Final Exam Review Study these practice questions for


















- Slides: 47

Final Exam Review Study these practice questions for your exam!!

A chemical bond resulting from an attraction between a cation and an anion is called…. A. Covalent bond B. Ionic bond C. Organic bond D. Acidic bond

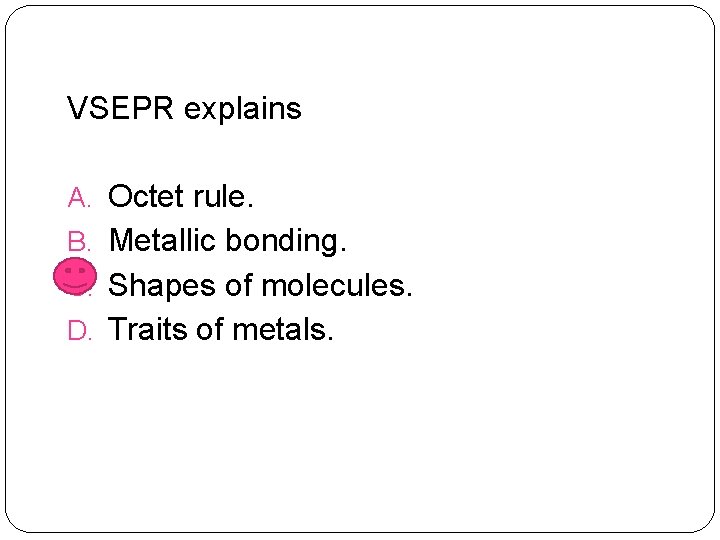
VSEPR explains A. Octet rule. B. Metallic bonding. C. Shapes of molecules. D. Traits of metals.

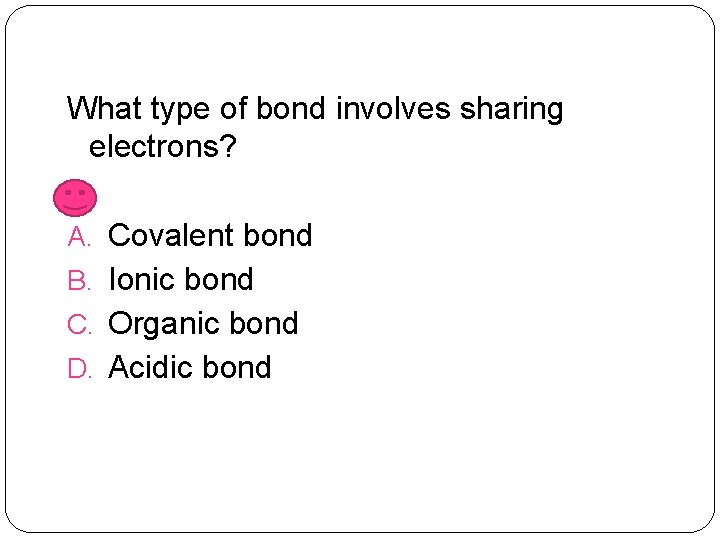
What type of bond involves sharing electrons? A. Covalent bond B. Ionic bond C. Organic bond D. Acidic bond

What is the overall charge of any compound? A. positive B. negative C. neutral

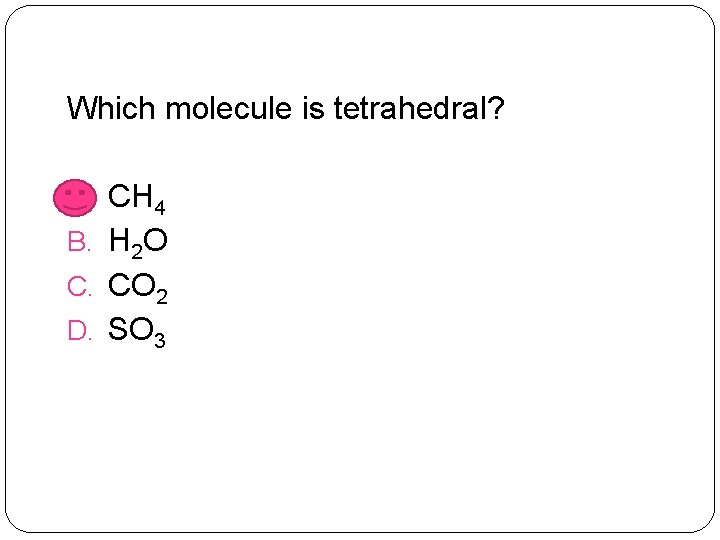
Which molecule is tetrahedral? A. CH 4 B. H 2 O C. CO 2 D. SO 3

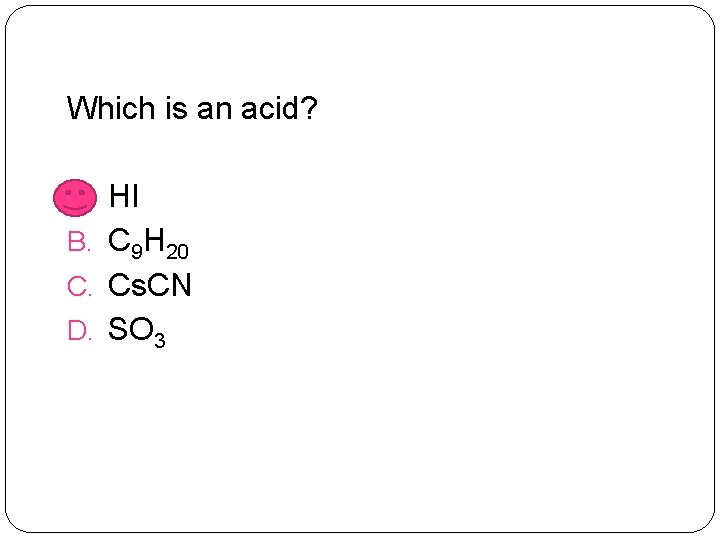
Which is an acid? A. HI B. C 9 H 20 C. Cs. CN D. SO 3

How many valence electrons does aluminum have? A. 3 B. 13 C. 26. 98 D. 41

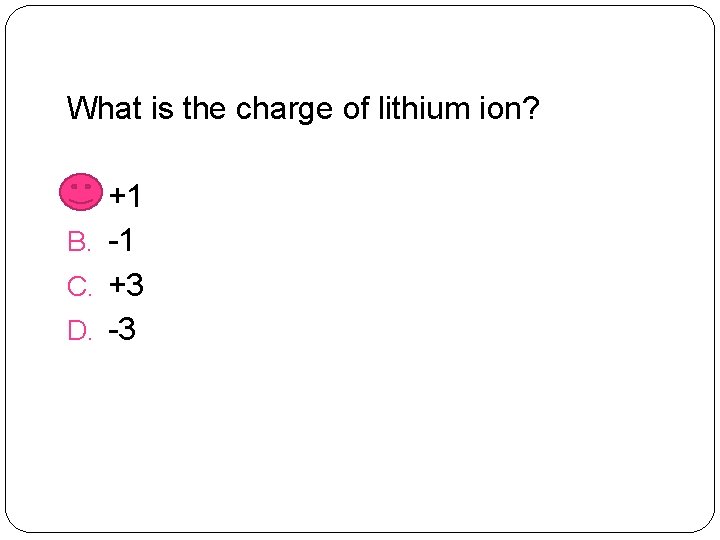
What is the charge of lithium ion? A. +1 B. -1 C. +3 D. -3

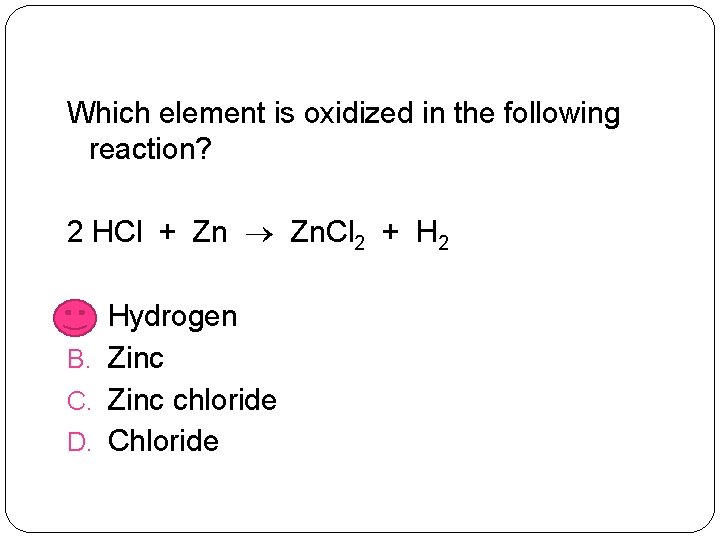
Which element is oxidized in the following reaction? 2 HCl + Zn Zn. Cl 2 + H 2 A. Hydrogen B. Zinc C. Zinc chloride D. Chloride

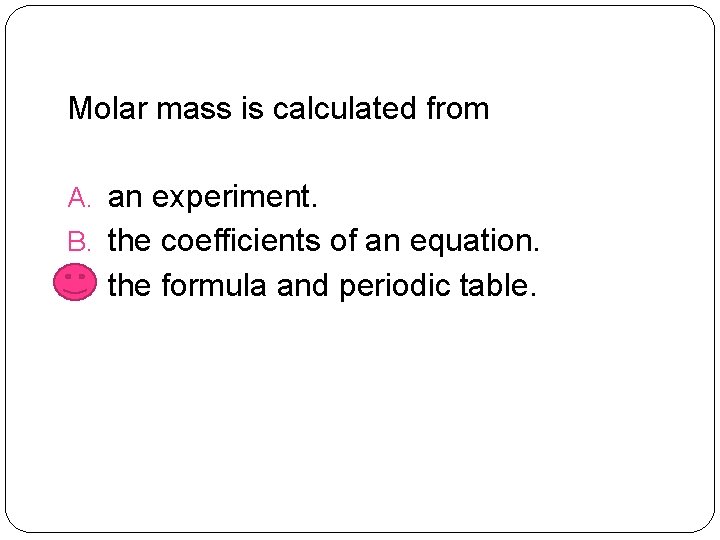
Molar mass is calculated from A. an experiment. B. the coefficients of an equation. C. the formula and periodic table.

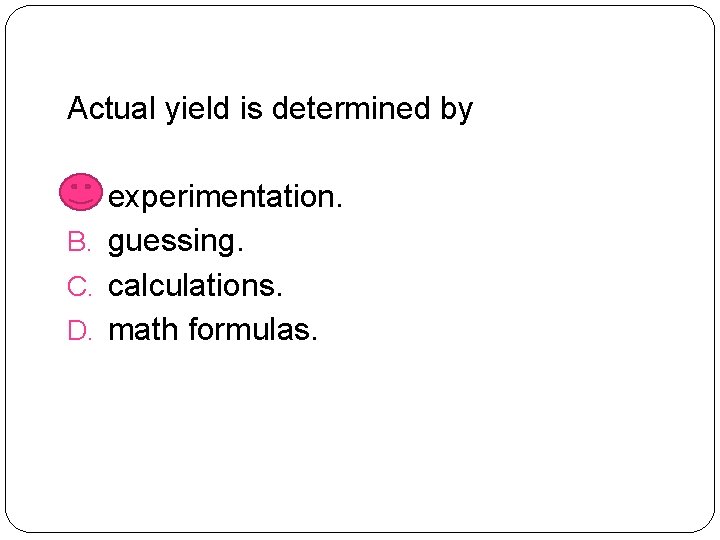
Actual yield is determined by A. experimentation. B. guessing. C. calculations. D. math formulas.

What is the p. H of a neutral solution? A. 0 B. 1 C. 7 D. 14

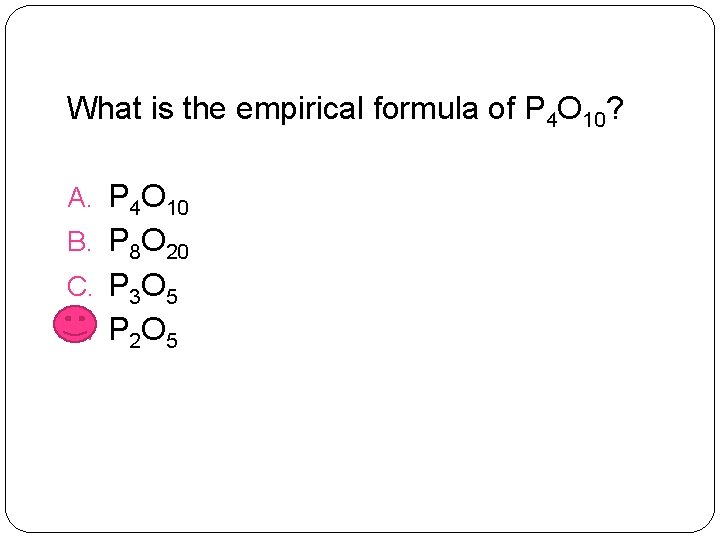
What is the empirical formula of P 4 O 10? A. P 4 O 10 B. P 8 O 20 C. P 3 O 5 D. P 2 O 5

Sodas have a p. H of 4. They are A. Acidic. B. Basic. C. Neutral.

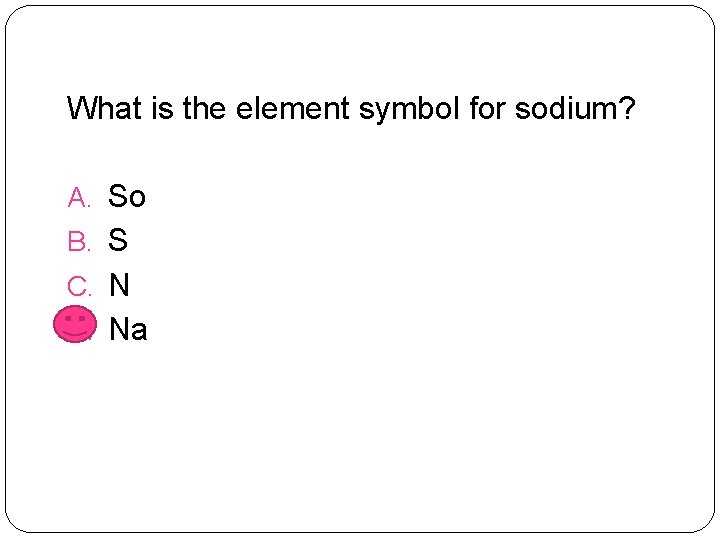
What is the element symbol for sodium? A. So B. S C. N D. Na

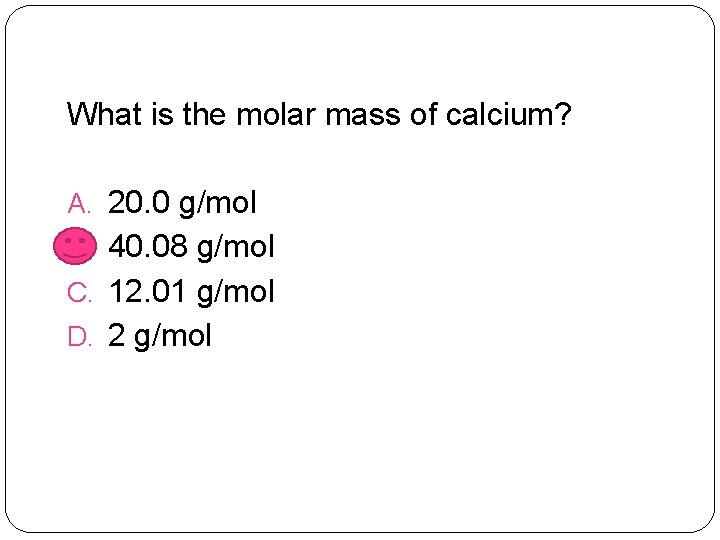
What is the molar mass of calcium? A. 20. 0 g/mol B. 40. 08 g/mol C. 12. 01 g/mol D. 2 g/mol

What is the universal solvent? A. salt B. oxygen C. water D. molarity

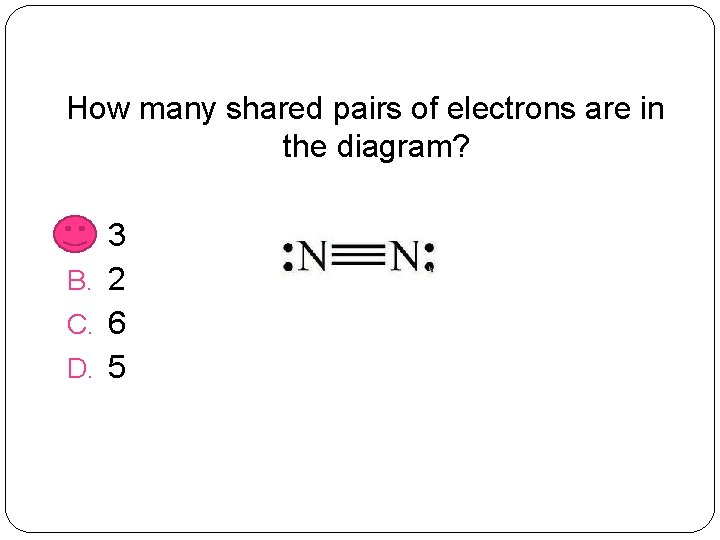
How many shared pairs of electrons are in the diagram? A. 3 B. 2 C. 6 D. 5

Acids react with bases to produce… A. Water and hydrogen gas B. Bases and acids. C. Salt and water D. Calcium

Which of the following is a noble gas? A. Neon B. Hydrogen C. Titanium D. Calcium

Sugar water is a(n) A. electrolyte. B. non-electrolyte.

Salt water is a(n) A. electrolyte. B. non-electrolyte.

Acids taste… A. Sour. B. Bitter. C. Sweet. D. Salty.

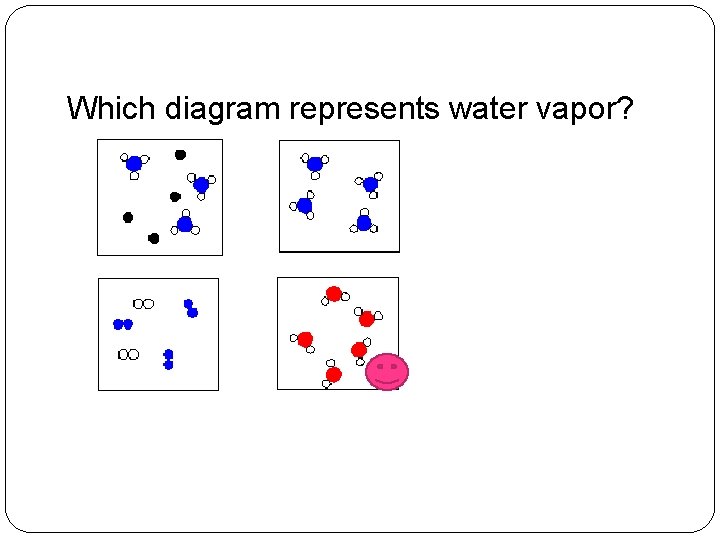
Which diagram represents water vapor?

What can be added to a solution to make the solute dissolve easier? A. heat B. ice cubes C. more solute D. more water

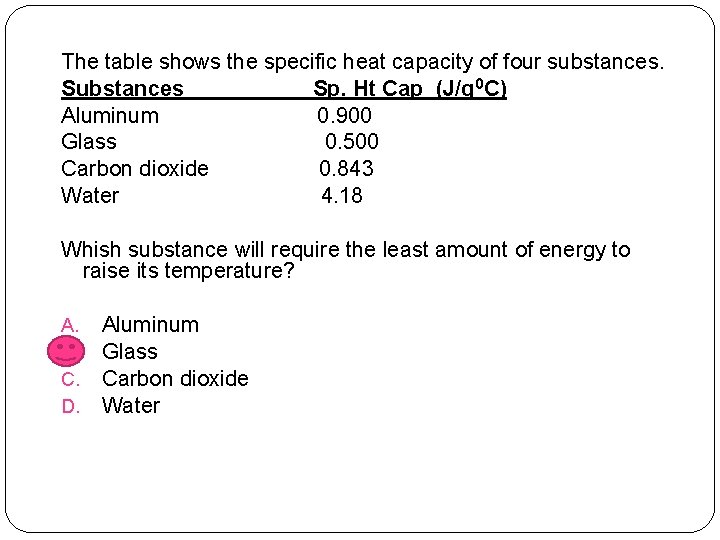
The table shows the specific heat capacity of four substances. Substances Sp. Ht Cap (J/g 0 C) Aluminum 0. 900 Glass 0. 500 Carbon dioxide 0. 843 Water 4. 18 Whish substance will require the least amount of energy to raise its temperature? A. B. C. D. Aluminum Glass Carbon dioxide Water

A process is endothermic if… A. The temperature increases. B. The temperature decreases. C. The solution turns red. D. The solution turns blue.

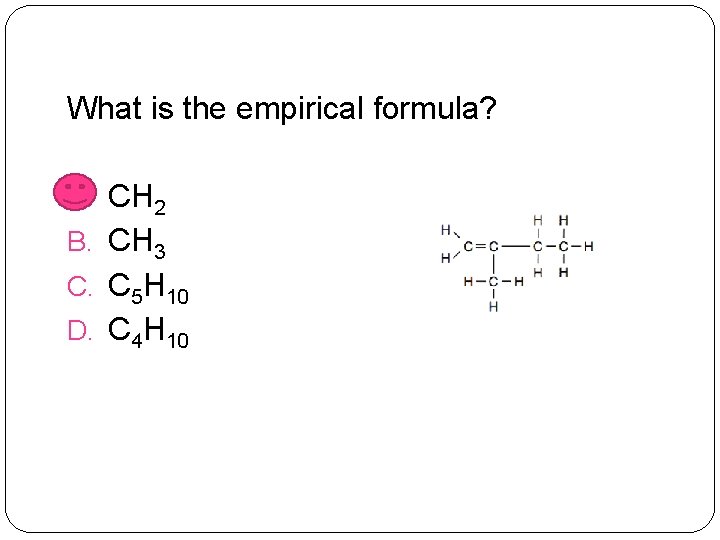
What is the empirical formula? A. CH 2 B. CH 3 C. C 5 H 10 D. C 4 H 10

What are the products of the reaction? Na. OH + H 3 PO 4 __? __ + __? __ A. Sodium hydroxide and hydrogen phosphate B. Phosphoric acid and water C. Sodium phosphate and water D. Sodium and phosphorus.

When the following equation is balanced, what is the coefficient of aluminum phosphate, Al. PO 4? __ Al(NO 3)3 + __ Ca 3(PO 4)2 __ Al. PO 4 + __ Ca(NO 3)2 A. B. C. D. 1 2 3 4

What does the (s) stand for in chemical equation? A. insoluble (solid) B. soluble (aqueous) C. dissolved in water D. gas

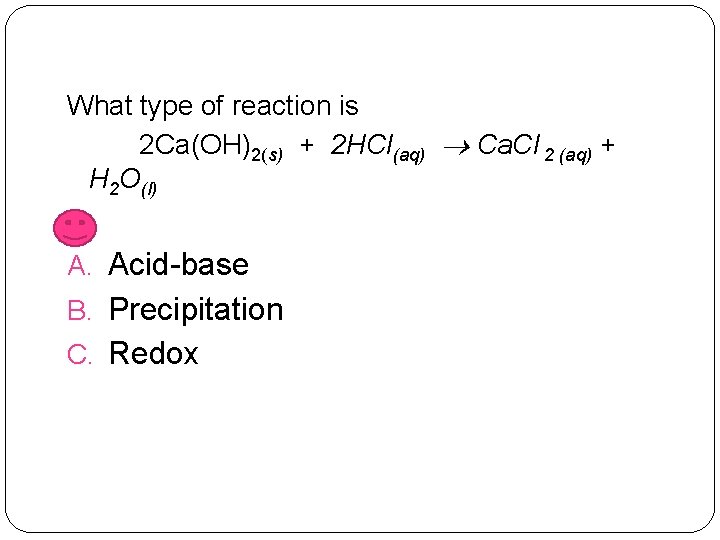
What type of reaction is 2 Ca(OH)2(s) + 2 HCl(aq) Ca. Cl 2 (aq) + H 2 O(l) A. Acid-base B. Precipitation C. Redox

What type of reaction occurs between sulfuric acid and silver nitrate? A. Acid-base B. Precipitation C. Redox

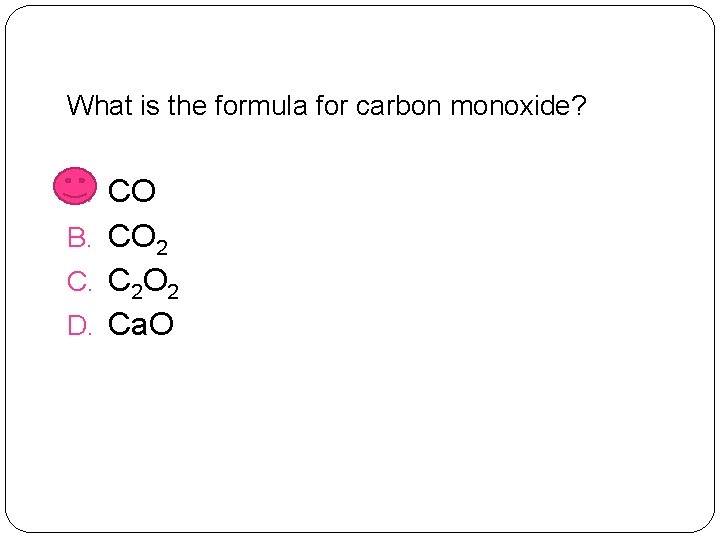
What is the formula for carbon monoxide? A. CO B. CO 2 C. C 2 O 2 D. Ca. O

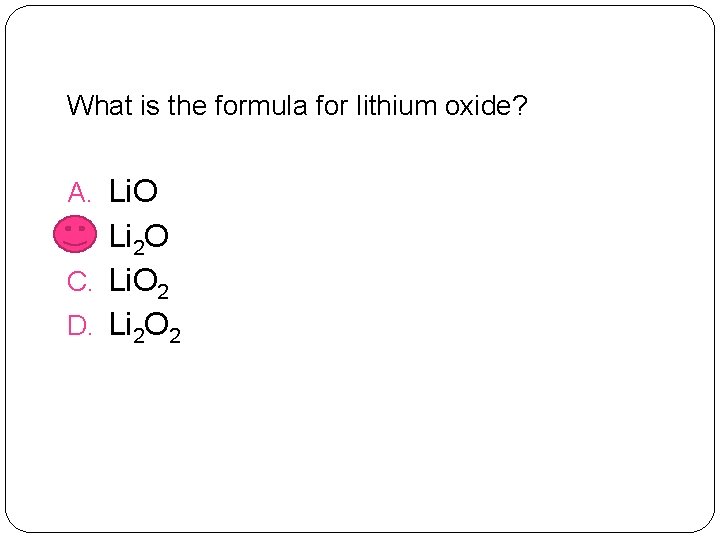
What is the formula for lithium oxide? A. Li. O B. Li 2 O C. Li. O 2 D. Li 2 O 2

If temperature increases, volume A. Increases. B. Decreases.

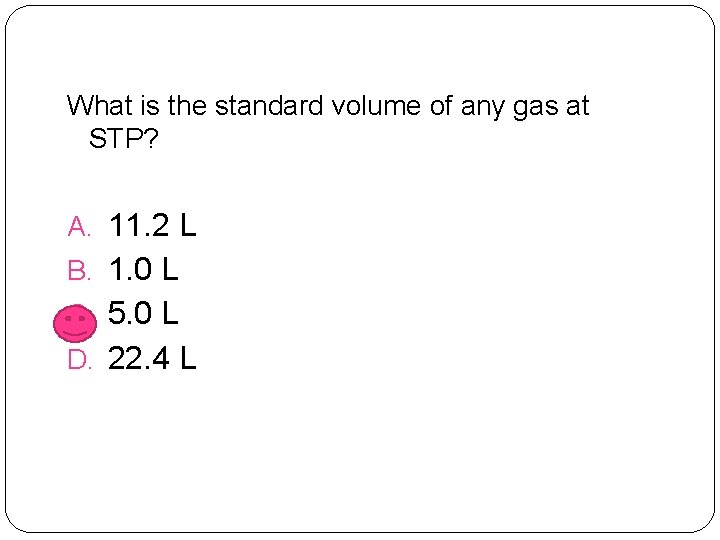
What is the standard volume of any gas at STP? A. 11. 2 L B. 1. 0 L C. 5. 0 L D. 22. 4 L

What would happen to a balloon placed in the freezer? A. The volume would increase. B. The volume would decrease. C. The volume remains constant. D. It depends on the gas inside the balloon.

What is the name of Zn. Cl 2? A. Zinc chloride B. Zinc (II) chloride C. Zinc (II) chloride (II) D. Zinc dichloride

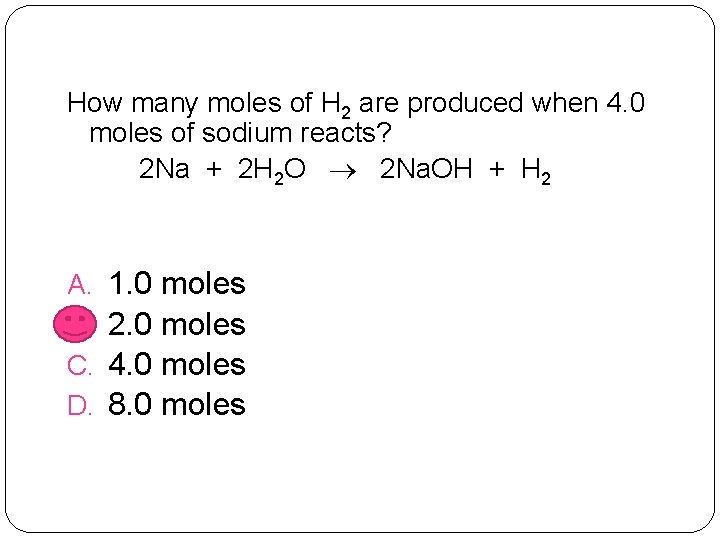
How many moles of H 2 are produced when 4. 0 moles of sodium reacts? 2 Na + 2 H 2 O 2 Na. OH + H 2 A. B. C. D. 1. 0 moles 2. 0 moles 4. 0 moles 8. 0 moles

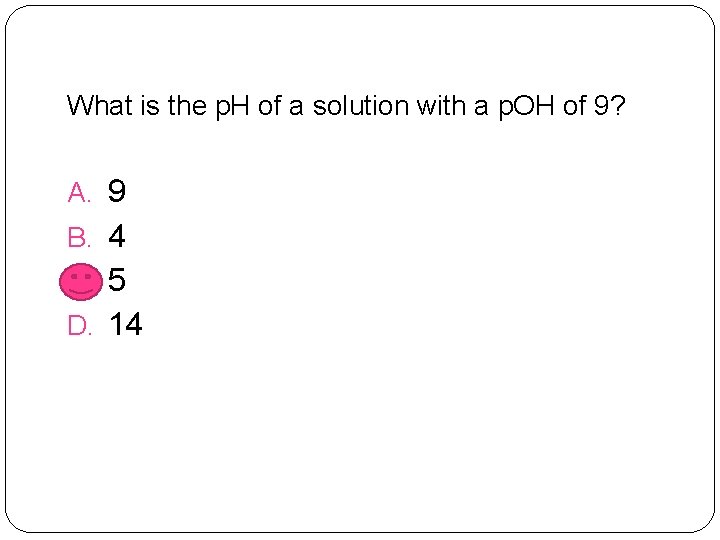
What is the p. H of a solution with a p. OH of 9? A. 9 B. 4 C. 5 D. 14

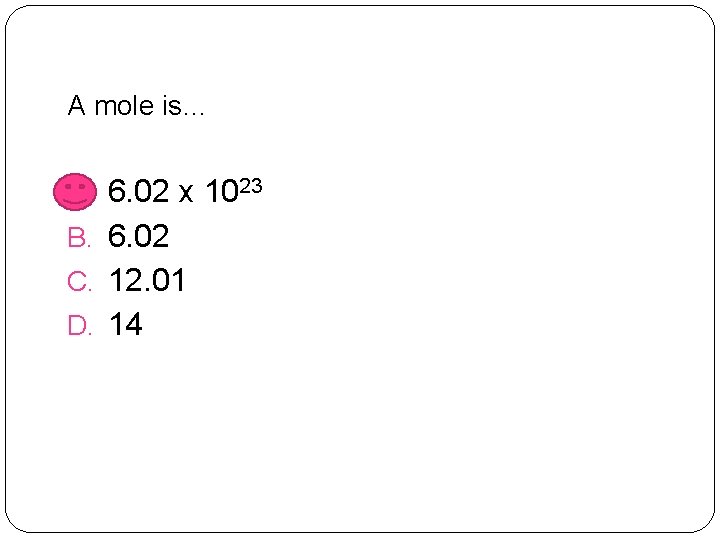
A mole is… A. 6. 02 x 1023 B. 6. 02 C. 12. 01 D. 14

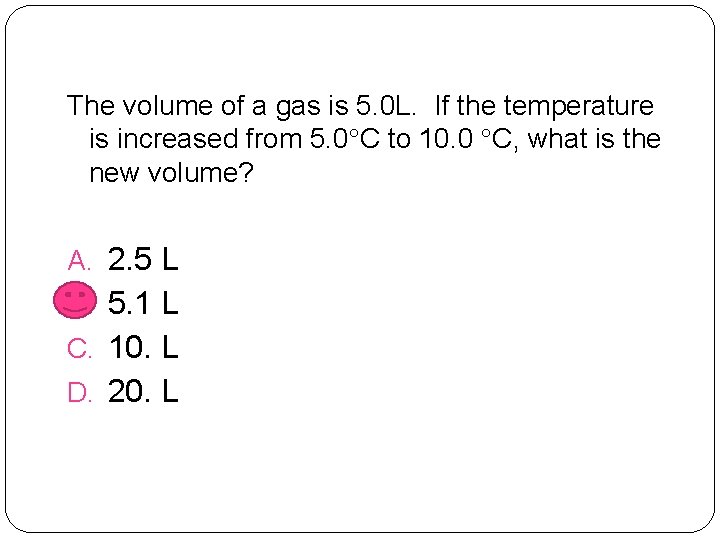
The volume of a gas is 5. 0 L. If the temperature is increased from 5. 0 C to 10. 0 C, what is the new volume? A. 2. 5 L B. 5. 1 L C. 10. L D. 20. L

What happens to volume of a gas during compression? A. increases B. decreases C. remains constant

Boiling point, freezing point, surface tension, density, and compressibility are… A. Physical changes B. Chemical changes C. Physical and chemical changes

If H is negative, the reaction is A. Endothermic. B. Exothermic.