General Chemistry Principles and Modern Applications Petrucci Harwood







- Slides: 29

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 4: Chemical Reactions Philip Dutton University of Windsor, Canada Prentice-Hall © 2002 Slide 1 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Contents 4 -1 4 -2 4 -3 4 -4 4 -5 Chemical Reactions and Chemical Equations and Stoichiometry Chemical Reactions in Solution Determining the Limiting reagent Other Practical Matters in Reaction Stoichiometry Focus on Industrial Chemistry Slide 2 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

4 -1 Chemical Reactions and Chemical Equations As reactants are converted to products we observe: – Color change – Precipitate formation – Gas evolution – Heat absorption or evolution Chemical evidence may be necessary. Slide 3 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

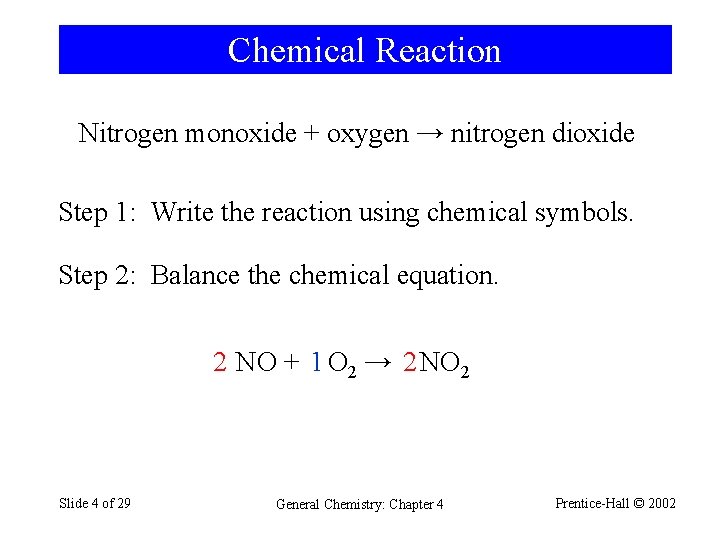
Chemical Reaction Nitrogen monoxide + oxygen → nitrogen dioxide Step 1: Write the reaction using chemical symbols. Step 2: Balance the chemical equation. 2 NO + 1 O 2 → 2 NO 2 Slide 4 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

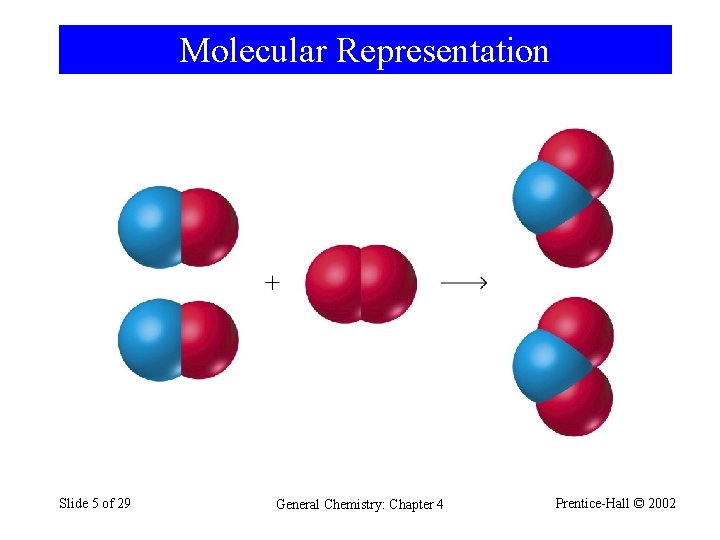
Molecular Representation Slide 5 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

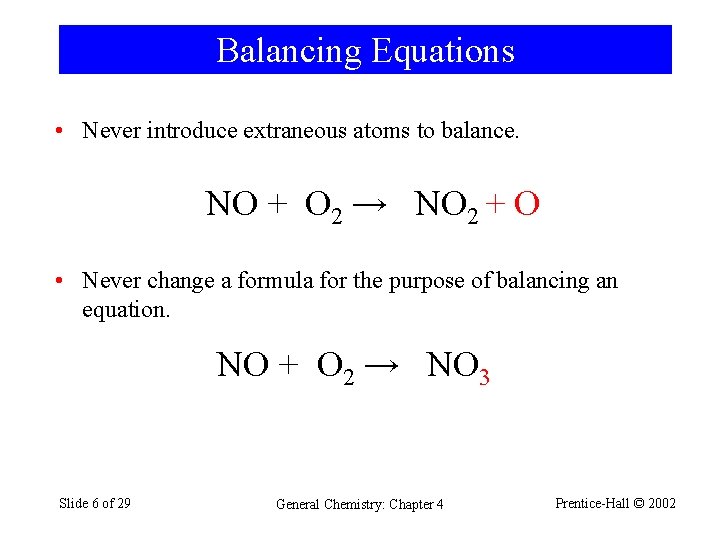
Balancing Equations • Never introduce extraneous atoms to balance. NO + O 2 → NO 2 + O • Never change a formula for the purpose of balancing an equation. NO + O 2 → NO 3 Slide 6 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Balancing Equation Strategy • Balance elements that occur in only one compound on each side first. • Balance free elements last. • Balance unchanged polyatomics as groups. • Fractional coefficients are acceptable and can be cleared at the end by multiplication. Slide 7 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

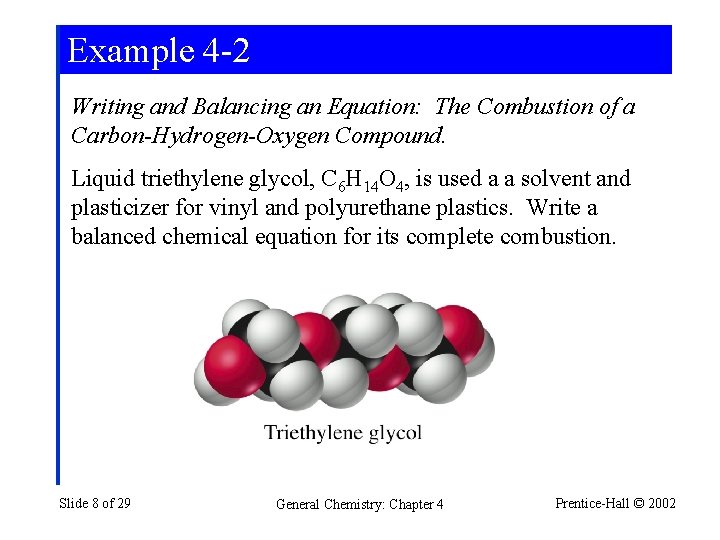
Example 4 -2 Writing and Balancing an Equation: The Combustion of a Carbon-Hydrogen-Oxygen Compound. Liquid triethylene glycol, C 6 H 14 O 4, is used a a solvent and plasticizer for vinyl and polyurethane plastics. Write a balanced chemical equation for its complete combustion. Slide 8 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

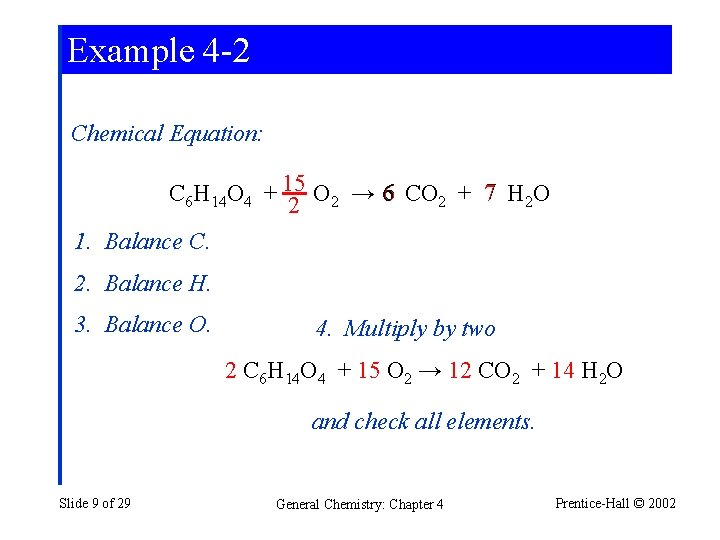
Example 4 -2 Chemical Equation: C 6 H 14 O 4 + 15 O 2 → 6 CO 2 + 7 H 2 O 2 1. Balance C. 2. Balance H. 3. Balance O. 4. Multiply by two 2 C 6 H 14 O 4 + 15 O 2 → 12 CO 2 + 14 H 2 O and check all elements. Slide 9 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

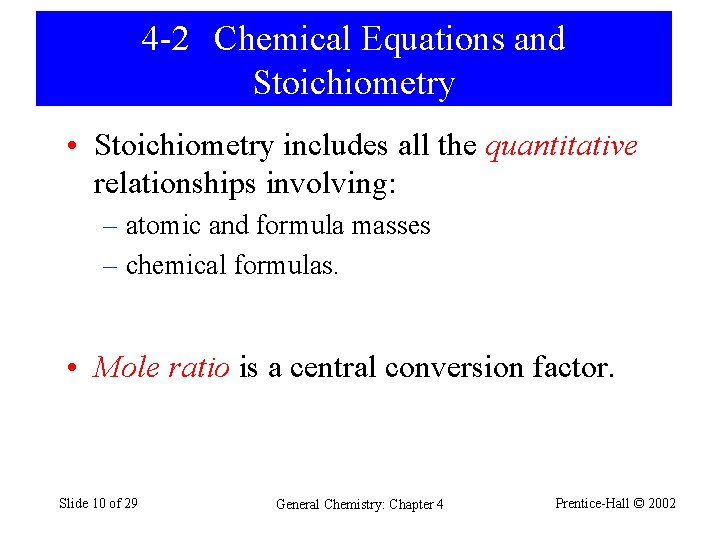
4 -2 Chemical Equations and Stoichiometry • Stoichiometry includes all the quantitative relationships involving: – atomic and formula masses – chemical formulas. • Mole ratio is a central conversion factor. Slide 10 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

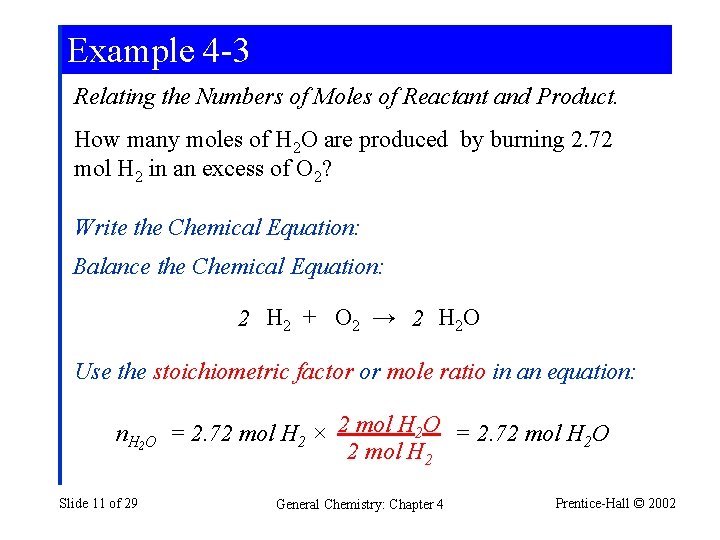
Example 4 -3 Relating the Numbers of Moles of Reactant and Product. How many moles of H 2 O are produced by burning 2. 72 mol H 2 in an excess of O 2? Write the Chemical Equation: Balance the Chemical Equation: 2 H 2 + O 2 → 2 H 2 O Use the stoichiometric factor or mole ratio in an equation: n. H 2 O = 2. 72 mol H 2 × 2 mol H 2 O = 2. 72 mol H 2 O 2 mol H 2 Slide 11 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

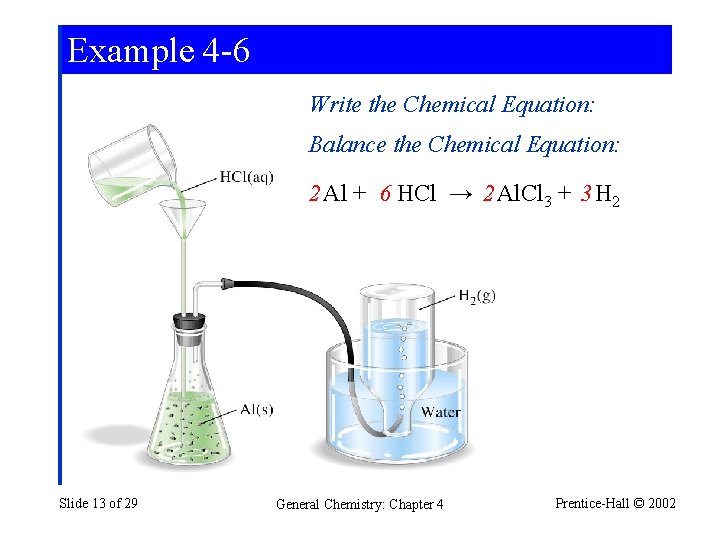
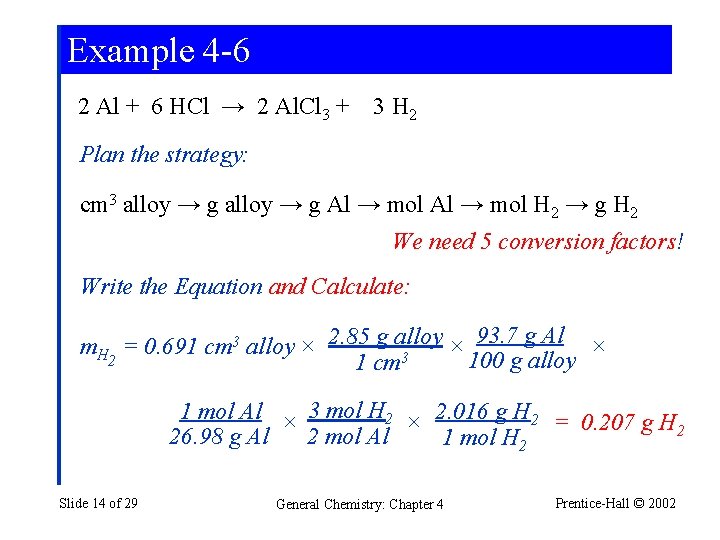
Example 4 -6 Additional Conversion Factors ina Stoichiometric Calculation: Volume, Density, and Percent Composition. An alloy used in aircraft structures consists of 93. 7% Al and 6. 3% Cu by mass. The alloy has a density of 2. 85 g/cm 3. A 0. 691 cm 3 piece of the alloy reacts with an excess of HCl(aq). If we assume that all the Al but none of the Cu reacts with HCl(aq), what is the mass of H 2 obtained? Slide 12 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Example 4 -6 Write the Chemical Equation: Balance the Chemical Equation: 2 Al + 6 HCl → 2 Al. Cl 3 + 3 H 2 Slide 13 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Example 4 -6 2 Al + 6 HCl → 2 Al. Cl 3 + 3 H 2 Plan the strategy: cm 3 alloy → g Al → mol H 2 → g H 2 We need 5 conversion factors! Write the Equation and Calculate: 93. 7 g Al × m. H = 0. 691 cm 3 alloy × 2. 85 g alloy × 2 100 g alloy 1 cm 3 1 mol Al × 3 mol H 2 × 2. 016 g H 2 = 0. 207 g H 2 2 mol Al 26. 98 g Al 1 mol H 2 Slide 14 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

4 -3 Chemical Reactions in Solution • Close contact between atoms, ions and molecules necessary for a reaction to occur. • Solvent – We will usually use aqueous (aq) solution. • Solute – A material dissolved by the solvent. Slide 15 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

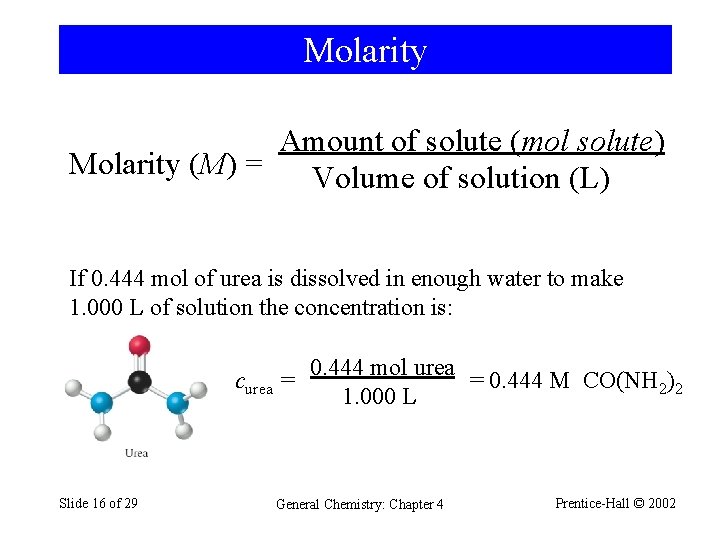
Molarity Amount of solute (mol solute) Molarity (M) = Volume of solution (L) If 0. 444 mol of urea is dissolved in enough water to make 1. 000 L of solution the concentration is: curea Slide 16 of 29 0. 444 mol urea = = 0. 444 M CO(NH 2)2 1. 000 L General Chemistry: Chapter 4 Prentice-Hall © 2002

Preparation of a Solution Weigh the solid sample. Dissolve it in a volumetric flask partially filled with solvent. Carefully fill to the mark. Slide 17 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

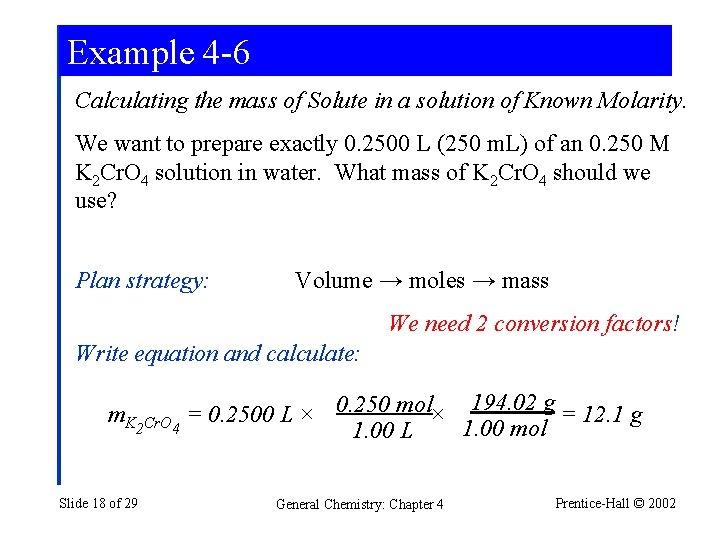
Example 4 -6 Calculating the mass of Solute in a solution of Known Molarity. We want to prepare exactly 0. 2500 L (250 m. L) of an 0. 250 M K 2 Cr. O 4 solution in water. What mass of K 2 Cr. O 4 should we use? Plan strategy: Volume → moles → mass We need 2 conversion factors! Write equation and calculate: m. K 0. 250 mol× 194. 02 g = 12. 1 g = 0. 2500 L × 2 Cr. O 4 1. 00 mol 1. 00 L Slide 18 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

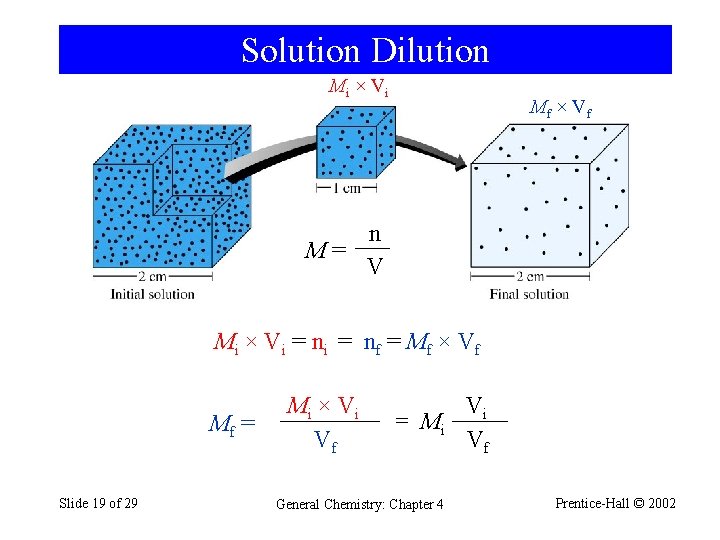
Solution Dilution Mi × V i Mf × V f n M= V Mi × Vi = nf = Mf × Vf Mf = Slide 19 of 29 Mi × V i Vf = Mi General Chemistry: Chapter 4 Vi Vf Prentice-Hall © 2002

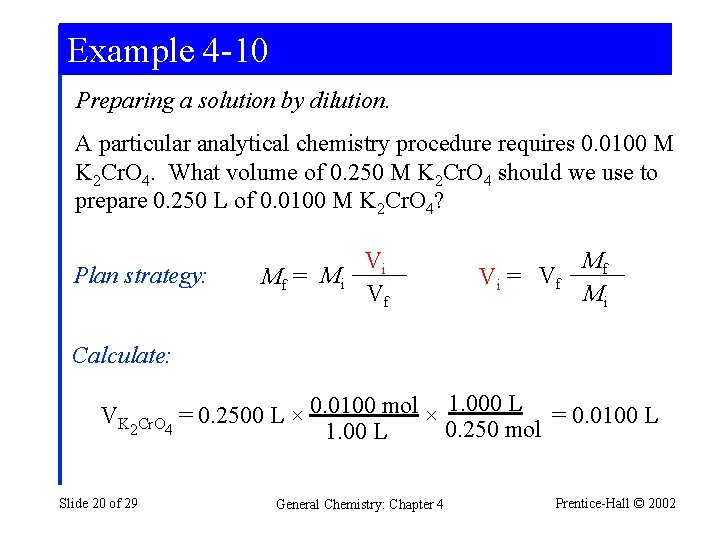
Example 4 -10 Preparing a solution by dilution. A particular analytical chemistry procedure requires 0. 0100 M K 2 Cr. O 4. What volume of 0. 250 M K 2 Cr. O 4 should we use to prepare 0. 250 L of 0. 0100 M K 2 Cr. O 4? Plan strategy: Vi Mf = Mi Vf Vi = Vf Mf Mi Calculate: VK 0. 0100 mol × 1. 000 L = 0. 0100 L = 0. 2500 L × 2 Cr. O 4 0. 250 mol 1. 00 L Slide 20 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

4 -4 Determining Limiting Reagent • The reactant that is completely consumed determines the quantities of the products formed. Slide 21 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

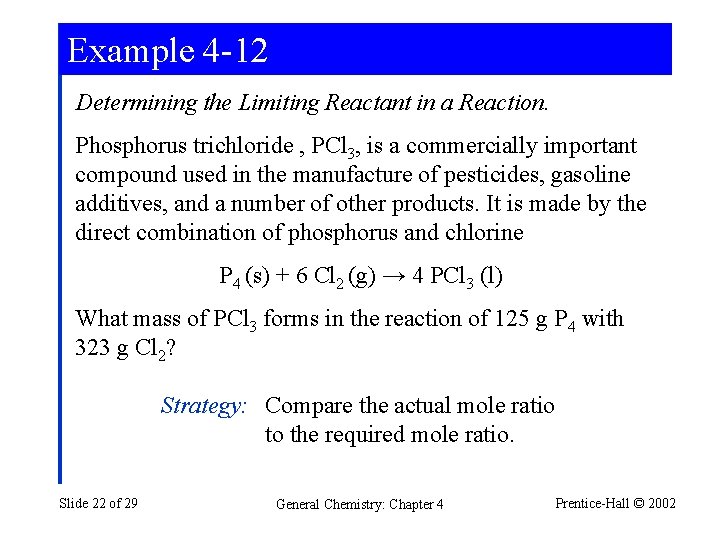
Example 4 -12 Determining the Limiting Reactant in a Reaction. Phosphorus trichloride , PCl 3, is a commercially important compound used in the manufacture of pesticides, gasoline additives, and a number of other products. It is made by the direct combination of phosphorus and chlorine P 4 (s) + 6 Cl 2 (g) → 4 PCl 3 (l) What mass of PCl 3 forms in the reaction of 125 g P 4 with 323 g Cl 2? Strategy: Compare the actual mole ratio to the required mole ratio. Slide 22 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

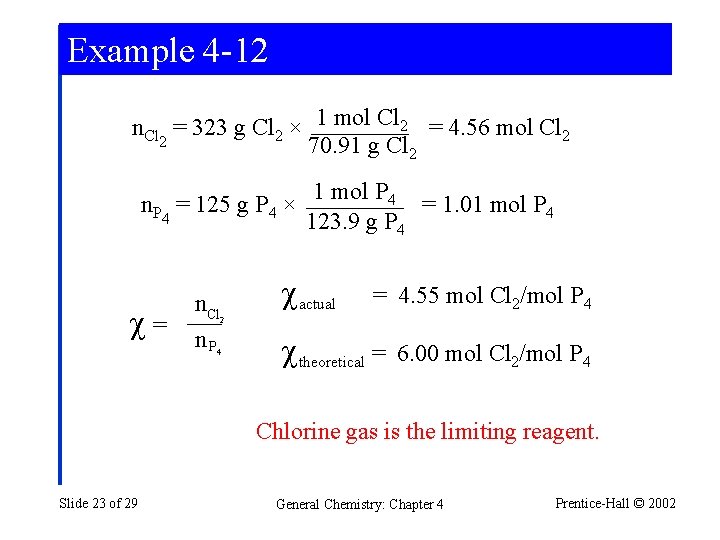
Example 4 -12 n. Cl = 323 g Cl 2 × 1 mol Cl 2 = 4. 56 mol Cl 2 2 70. 91 g Cl 2 n. P = 125 g P 4 × 4 = n. Cl n. P 1 mol P 4 = 1. 01 mol P 4 123. 9 g P 4 actual = 4. 55 mol Cl 2/mol P 4 2 4 theoretical = 6. 00 mol Cl 2/mol P 4 Chlorine gas is the limiting reagent. Slide 23 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

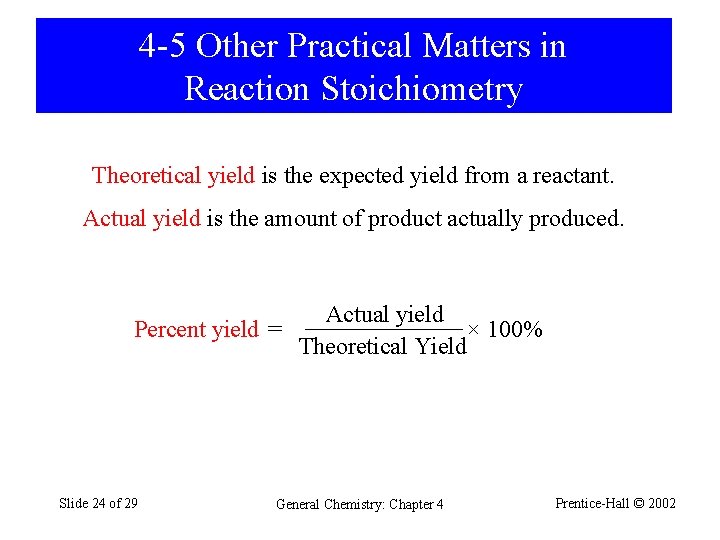
4 -5 Other Practical Matters in Reaction Stoichiometry Theoretical yield is the expected yield from a reactant. Actual yield is the amount of product actually produced. Actual yield Percent yield = × 100% Theoretical Yield Slide 24 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Theoretical, Actual and Percent Yield • When actual yield = % yield the reaction is said to be quantitative. • Side reactions reduce the percent yield. • By-products are formed by side reactions. Slide 25 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Consecutive Reactions, Simultaneous Reactions and Overall Reactions • Multistep synthesis is often unavoidable. • Reactions carried out in sequence are called consecutive reactions. • When substances react independently and at the same time the reaction is a simultaneous reaction. Slide 26 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

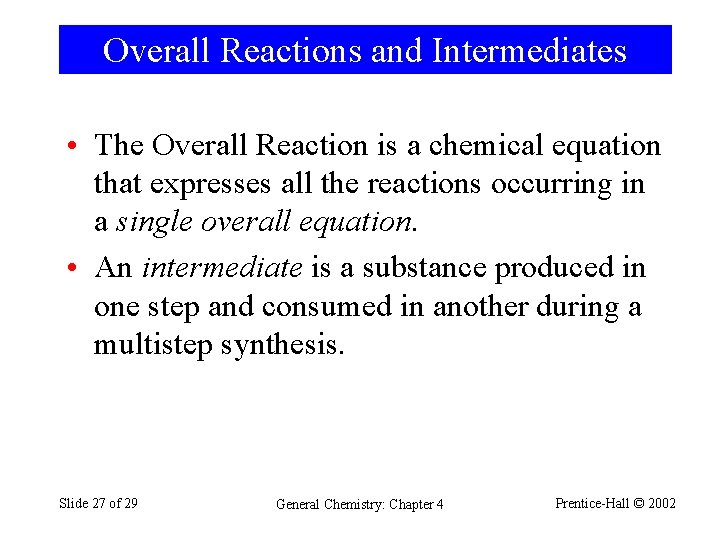
Overall Reactions and Intermediates • The Overall Reaction is a chemical equation that expresses all the reactions occurring in a single overall equation. • An intermediate is a substance produced in one step and consumed in another during a multistep synthesis. Slide 27 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

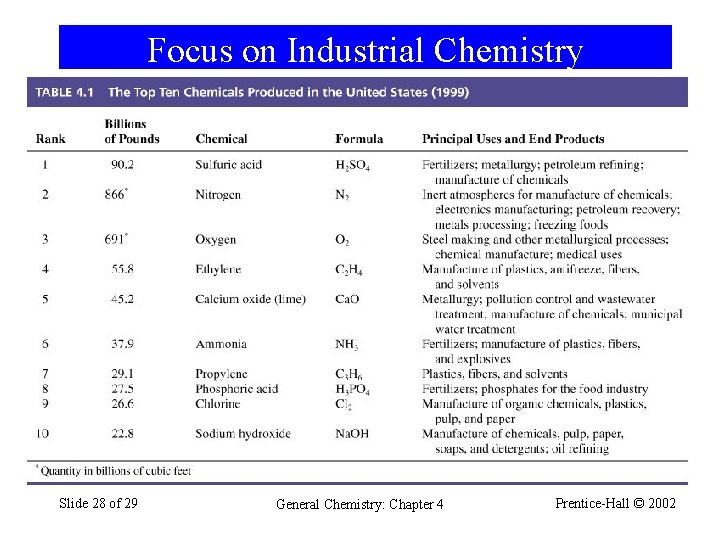
Focus on Industrial Chemistry Slide 28 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002

Chapter 4 Questions 1, 6, 12, 25, 39, 45, 53, 65, 69, 75, 84, 94, 83, 112 Slide 29 of 29 General Chemistry: Chapter 4 Prentice-Hall © 2002
 Normal koşullar
Normal koşullar Klorpentan
Klorpentan Quimica general petrucci 11 edicion pdf
Quimica general petrucci 11 edicion pdf Alan petrucci
Alan petrucci Petrucci
Petrucci Petrucci
Petrucci Doug harwood
Doug harwood Yankee city araştırması nedir
Yankee city araştırması nedir Crusher hire harwood
Crusher hire harwood Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen nuttall
Gwen nuttall Joe harwood
Joe harwood Leigh harwood
Leigh harwood Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Electrical engineering
Electrical engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Irradiated food
Irradiated food Smart materials names
Smart materials names Modern chemistry chapter 9
Modern chemistry chapter 9 Love chemical formula
Love chemical formula Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Chapter 14 acids and bases
Chapter 14 acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 review solutions section 2
Chapter 12 review solutions section 2 Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions