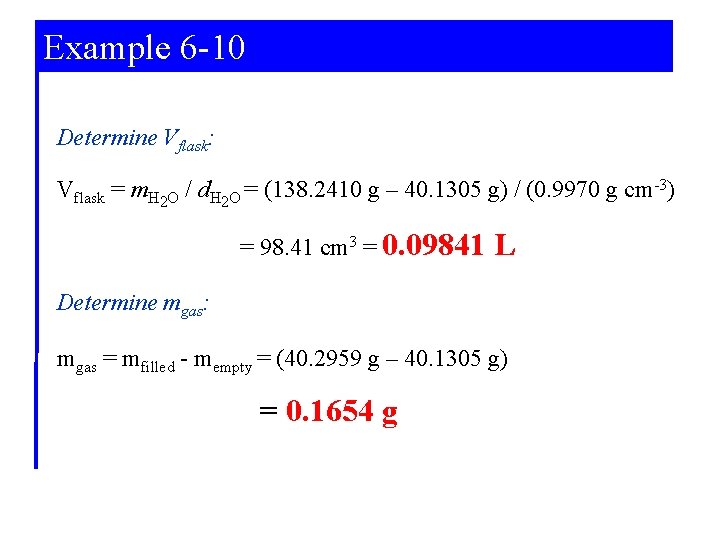
Example 6 -10 Determining a Molar Mass with the Ideal Gas Equation. Polypropylene is an important commercial chemical. It is used in the synthesis of other organic chemicals and in plastics production. A glass vessel weighs 40. 1305 g when clean, dry and evacuated; it weighs 138. 2410 when filled with water at 25°C (δ=0. 9970 g cm-3) and 40. 2959 g when filled with propylene gas at 740. 3 mm Hg and 24. 0°C. What is the molar mass of polypropylene? Strategy: Determine Vflask Determine mgas Use the Gas Equation.

Example 6 -10 Determine Vflask: Vflask = m. H 2 O / d. H 2 O = (138. 2410 g – 40. 1305 g) / (0. 9970 g cm-3) = 98. 41 cm 3 = 0. 09841 L Determine mgas: mgas = mfilled - mempty = (40. 2959 g – 40. 1305 g) = 0. 1654 g

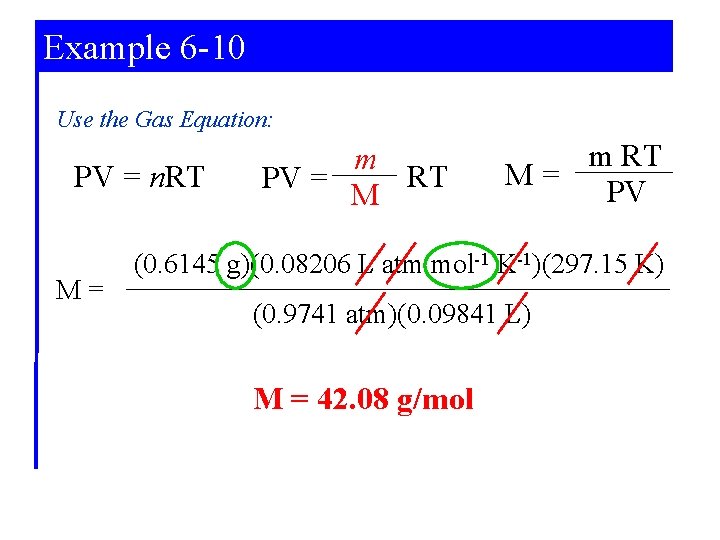
Example 6 -10 5 -6 Use the Gas Equation: PV = n. RT M= m RT PV = M m RT M= PV (0. 6145 g)(0. 08206 L atm mol-1 K-1)(297. 15 K) (0. 9741 atm)(0. 09841 L) M = 42. 08 g/mol

Gas Densities PV = n. RT and m m , n= d= M V m RT PV = M Rearranging to obtain density we get: m MP =d= V RT

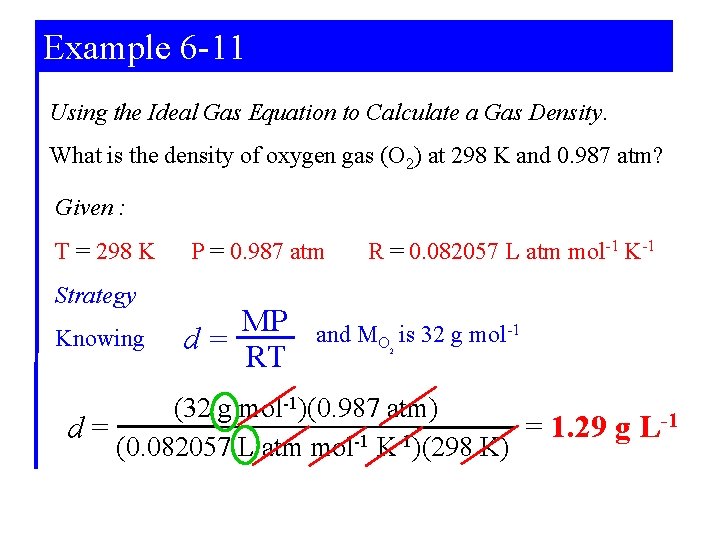
Example 6 -11 Using the Ideal Gas Equation to Calculate a Gas Density. What is the density of oxygen gas (O 2) at 298 K and 0. 987 atm? Given : T = 298 K Strategy Knowing P = 0. 987 atm MP d= RT R = 0. 082057 L atm mol-1 K-1 and MO is 32 g mol-1 2 (32 g mol-1)(0. 987 atm) -1 = 1. 29 g L d= (0. 082057 L atm mol-1 K-1)(298 K)
 Gazlarda yoğunluk formülü
Gazlarda yoğunluk formülü Organik kimya adlandırma öncelik sırası
Organik kimya adlandırma öncelik sırası Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Harwood imalat işletmesi araştırması
Harwood imalat işletmesi araştırması Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen harwood: selected poems
Gwen harwood: selected poems Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Pearson engineering
Pearson engineering