Final Exam Review Take these Notes Study them








































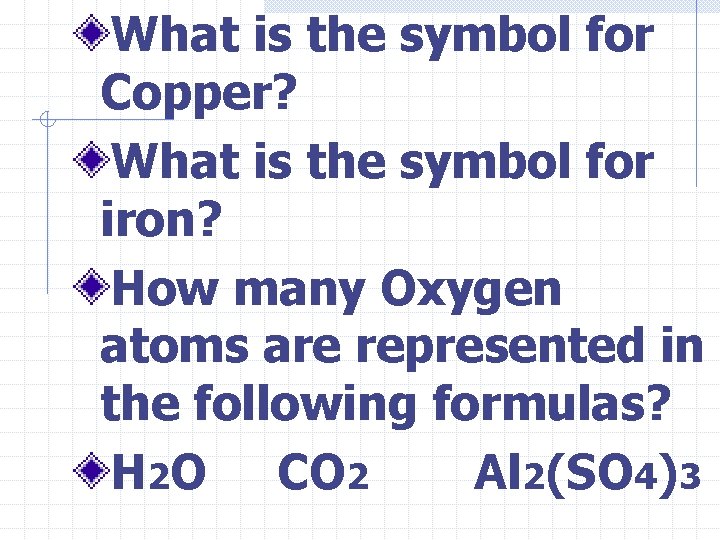


























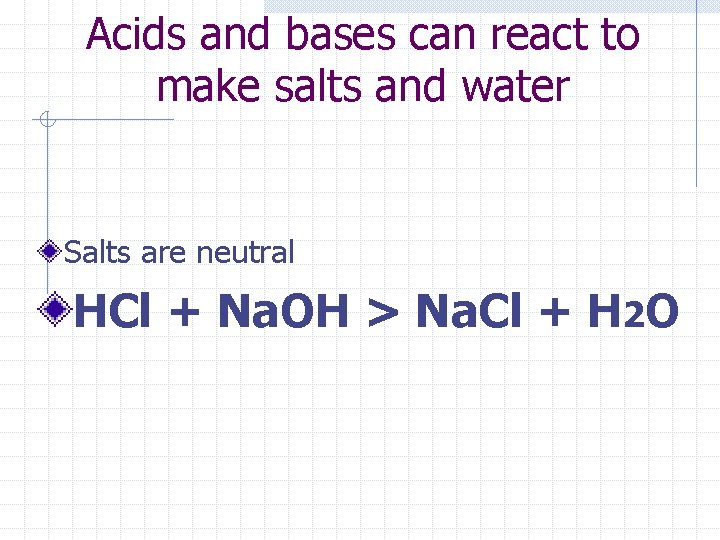










- Slides: 141

Final Exam Review Take these Notes! Study them, ask questions!!!

Unit 1 Force and Motion Speed and velocity Newton’s first Newton’s second Newton’s third law Recognizing laws of motion Analyzing motion using graphs Mass verses weight Gravity Work and power Simple machines

Speed and Velocity An object is in motion when it’s distance from a fixed object is changing. Ex me & desk walk from point of reference Speed is the rate at which motion occurs. A “rate” is the change of something per unit time Ex: m/s Speed = d/t Velocity = distance / time and the direction Do the problems on the next page.

A. B. C. D. Which of the following involves velocity? A bicyclist traveled 68. 7 meters A Bear ran 42 feet in only 3 seconds A tornado traveled 3 miles toward Walter hill in 2 minutes. Todd beat Mary in the 100 yard dash by 3 seconds.

An ice cream truck traveled 1. 7 km in only 3 minutes, what was the truck’s velocity in km/min and km/s? The speed is. 57 km/min or. 0094 km/s but the velocity is unknown because you need a direction like. 57 km/min toward the playground or to the east, etc.

Newton’s first law of motion An object at rest remains at rest and an object in motion remains in motion unless acted upon by an unbalanced force Remember Newton’s I = The law of inertia The tendency of objects to resist a change in their motion In order to change anything's present motion an unbalanced force must act on it

It takes a force to start an object moving Rock, car, book, ball etc It takes a force to slow or stop an object from moving Ex: friction with floor, road, table, air Forces can be balanced or unbalanced. Ex: tug of war, terminal velocity Mass is a measure of inertia. Inertia is the tendency for objects to resist a change in their motion so it makes sense that something with more mass would have more inertia. The > The mass the > the inertia

Ex: question How does Newton’s first law relate to a person crashing a bike into a wall? The bike has inertia, it wants to continue going forward at the same rate of speed until the wall exerts a force on the bike to stop it. What about the person on the bike? What would stop the bike if there was no wall?

Newton’s second law Pedal bike hard or easy to speed up fast? Force is related to acceleration The force on an object is equal to the mass of the object and the objects acceleration Force = Mass x Acceleration is the rate at which velocity changes so remember, acceleration happens whenever something speeds up, slows down, or changes direction. Ex: car speeding up, slowing down or turning. Like the Ferris Wheel question.

A = Δv/t The units of acceleration are m/s^2 or Draw graph now m/s/s A car is moving 3 m/s after 1 second, at the end of 2 seconds it is moving at a rate of 6 m/s. this does not mean that it traveled 6 m in 2 seconds! This means that it is traveling 6 m/s at the end of 2 seconds. At three seconds the object is traveling at 9 m/s then the acceleration is a = vf-vi a = 9 -0 = 3 m/s/s t 3

Ex: A person pushes a shopping cart, which one of the following will result in an increase of the shopping cart’s acceleration? Increasing the amount of groceries in the cart n Decreasing the force on the cart n Decreasing the distance traveled by the cart n Increasing the force on the cart n

Newton’s Third Law For every action force there is an equal and opposite reaction force.

Action–reaction forces are not balanced forces. n n Balanced forces act on one object in different directions Ex: tug of war and rope Action- reaction forces act on two different objects Ex: a person on roller skates and a wall The rocket forces hot gases downward and the hot gases must exert an equal and opposite reaction on the rocket

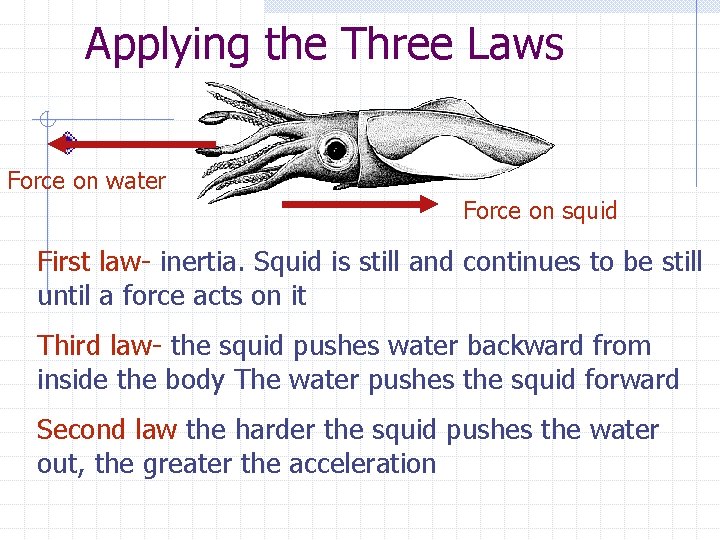
Applying the Three Laws Force on water Force on squid First law- inertia. Squid is still and continues to be still until a force acts on it Third law- the squid pushes water backward from inside the body The water pushes the squid forward Second law the harder the squid pushes the water out, the greater the acceleration

Ex: question A man is stuck out on very slippery ice on a frozen lake. He is close to shore but cannot reach it. He throws his backpack away from shore and he slides into the shore himself. Explain how the three laws of motion apply in this situation.

Analyzing Motion Using Graphs A line graph compares two variables. When looking at a graph, make sure you know exactly what the variables are on each axis. Look at the following graphs.



Mass Versus Weight The force that pulls objects toward the earth is called gravity All objects have weight due to gravity Weight is a measure of the force of gravity exerted on an object F=mxa so W=mxg Because of the equation, Weight varies with different masses and gravities.

W=mxg Acceleration due to gravity (g) on earths surface is 9. 8 m/s/s or 9. 8 m/s^2 Draw picture 4. 9, 19. 6, 44. 1 Weight is not the same as mass

Weight is a measure of the force of gravity exerted on an object Newtons Mass is a measure of how much matter is in an object Kilograms Weight changes from place to place but mass never changes!

Example Question An object on Earth has a mass of 10 kg. What is the objects weight? n n n 98 lb 98 N 109. 8 N 980 lb

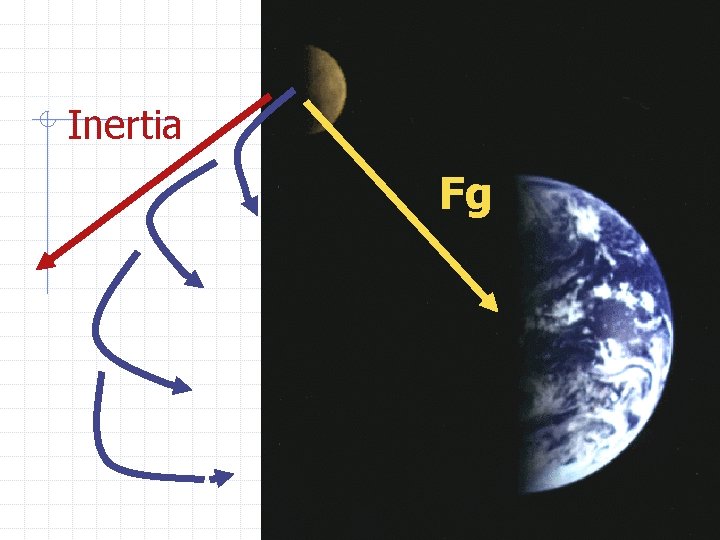
Gravity in Action: of Satellites and Tides Satellites are objects that travel around other objects in space Satellites travel at constant speeds but are always accelerating. Gravity and inertia

Inertia Fg

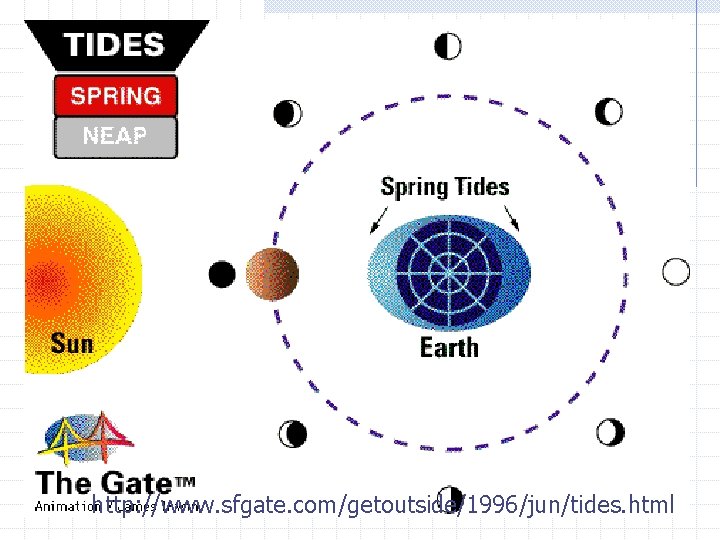
http: //www. sfgate. com/getoutside/1996/jun/tides. html

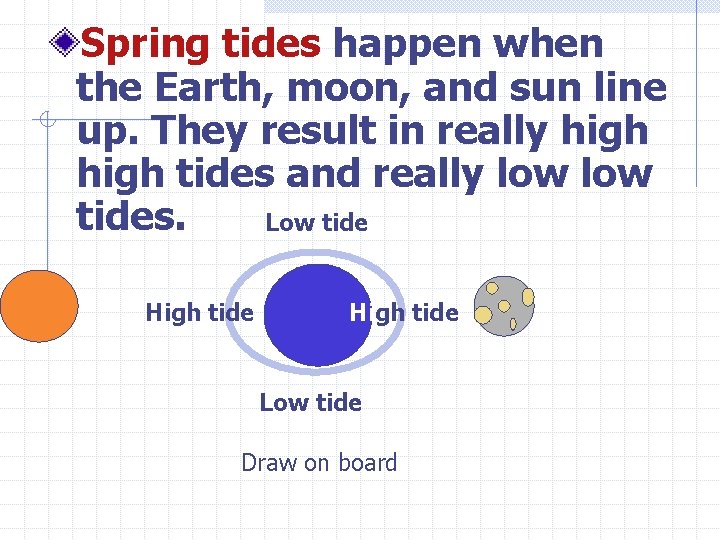
Spring tides happen when the Earth, moon, and sun line up. They result in really high tides and really low tides. Low tide High tide Low tide Draw on board

Neap tides happen when the earth, and moon make a right angle with the sun and result in lower than normal high tides and higher than normal low tides High tide Low tide

Work and Power Work equals force times distance N x m = 1 Nm = 1 Joule W=Fxd Two things must happen for work to be done: n n The object must move some distance The object must move in the same direction as the force. click to show unit then do Ex: books being carried

Power You can do the same amount of work in different times, do the work fast or slow Power is the rate at which work is done Power equals work divided by time P = w/t the Watt is the SI unit for power 1 W = 1 j/s

Ex: Problem Mr. D’s Jeep exerts a force of 1000 N to pull a boat 20 m in 8 s. What is the power output of the jeep in k. W? 2. 5 k. W

Simple machines A machine is a device that makes work easier to do. Some machines decrease the amount of force you must exert at a given time Some machines decrease the distance over which you need to exert the force And Some change the direction in which you exert the force

6 Simple Machines in 2 Families Inclined plane family Lever family Inclined plane Simple lever n Wedge screw n n First class Second class Third class Wheel and axle Pulley

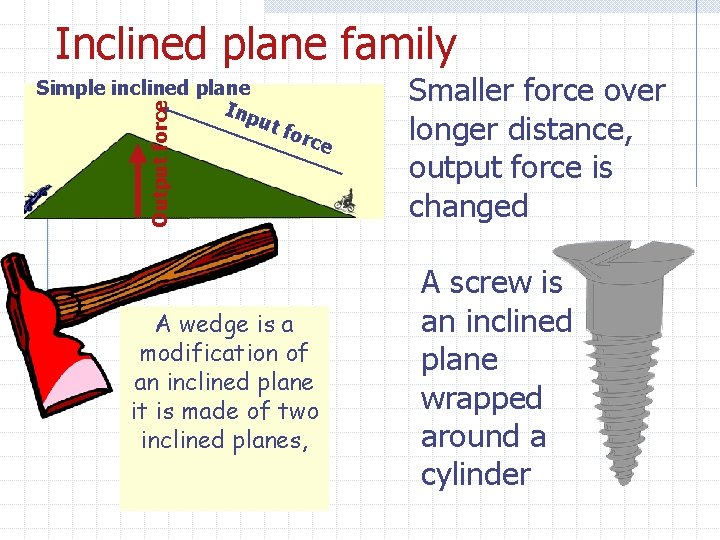
Inclined plane family Output force Simple inclined plane Inp ut for ce A wedge is a modification of an inclined plane it is made of two inclined planes, Smaller force over longer distance, output force is changed A screw is an inclined plane wrapped around a cylinder

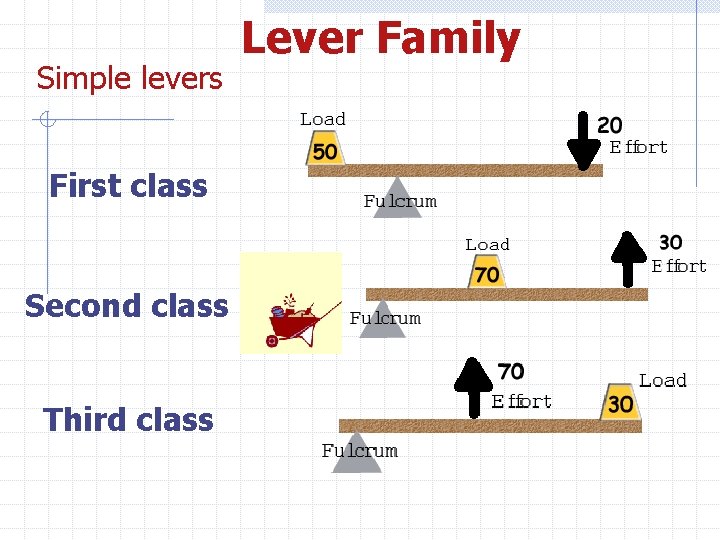
Simple levers First class Second class Third class Lever Family

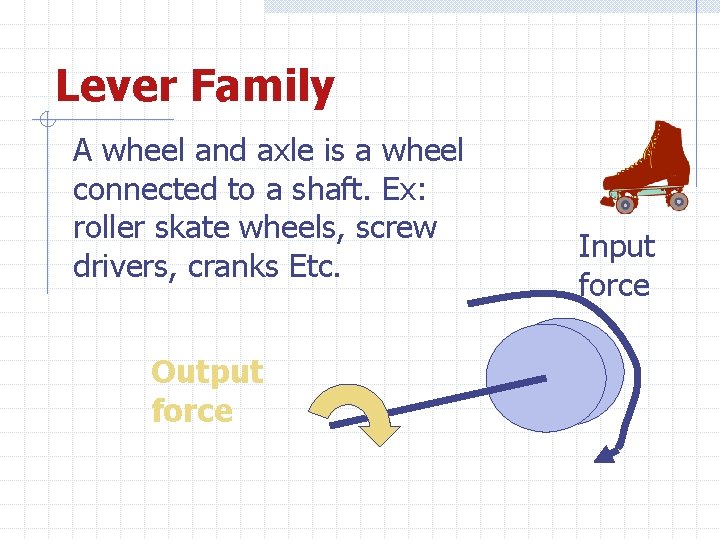
Lever Family A wheel and axle is a wheel connected to a shaft. Ex: roller skate wheels, screw drivers, cranks Etc. Output force Input force

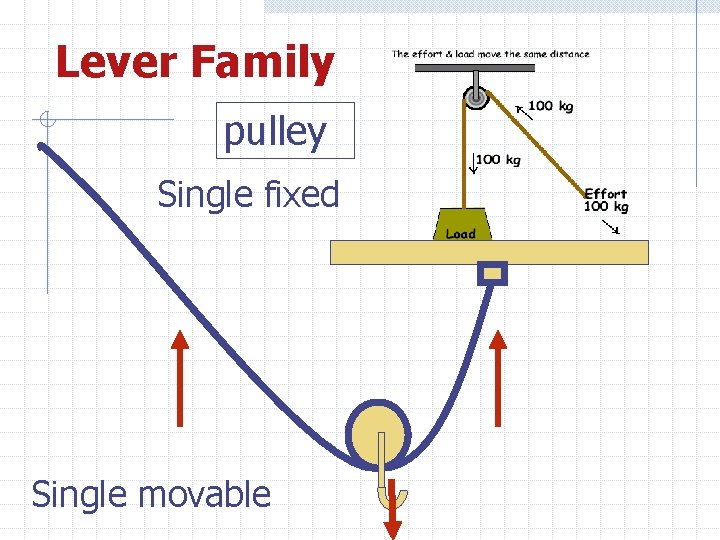
Lever Family pulley Single fixed Single movable

The Structure and Properties of Matter Measuring matter Phases of matter Atomic structure Elements and compounds Chemical symbols and formulas The periodic table Mixtures Solutions

Measuring matter Mass is the amount of matter in an object Mass is measured on a balance The SI unit of mass is grams or kilograms

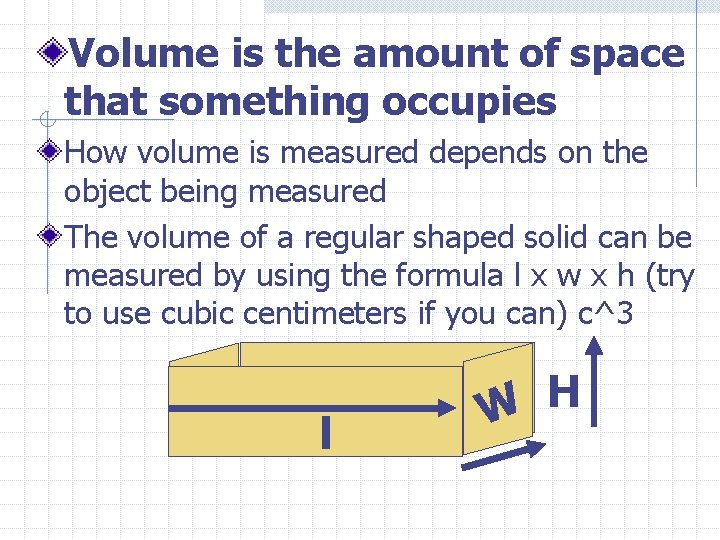
Volume is the amount of space that something occupies How volume is measured depends on the object being measured The volume of a regular shaped solid can be measured by using the formula l x w x h (try to use cubic centimeters if you can) c^3 l H W

The volume of liquids can be measured in a graduated cylinder. The volume of an irregular solid can be measured using a graduated cylinder and a liquid. Do this for them

Density = mass divided by volume D=m/v g/cm^3 Two objects might have the same mass but occupy very different volumes. Ex: 1 kg feathers and 1 kg lead Two objects might have the same volumes but very different masses Ex: brick and sponge

Density continued. Density is a characteristic physical property. The density of something determines if something will float in water or not. The density of water is 1 g/cm^3

Example Question A sample of pumice rock occupies a volume of 20. 1 cm^3 And has a mass of 18 g. What is it’s density? . 90 g/cm^3 Will the rock float in water? yes

Phases of matter Solid Liquid Gas Plasma

a Solid has: a Definite volume and definite shape n. Particles tightly packed but can vibrate in place

A liquid has A definite volume but no definite shape Particles still close together but with enough energy to overcome some of their intermolecular attraction to each other. Free to slide around each other

A gas has no definite volume and no definite shape Gasses will expand to fill their container because they have enough kinetic Energy to overcome almost all of their intermolecular attraction to each other

A Plasma Exists only at very high temperatures Is similar to a gas but contains pieces of particles and not all whole particles of matter Can be found in fluorescent light bulbs Naturally occurring plasmas include Fire, northern lights, lightning. The stars

Example question: A sample of matter occupies the same amount of space no matter what it’s container may be, yet it always takes the shape of it’s container. What phase of matter is it in?

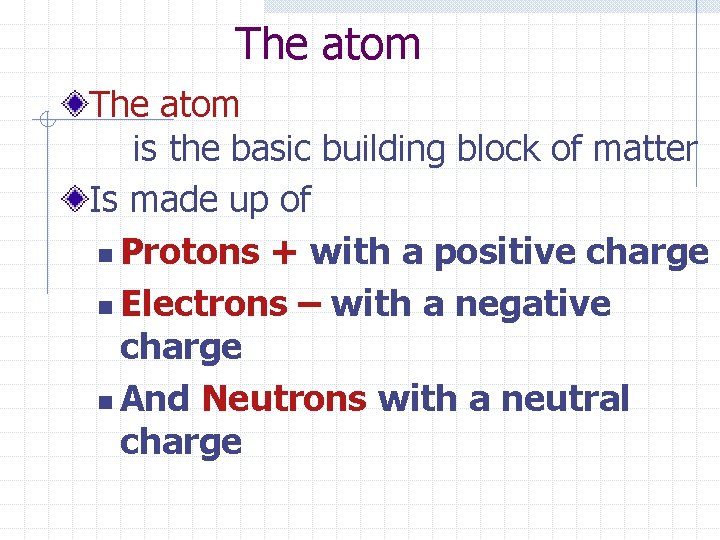
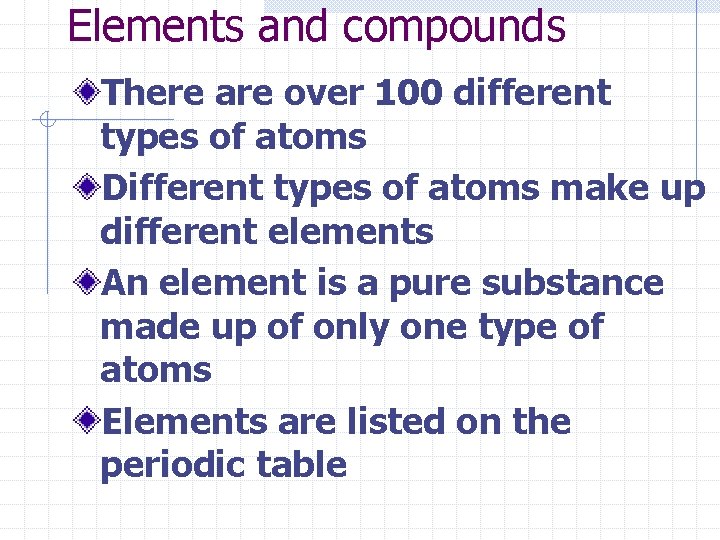
The atom is the basic building block of matter Is made up of n Protons + with a positive charge n Electrons – with a negative charge n And Neutrons with a neutral charge

Electron cloud electrons nucleus Protons and neutrons

N P+ E- Neutrons are slightly more massive than protons Protons are 1, 836 time more massive than electrons So almost all of the mass of an atom is located in the nucleus While almost all of the volume of an atom is the surrounding electron cloud

Elements and compounds There are over 100 different types of atoms Different types of atoms make up different elements An element is a pure substance made up of only one type of atoms Elements are listed on the periodic table

Common Elements Helium, oxygen, carbon, nitrogen, gold, silver, copper, aluminum

Pure Substances Elements are pure substances Compounds are pure substances they are made of two or more elements Compounds can be given in single formulas

Common Compounds Water, carbon dioxide sugar rubbing alcohol, ammonia, sodium chloride, baking soda, hydrogen peroxide

Elements and compounds are pure substances Which of these examples best represents an element A-helium in a balloon B-the air you breathe C-a gravel driveway C-salt


Objectives for the Day 2. 1 a Select a pure substance, which is an element or compound, from a list of choices 2. 2 c Recognize symbols for common elements (H, He, LI…)Or formulas for common compounds given a list.

Elements and compounds TN standard 2. 1 a & 2. 2 c There are two categories of matter: pure substances and mixtures Pure substances are the same throughout and do not vary from sample to sample. For example: a sample of pure silver is made of all silver atoms and two different samples of pure silver would be the same.

Elements and compounds are pure substances An Element is a pure substance made up of only one type of atom Ex: gold, iron, hydrogen etc. A compound is a pure substance made up of atoms of two or more elements that are chemically bonded together and take on their own properties

Examples of compounds include: Water, carbon dioxide, sugar, sodium chloride Etc. Overhead examples Many compounds and some elements exist as molecules. A molecule is the smallest unit of an element or compound that retains all of the properties of that element or Example tubes of H and O compound 2 2

Pure Substances Elements Are represented by chemical symbols that are one or two letters long and have the first letter capitalized. Examples: Aluminum n Carbon n Copper n Al C Cu Compounds Are represented by chemical formulas which are made up of symbols and numbers Sodium Chloride Na. Cl n Hydrogen peroxide H 2 O 2 n Glucose C 6 H 12 O 6 n

Chemical formulas have two kinds of numbers The Subscript tells us how many of the preceding there are. C 6 H 12 O 6 Al 2(SO 3)3 Coefficients tell us how many of everything after them there are until we get to a space 6 H 20 + 6 CO 2 > C 6 H 12 O 6 + 6 O 2

Example Question: Which of the following best represents an element? n The air you breath n A gravel driveway n Sodium chloride n The helium in a balloon
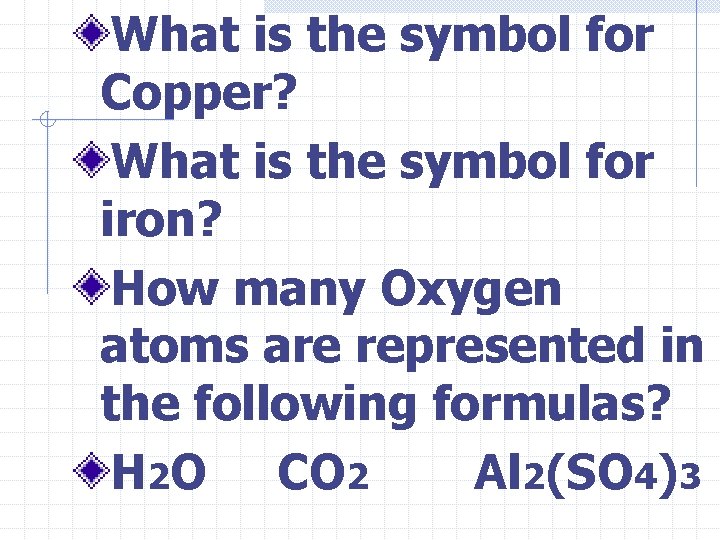
What is the symbol for Copper? What is the symbol for iron? How many Oxygen atoms are represented in the following formulas? H 2 O CO 2 Al 2(SO 4)3

Work in Groups of Three in Your Rows Do Questions 1 -9 of the Practice Worksheet

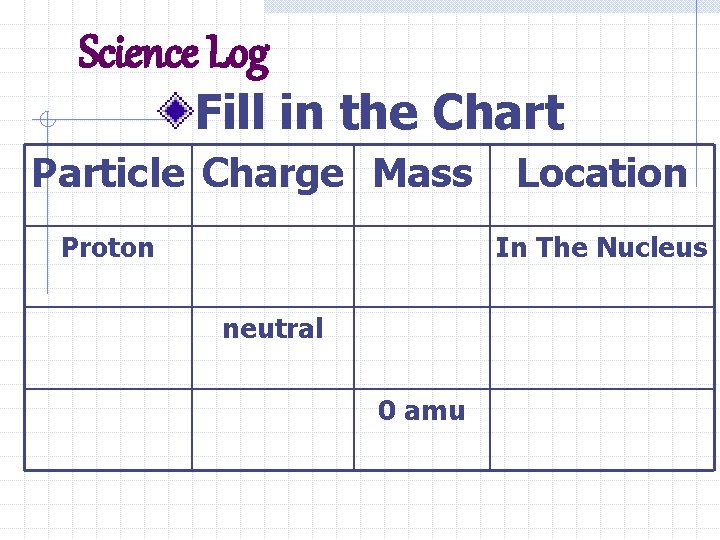
Science Log Fill in the Chart Particle Charge Mass Proton Location In The Nucleus neutral 0 amu

Two New Objectives 2. 2 a Identify an element as a metal, non-metal, or metalloid using the periodic table 2. 3 a Identify the atomic number, atomic mass, number of protons, number of neutrons, and electrons in an atom of a given element using the periodic table

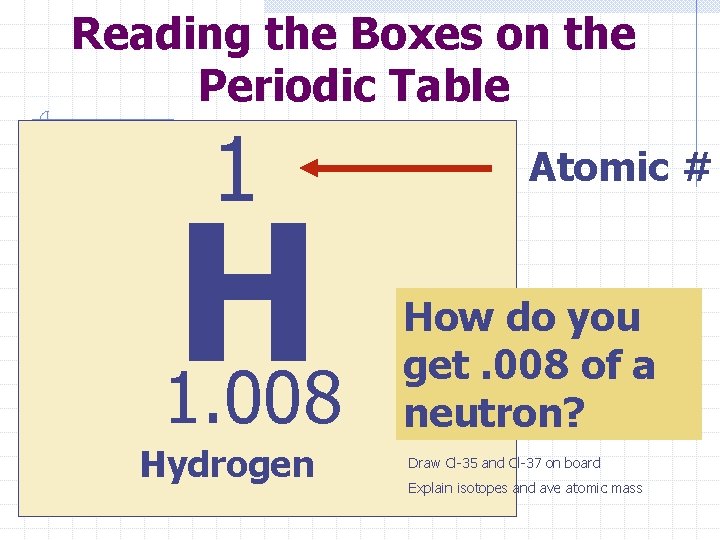
The periodic table Elements are 2. 2 a arranged on Tn standard & 2. 3 a the periodic table in order of increasing atomic number The Atomic # is the number of protons an atom has. For example: element 1 is Hydrogen and it has 1 proton Look at periodic table for more examples

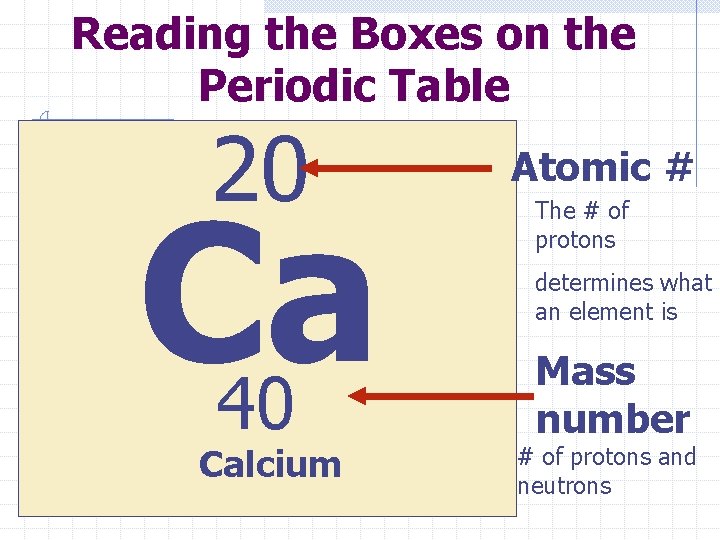
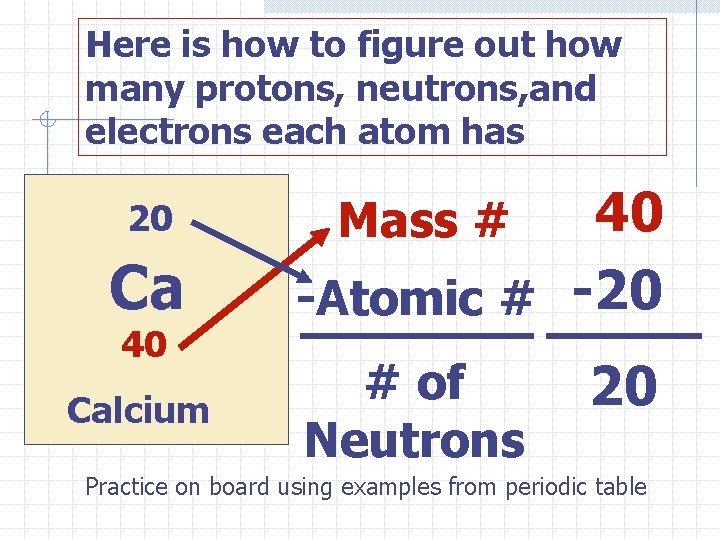
Reading the Boxes on the Periodic Table 20 Ca 40 Calcium Atomic # The # of protons determines what an element is Mass number # of protons and neutrons

Here is how to figure out how many protons, neutrons, and electrons each atom has 20 Ca 40 Calcium 40 -Atomic # -20 Mass # # of Neutrons 20 Practice on board using examples from periodic table

Reading the Boxes on the Periodic Table 1 H 1. 008 Hydrogen Atomic # How do you get. 008 of a neutron? Draw Cl-35 and Cl-37 on board Explain isotopes and ave atomic mass

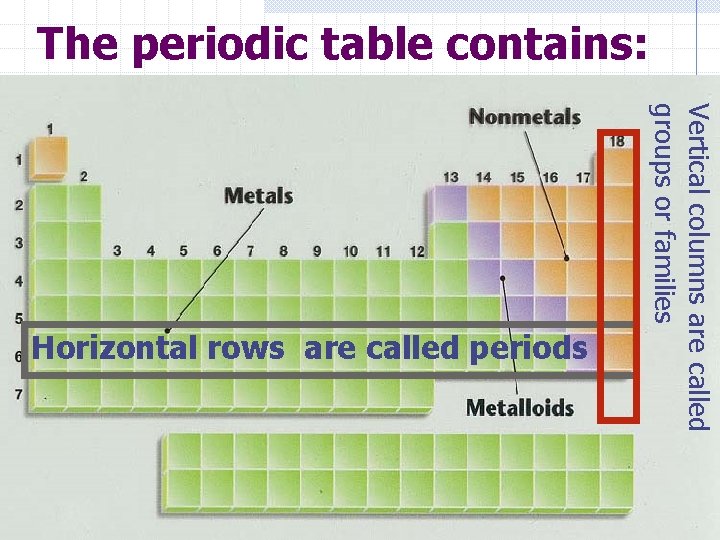
The periodic table contains: Vertical columns are called groups or families Horizontal rows are called periods

Compare and contrast metals & non Luster (shininess) Good conductors of heat and electricity High density (heavy for their size) High melting point Ductile (most metals can be drawn out into thin wires) Malleable (most metals can be hammered into thin sheets) No luster (dull appearance) Poor conductor of heat and electricity Brittle (breaks easily) Not ductile Not malleable Low density Low melting point

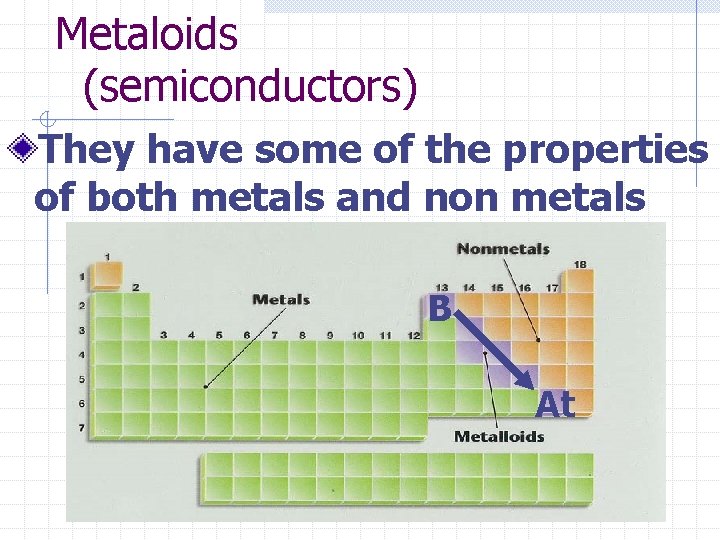
Metaloids (semiconductors) They have some of the properties of both metals and non metals B At

Example question: According to the periodic table, classify the following into categories of metal, non-metal, or metalloid/semiconductor n Boron B n Strontium Sr n Lead Pb n Krypton Kr

s tal Halogens Noble Gases on et th M il M ar a i. E k l al A lk A me et he r. N al s l a Ot Also know: s Transition Metals Othe r Me tals

Work in groups of three in your rows Do Questions 10 -17 of the practice worksheet

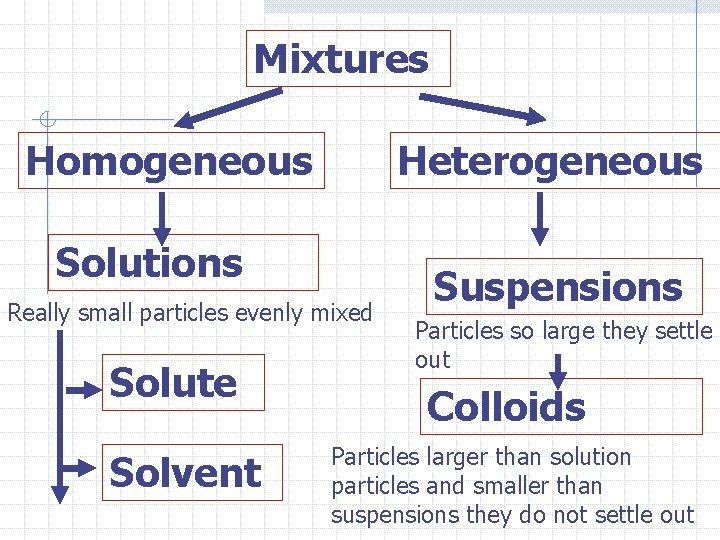
Mixtures State objective 2. 1 b. Identify a substance as a compound or mixture given a description of the substance A mixture is a blend of more than one pure substance Unlike a pure substance, a mixture can vary from sample to sample. Ex: dirt, fruit salad etc.

Two Types of Mixtures Homogeneous mixtures Are the same throughout and can be called solutions Ex: water and alcohol, Salt and water, Heterogeneous mixtures Are not the same throughout Ex: marbles and sand, dirt, Caesar salad

Example question: Which of these is a mixture you might find at the beach? n Carbon n Oxygen n Sunlight n Sand

Solutions Tn State objective 2. 1 d distinguish between elements, compounds, solutions, colloids, and suspensions given an example A solution is a homogeneous mixture in the same phase. You cannot tell where one thing is and another begins in a solution Ex: Saline solution

Mixtures Homogeneous Heterogeneous Solutions Really small particles evenly mixed Solute Solvent Suspensions Particles so large they settle out Colloids Particles larger than solution particles and smaller than suspensions they do not settle out

In a solution, one thing is dissolved in another. The solute, is the stuff that is dissolved The solvent is the substance that does the dissolving. Ex; sugar or salt in water

Example question: Which of these can be used to identify a suspension. A solute is dissolved in a solvent n Particles are small n Particles settle if undisturbed n A solid is dissolved in a liquid n

Tyler

Interactions of matter Physical and chemical changes Chemical reactions and equations Balancing chemical equations Types of chemical reactions Endothermic and exothermic reactions Acids bases and salts Acid rain.

Physical and Chemical Changes A physical change alters the appearance of matter without altering the composition of the matter. Ex: water changes phases of matter but it is still all H 2 O

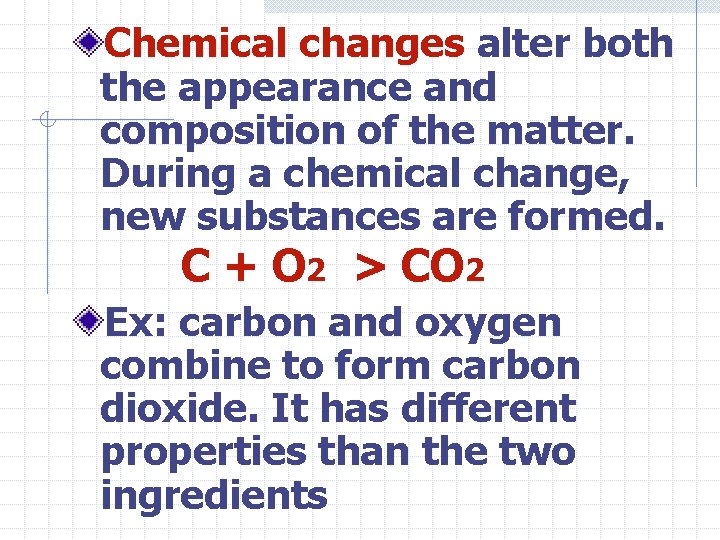
Chemical changes alter both the appearance and composition of the matter. During a chemical change, new substances are formed. C + O 2 > CO 2 Ex: carbon and oxygen combine to form carbon dioxide. It has different properties than the two ingredients

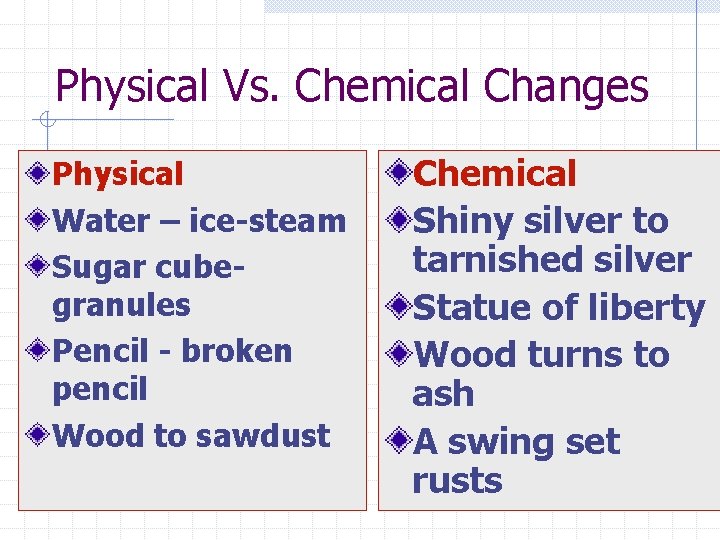
Physical Vs. Chemical Changes Physical Water – ice-steam Sugar cubegranules Pencil - broken pencil Wood to sawdust Chemical Shiny silver to tarnished silver Statue of liberty Wood turns to ash A swing set rusts

Things That Tell Us a Chemical Change Has Happened Production of gas Baking soda and vinegar Change in color Leaves turn in the fall Formation of a precipitate Like when we put two clear liquids together and solids fell out

Ex: Problem Classify the following as chemical or physical changes. n Splitting logs n Water evaporating from a puddle n A chair is painted n Dough bakes into bread

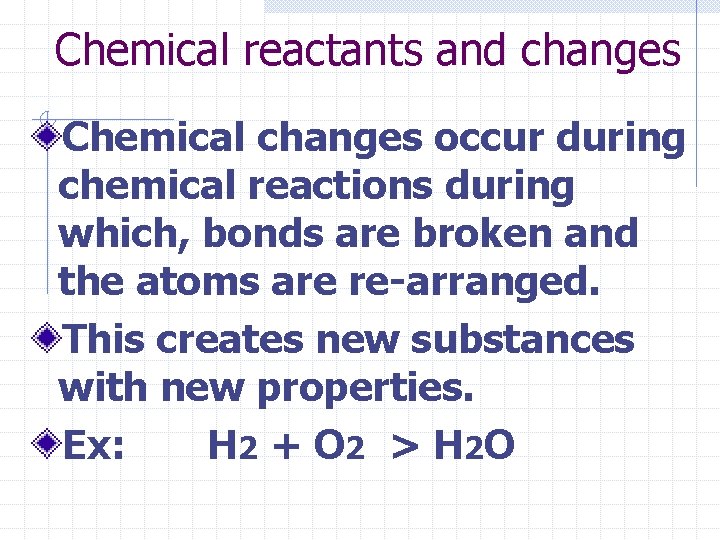
Chemical reactants and changes Chemical changes occur during chemical reactions during which, bonds are broken and the atoms are re-arranged. This creates new substances with new properties. Ex: H 2 + O 2 > H 2 O

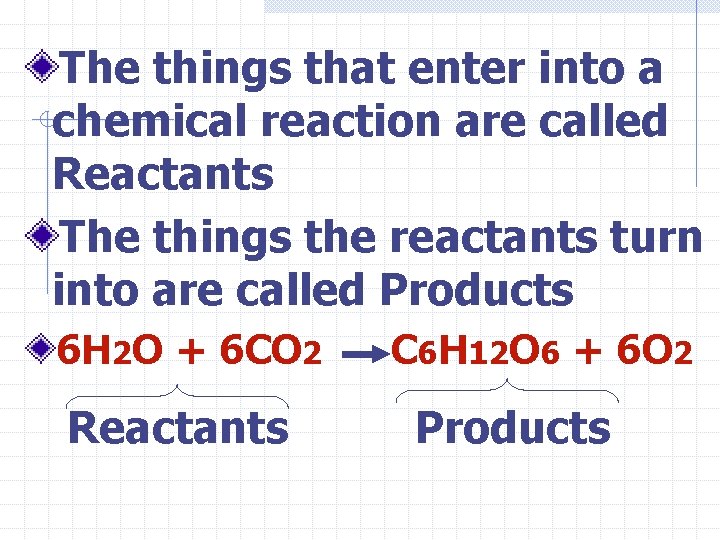
The things that enter into a chemical reaction are called Reactants The things the reactants turn into are called Products 6 H 2 O + 6 CO 2 Reactants C 6 H 12 O 6 + 6 O 2 Products

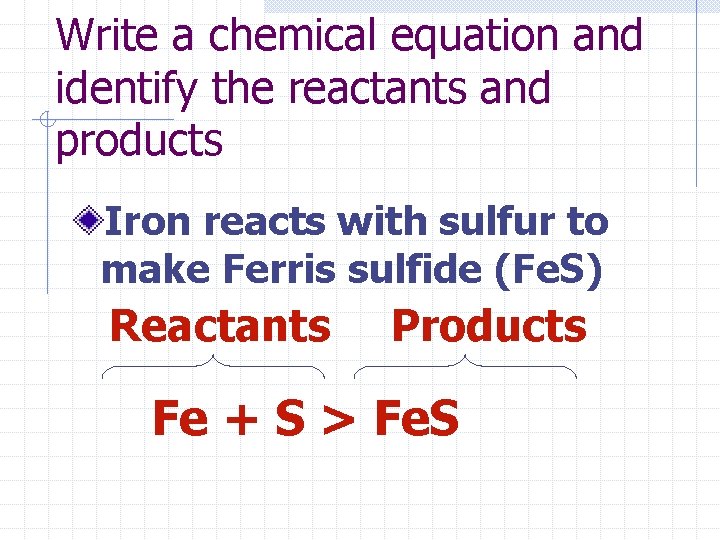
Write a chemical equation and identify the reactants and products Iron reacts with sulfur to make Ferris sulfide (Fe. S) Reactants Products Fe + S > Fe. S

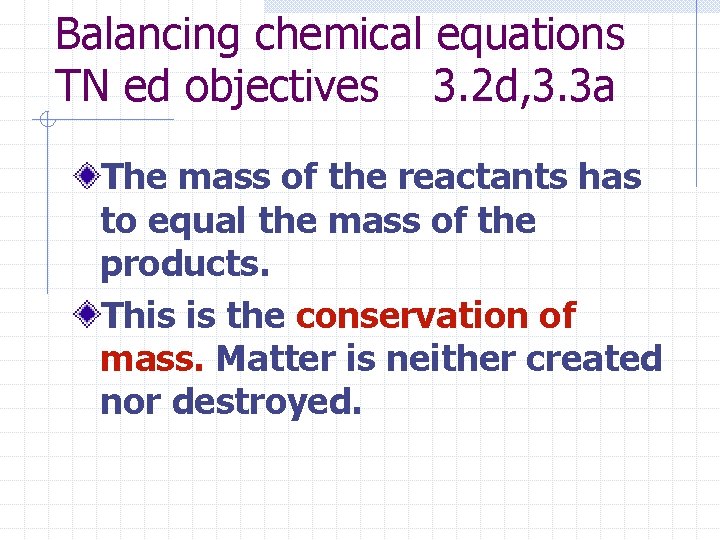
Balancing chemical equations TN ed objectives 3. 2 d, 3. 3 a The mass of the reactants has to equal the mass of the products. This is the conservation of mass. Matter is neither created nor destroyed.

Balance the following equations H 2 + O 2 > H 2 O 2 H 2 + O 2 > H 2 O Mg + O 2 > Mg. O

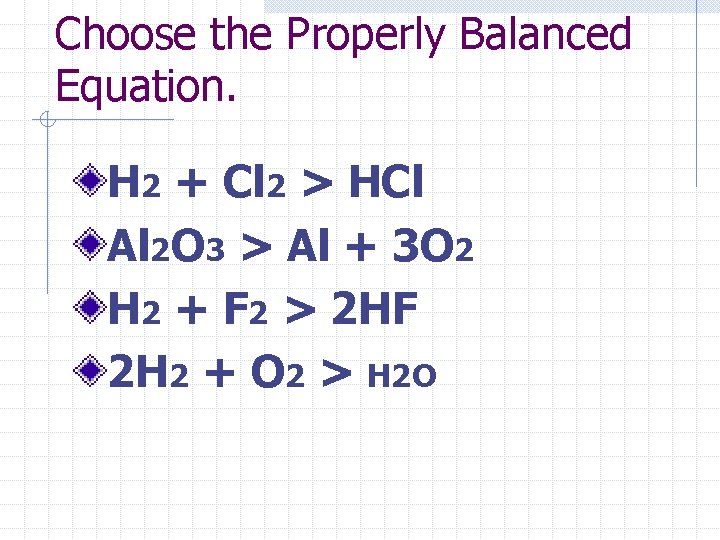
Choose the Properly Balanced Equation. H 2 + Cl 2 > HCl Al 2 O 3 > Al + 3 O 2 H 2 + F 2 > 2 HF 2 H 2 + O 2 > H 2 O

Types of chemical reactions 3. 2 b, 3. 3 b Single displacement Double displacement Synthesis Decomposition

Single displacement A + BX > AX + B Na + HCl > Na. Cl + H 2 Double displacement AX + BY > BX + AY HCl + Na. OH > Na. Cl + H 2 O

Synthesis – two or more substances come together to form a new, more complicated compound A + B > AB Ca + 2 Br > Ca. Br 2

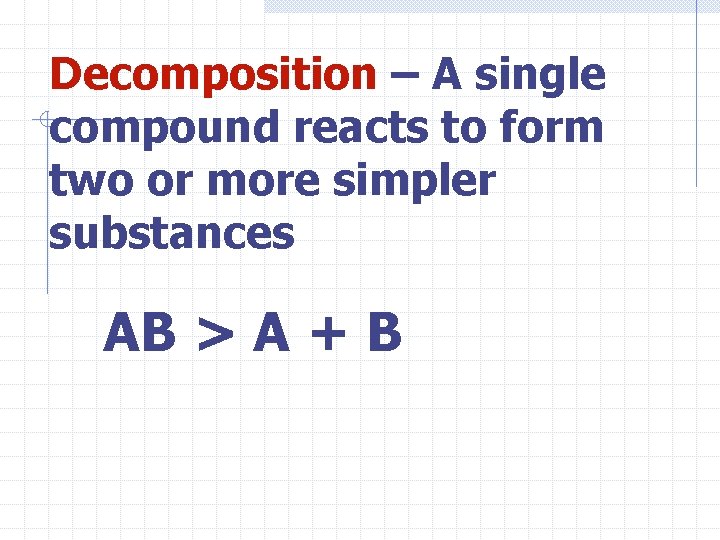
Decomposition – A single compound reacts to form two or more simpler substances AB > A + B

Example question What type of chemical reaction is this. H 2 CO 3 > CO 2 + H 2 O

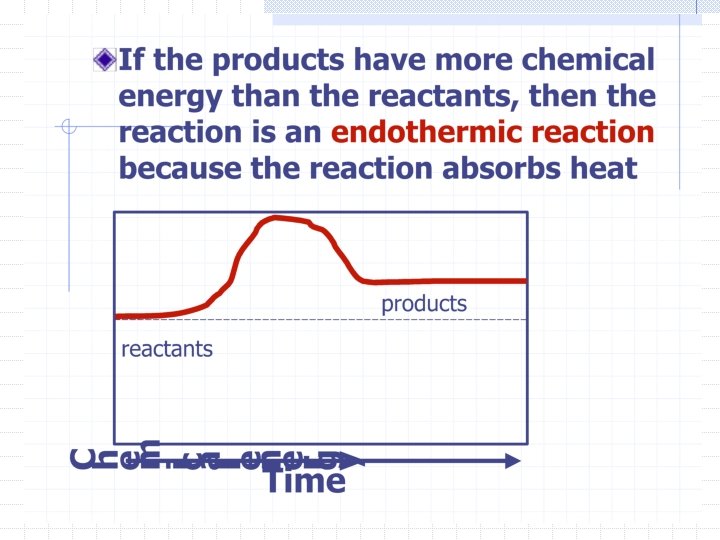
Endothermic and Exothermic Reactions All chemical reactions cause a change in energy Energy is the ability to do work or cause change Energy is stored in the chemical bonds that hold matter together.

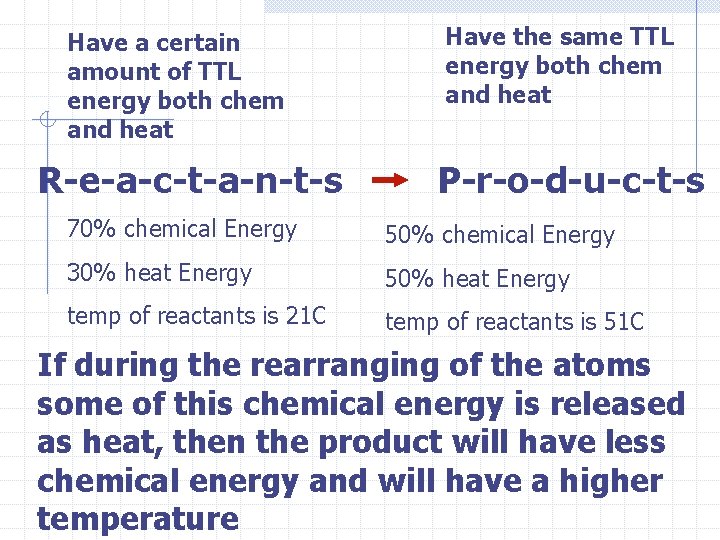
Chemical energy and heat energy are involved in a chemical reaction Some of the energy of the reactants is stored in the bonds as chemical energy Some of the energy is tied to the temperature of the reactants

Have a certain amount of TTL energy both chem and heat R-e-a-c-t-a-n-t-s Have the same TTL energy both chem and heat P-r-o-d-u-c-t-s 70% chemical Energy 50% chemical Energy 30% heat Energy 50% heat Energy temp of reactants is 21 C temp of reactants is 51 C If during the rearranging of the atoms some of this chemical energy is released as heat, then the product will have less chemical energy and will have a higher temperature

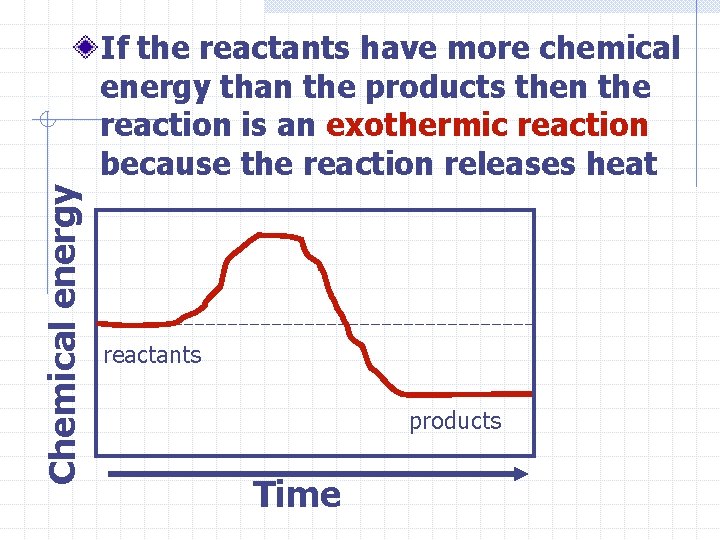
Chemical energy If the reactants have more chemical energy than the products then the reaction is an exothermic reaction because the reaction releases heat reactants products Time


So what does this mean? If the temperature of the products is higher than the temperature of the reactants then heat was released and the reaction is exothermic

Example question The reaction between baking soda an vinegar is exothermic. What must be true about the temperatures of the substances involved in the reaction? n n The temperature of the reactants is the same as the products The temperature of the products is lower than that of the reactants The temperature of the products is higher than that of the reactants The temperature of the reactants is replaced with the temperature of the reactants

Acids corrode metals Acids Bases and Salts An acid is something that increases the H+ concentration making more hydronium ions H 3 O+ Acids taste sour. ABR An Indicator is a substance that changes color in the presence of an acid or base For example: litmus paper

Bases feel slippery and taste bitter Bases have OH or the hydroxide ion on their formulas Sodium hydroxide is drain cleaner, While ammonium hydroxide is a household cleaner

Bases don’t react with metals but they will react with fats and oils to make soap
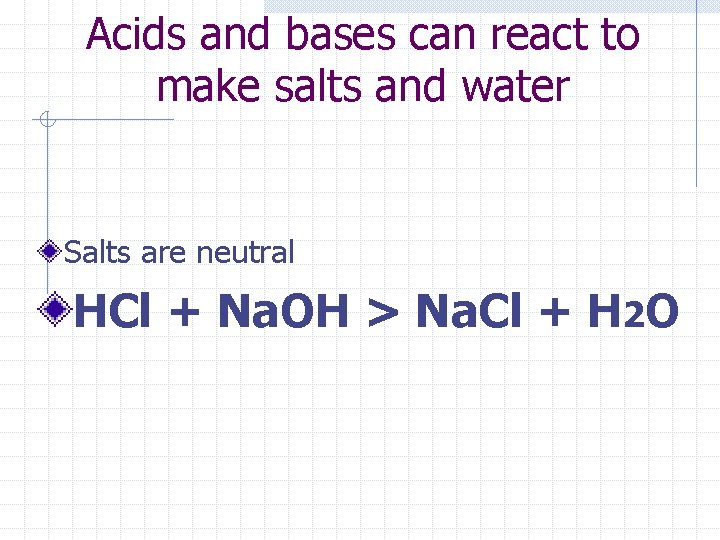
Acids and bases can react to make salts and water Salts are neutral HCl + Na. OH > Na. Cl + H 2 O

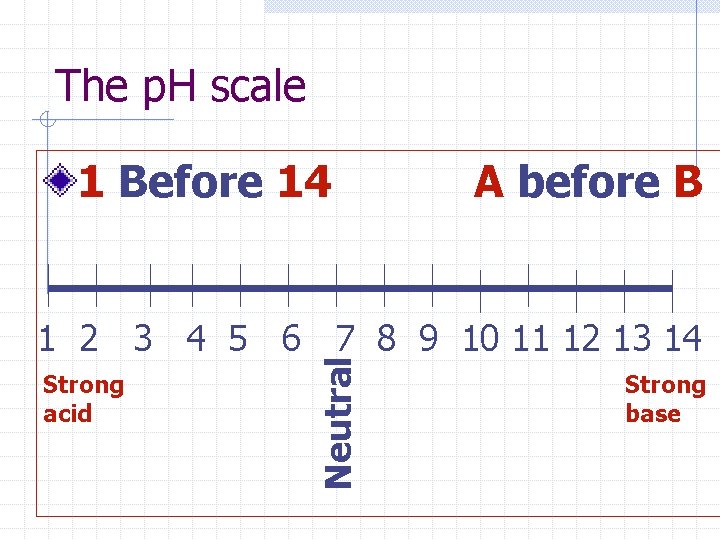
The p. H scale 1 Before 14 A before B Strong acid Neutral 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Strong base

Ex: Question The p. H of an egg is between 7. 6 and 8. 0. How would you describe an egg? n Weak acid n Strong base n Strong acid n Weak base

Acid Rain Many industrial processes (factories) release large amounts of chemicals into the air. Some of these chemicals combine with the water in the air to form acids. The acidic water then fall to Earth in the form of acid rain.

Some of the acidic rain can have p. H levels of as low as 3. 5!!! More acidic than a tomatoes!

Energy The nature of energy Types of waves Characteristics of waves Wave interactions Thermal energy and heat Temperature scales Nuclear energy Electrical energy

The nature of energy Energy is the ability to do work or cause change When you do work, you transfer energy to the object. For ex; move a ball from floor to over head. Throw a ball Etc.

Different Forms of Energy Chemical energy – is the energy stored in the bonds that hold compounds together. Thermal energy is the total energy of the particles of a sample of matter Heat is the transfer of thermal energy from one thing to another.

Different Forms of Energy Electrical energy is the energy produced by moving electric charges Electromagnetic energy is light energy radiation from the EM spectrum Nuclear energy is energy stored in the nucleus of an atom

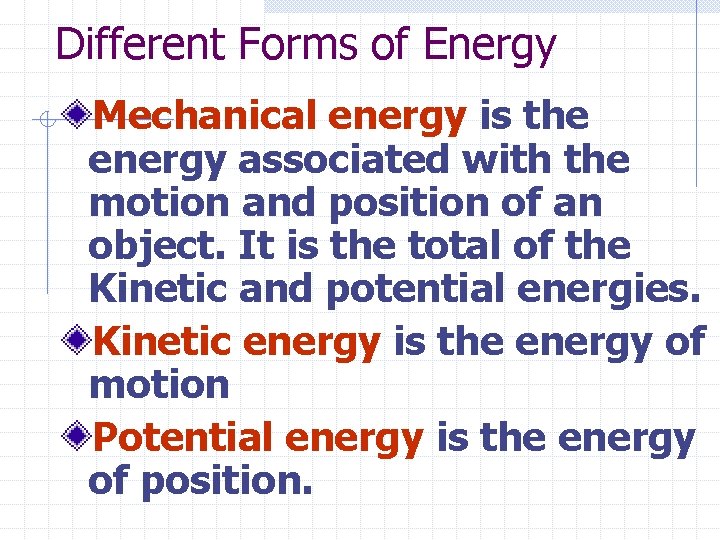
Different Forms of Energy Mechanical energy is the energy associated with the motion and position of an object. It is the total of the Kinetic and potential energies. Kinetic energy is the energy of motion Potential energy is the energy of position.

No matter what kind of energy you are talking about, one thing is sure. Energy is conserved. This means that energy does not just disappear. It changes into other types of energy. A clock pendulum will eventually stop. But the energy was not destroyed.

Energy From Sun to Plants to Fire to People. Energy Is Changed From Form to Form A researcher determined the amount of electrical energy that entered a toaster. Then figured the amount of thermal energy produced. The thermal energy was less than the electrical energy, what could be the reason for the difference? Some of the energy was changed into electromagnetic energy of light.

Waves Most waves require a medium. They are called mechanical waves. Ex: sound, seismic, surface waves etc. Some waves do not require a medium and can travel through empty space, (vacuum). They are called electromagnetic waves or light of all wavelengths

The Medium is The matter that a wave travels through A wave is a disturbance that transfers energy from one place to another Waves are produced by vibrations

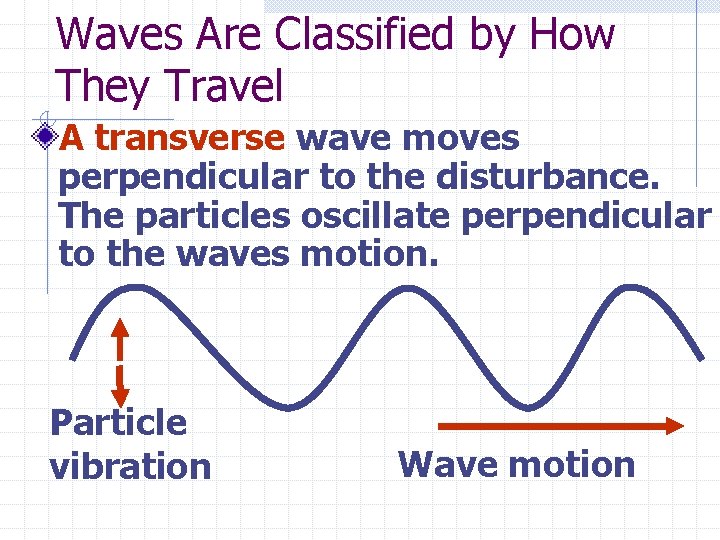
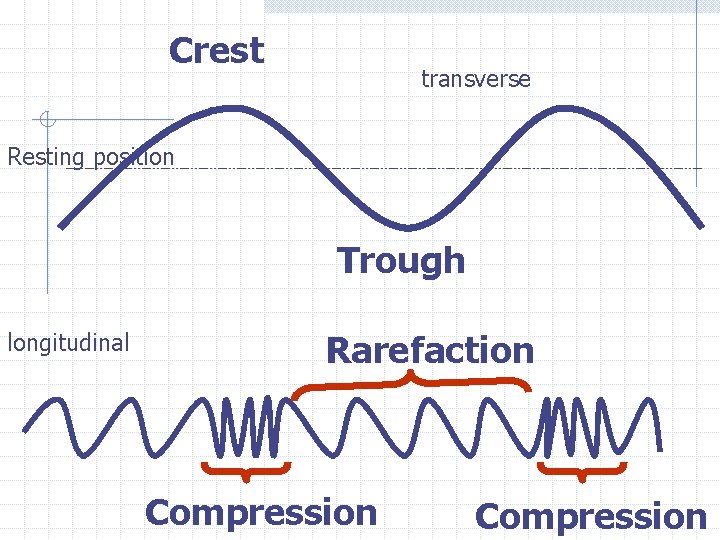
Waves Are Classified by How They Travel A transverse wave moves perpendicular to the disturbance. The particles oscillate perpendicular to the waves motion. Particle vibration Wave motion

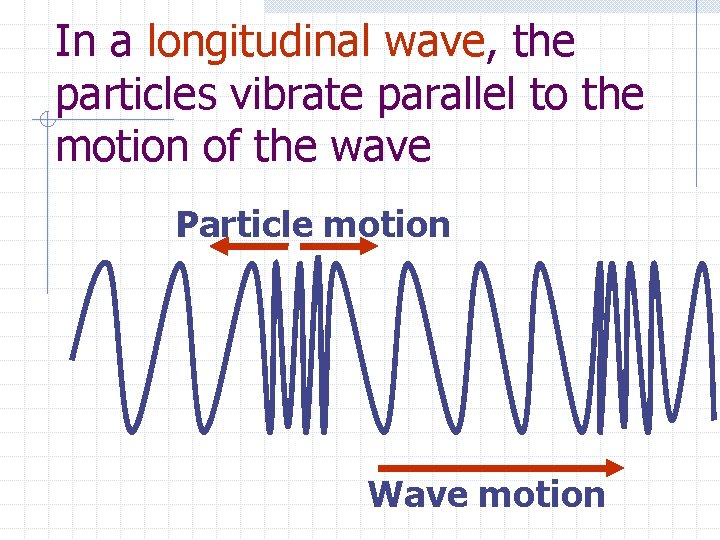
In a longitudinal wave, the particles vibrate parallel to the motion of the wave Particle motion Wave motion

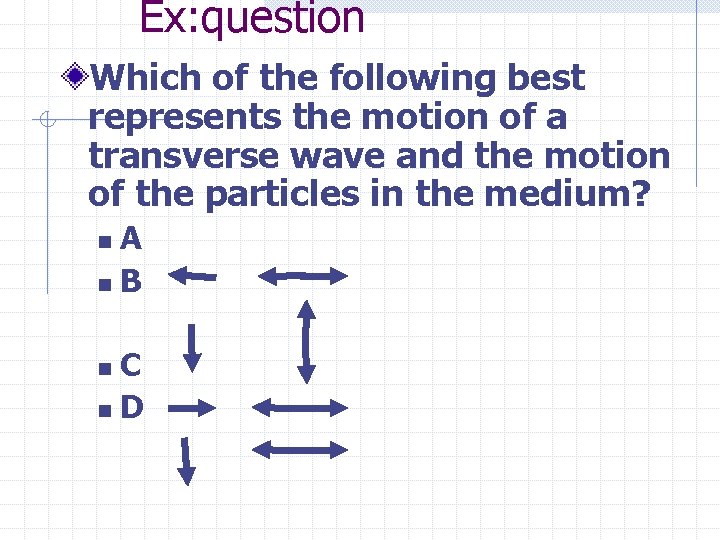
Ex: question Which of the following best represents the motion of a transverse wave and the motion of the particles in the medium? A n. B n C n. D n

Crest transverse Resting position Trough longitudinal Rarefaction Compression

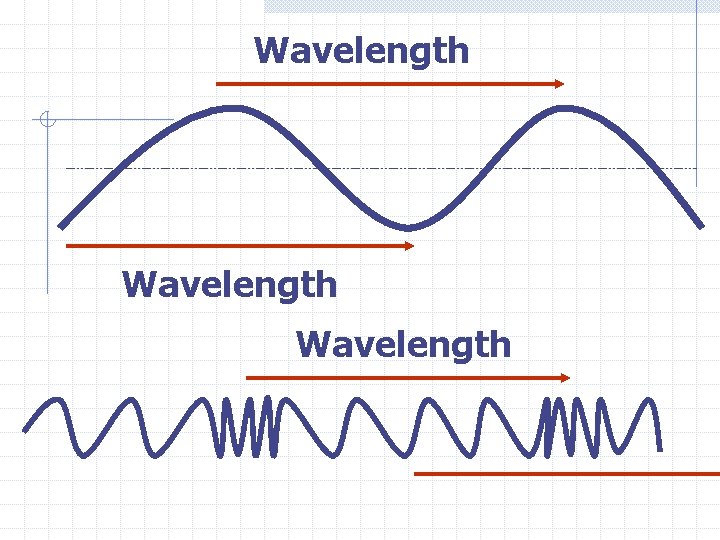
Wavelength

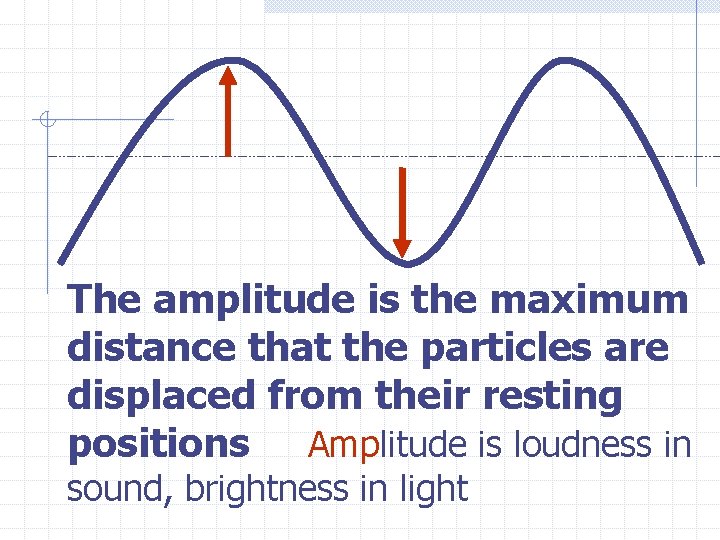
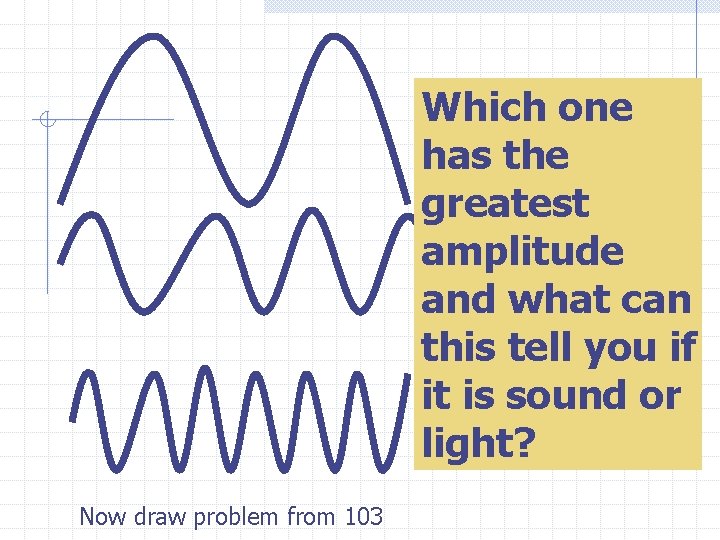
The amplitude is the maximum distance that the particles are displaced from their resting positions Amplitude is loudness in sound, brightness in light

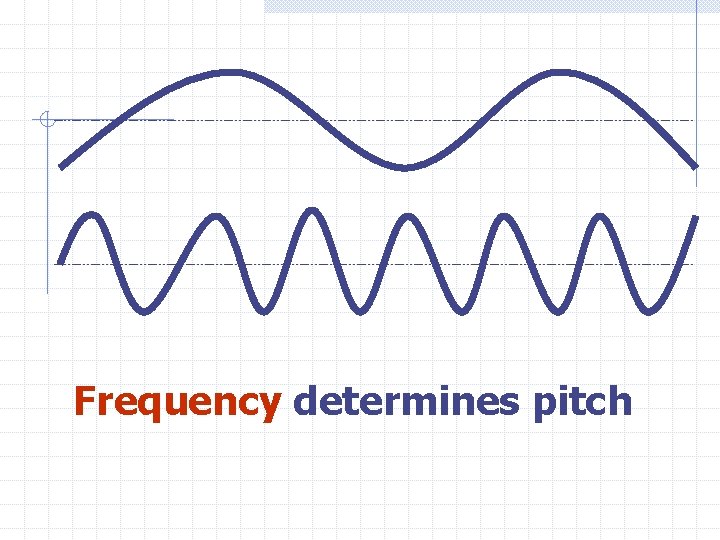
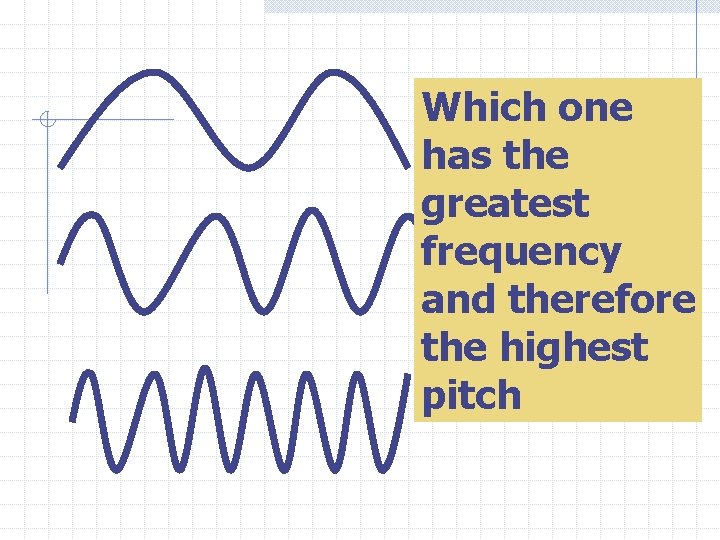
Frequency is the number of waves that pass a given point in a given time. For Ex: If 32 waves go by a place in 1 second then the frequency is 32 Hz. Each Hz is one vibration(wave) per second

Frequency determines pitch

Which one has the greatest frequency and therefore the highest pitch

Which one has the greatest amplitude and what can this tell you if it is sound or light? Now draw problem from 103

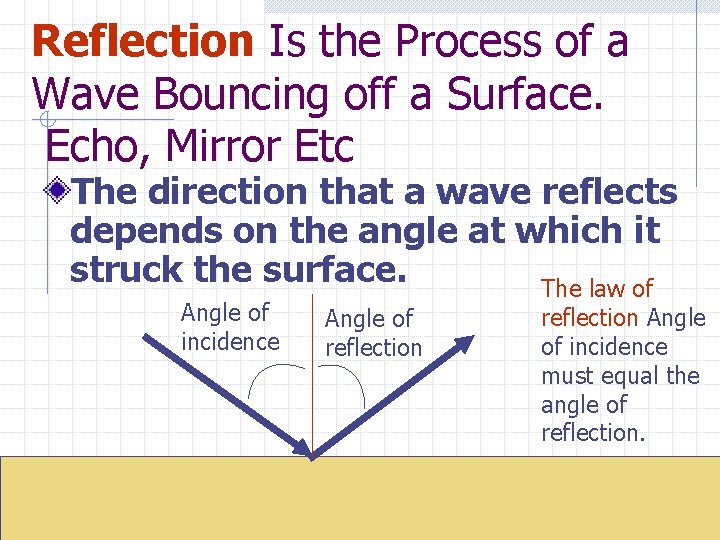
Reflection Is the Process of a Wave Bouncing off a Surface. Echo, Mirror Etc The direction that a wave reflects depends on the angle at which it struck the surface. The law of Angle of incidence Angle of reflection Angle of incidence must equal the angle of reflection.

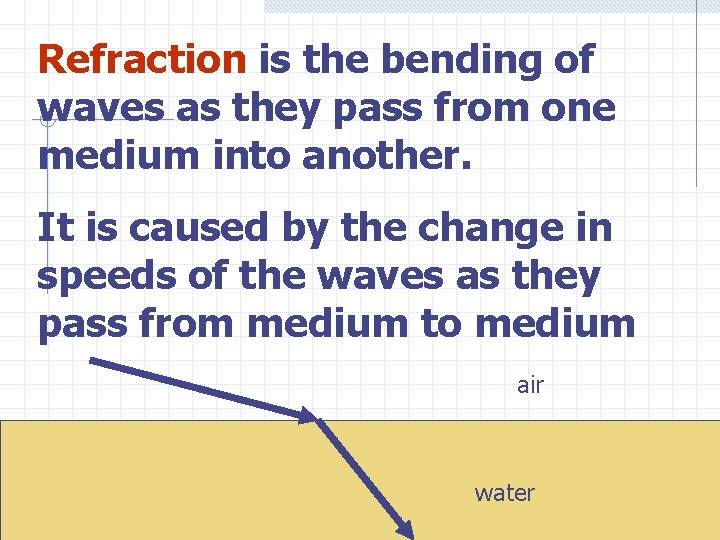
Refraction is the bending of waves as they pass from one medium into another. It is caused by the change in speeds of the waves as they pass from medium to medium air water

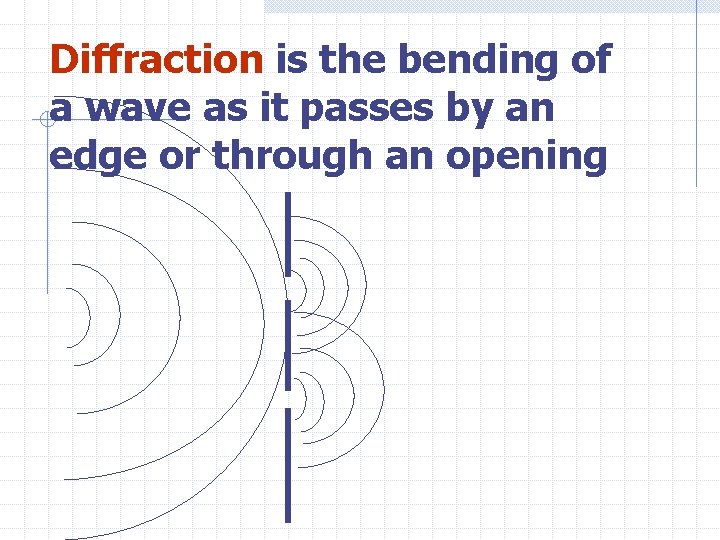
Diffraction is the bending of a wave as it passes by an edge or through an opening