PHYSICAL SCIENCE FINAL EXAM REVIEW JEOPARDY 1 Partners



































- Slides: 49

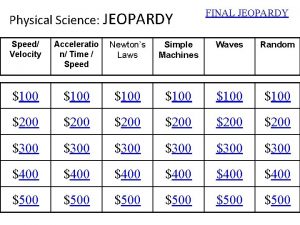
PHYSICAL SCIENCE FINAL EXAM REVIEW JEOPARDY 1

• Partners of 3 • May use notebooks & BRAINS. • All correct answers get points. • NO points for incorrect answers. • Do not put up answers until time is called. • Do not put down answers until scores are recorded. • Winning Groups Gets • 1 st 10 Extra Credit Points & Candy. • 2 nd & 3 rd Candy 1 Volunteer to keep score on white board. - Must have a good grade and few absences - Get candy! 2

Four materials are put into small containers. These materials are then moves from the small containers into larger containers. Which material will spread out to completely fill a larger container? A. air B. ice C. sand D. water 3

What happens to the particles of a sample of water as it is changed into ice? A. they move faster B. they spread apart C. they have higher thermal energy D. they become more compact 4

How does the kinetic energy of the particles of a solid compare to the kinetic energy of the particles of a gas? A. particles of a solid have greater kinetic energy than particles of a gas B. particles of a solid have less kinetic energy than particles of a gas C. particles of a solid have the same kinetic energy as particles of a gas D. particles of a solid do not have kinetic energy and particles of a gas do 5

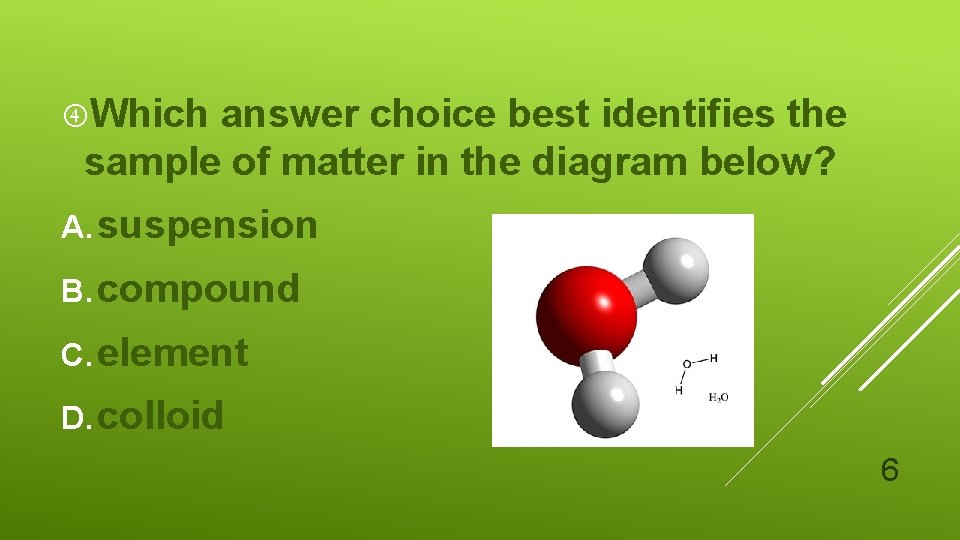
Which answer choice best identifies the sample of matter in the diagram below? A. suspension B. compound C. element D. colloid 6

Which element is correctly identified as a metal, nonmetal, or metalloid? A. magnesium (Mg) - nonmetal B. helium (He) - metal C. germanium (Ge) - metalloid D. chlorine (Cl) - metal 7

Which of the following is an example of a compound? A. sodium (Na) B. oxygen (O 2) C. chlorine (Cl) D. water (H 2 O) 8

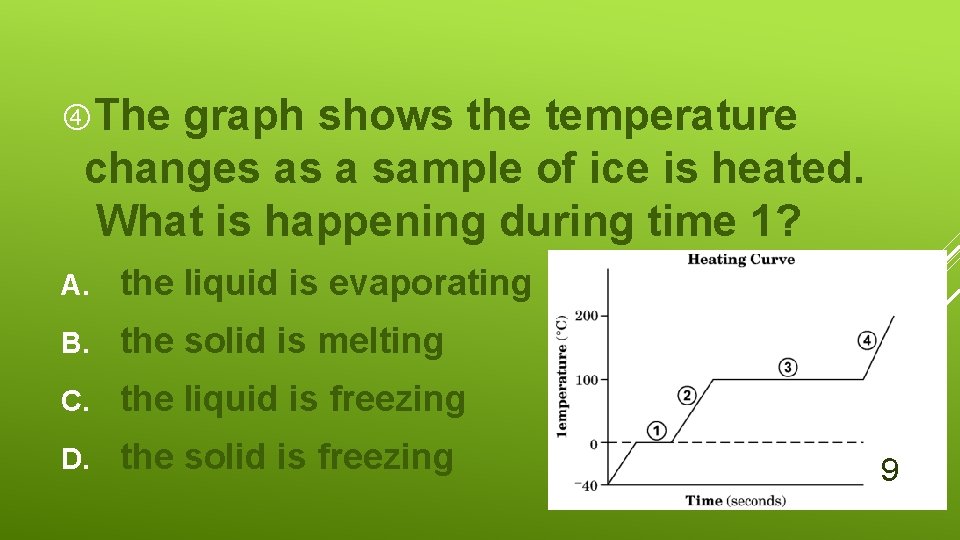
The graph shows the temperature changes as a sample of ice is heated. What is happening during time 1? A. the liquid is evaporating B. the solid is melting C. the liquid is freezing D. the solid is freezing 9

Which of the following is an example of a physical change? A. lighting a match B. burning wood C. breaking a glass D. rusting of iron 10

Which of the following lists three states of water in order of increasing kinetic energy? A. ice, vapor, liquid B. liquid, ice, vapor C. vapor, liquid, ice D. ice, liquid, vapor 11

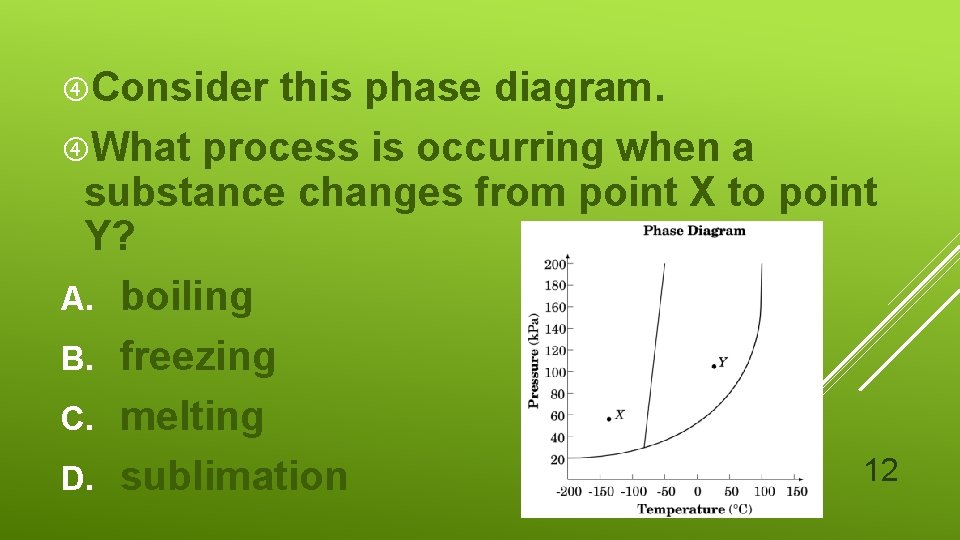
Consider this phase diagram. What process is occurring when a substance changes from point X to point Y? A. boiling B. freezing C. melting 12 D. sublimation

A solubility curve graph shows changes in solubility based on changes in _____. A. temperature B. pressure C. volume D. time 13

The graph below shows the solubility curves for various ionic compounds. What is the solubility of Cs. NO 3 at 52°C? A. 40 grams B. 26 grams C. 31 grams 14 D. 50 grams

The atomic number of an element indicates which of the following? A. the number of neutrons in the atom B. the number of protons in the atom C. the sum of the neutrons and protons in the atom D. the sum of the protons and electrons 15 in the atom

Why are the noble gases so unreactive? A. Their outer shell has 1 electron. B. Their outer shell has 2 electrons. C. Their outer shell is filled. D. Their outer shell needs one electron 16 to be full.

If an atom has 42 protons, 42 electrons and 55 neutrons what is its mass number? A. 97 B. 42 C. 55 D. 139 17

Which best represents how electrons are arranged in the energy levels of a carbon atom? A. first energy level = 1 electrons; second energy level = 5 electrons B. first energy level = 2 electrons; second energy level = 4 electrons C. first energy level = 3 electrons; second energy level = 3 electrons D. first energy level = 4 electrons; second energy level = 2 electrons 18

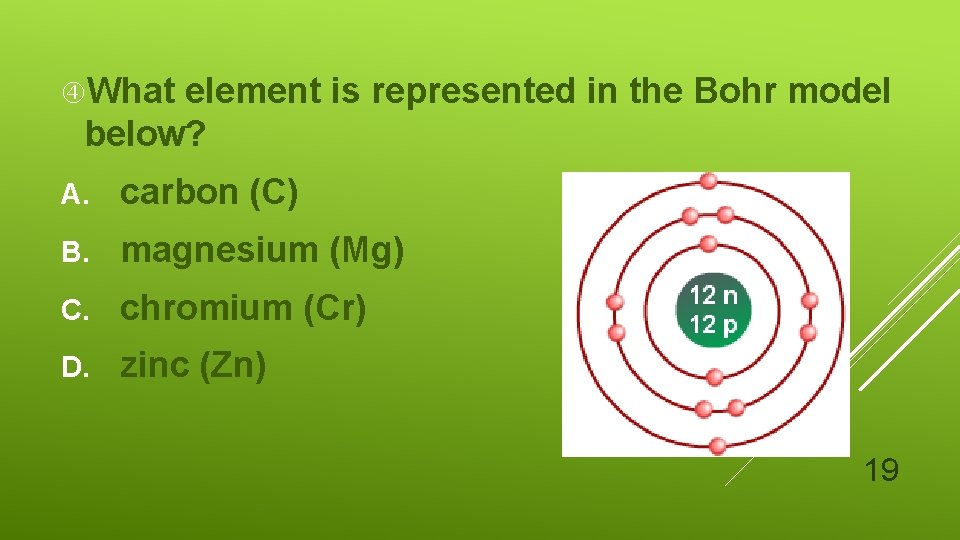
What element is represented in the Bohr model below? A. carbon (C) B. magnesium (Mg) C. chromium (Cr) D. zinc (Zn) 19

Which of the compounds is formed by a covalent bond? A. Na. Cl B. Ca. Cl 2 C. Na. Br D. C 6 H 12 O 6 20

Barium (Ba) and iodine (I) combine to form an ionic compound. What is the chemical formula for this compound? A. Ba. I B. Ba. I 2 C. Ba 2 I D. Ba 2 I 2 21

Which is the correct formula for dinitrogen pentoxide? A. N 4 O B. NO 2 C. N 2 O 5 D. NO 4 22
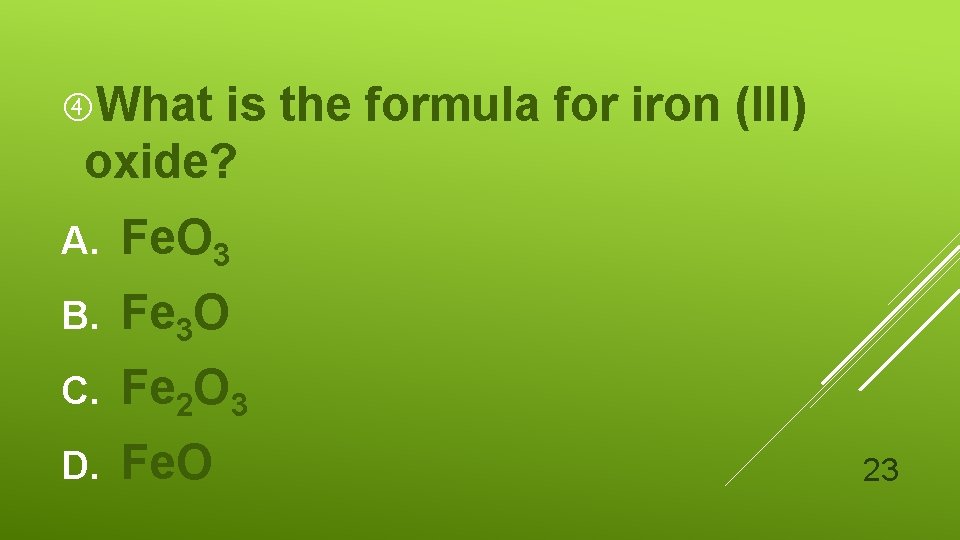
What is the formula for iron (III) oxide? A. Fe. O 3 B. Fe 3 O C. Fe 2 O 3 D. Fe. O 23

Common soaps are often basic. Which of these is a property of a chemical base? A. sour taste B. p. H equal to 6 C. turns litmus paper blue D. forms bubbles with limestone 24

In a _____ reaction, an acid and a base produce a salt and water. A. neutralization B. decomposition C. dilution D. concentration 25

The equation below shows a balanced neutralization reaction. Li. OH + HF → Li. F + H 2 O Which solution has a p. H greater than 7? A. H 2 O, because it is neutral B. HF, because it is an acid C. Li. F, because it is a salt D. Li. OH, because it is a base 26

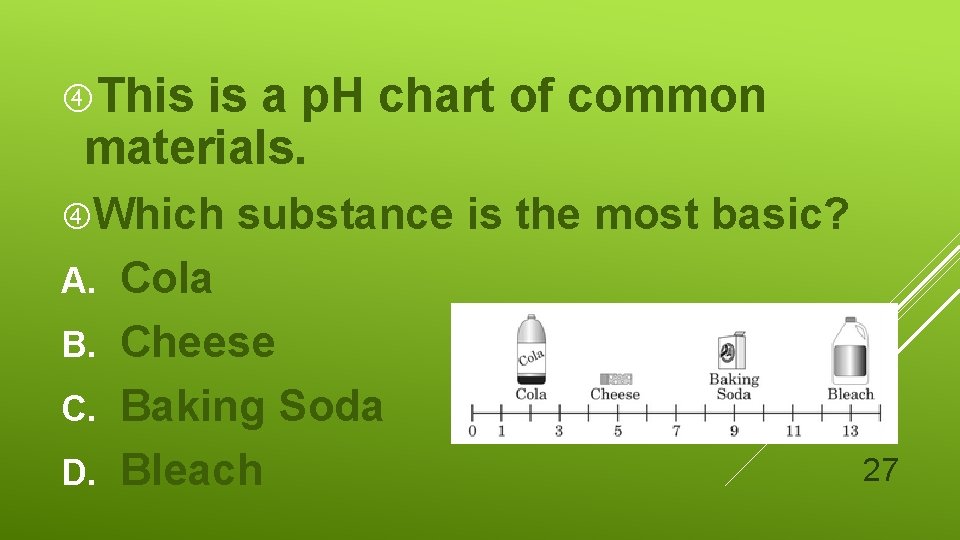
This is a p. H chart of common materials. Which substance is the most basic? Cola B. Cheese C. Baking Soda D. Bleach A. 27

A scientist uses litmus paper to measure the p. H of several different solutions. Which solution turns the litmus paper red? A. Na. OH B. Na. Cl C. HCl D. NH 3 28

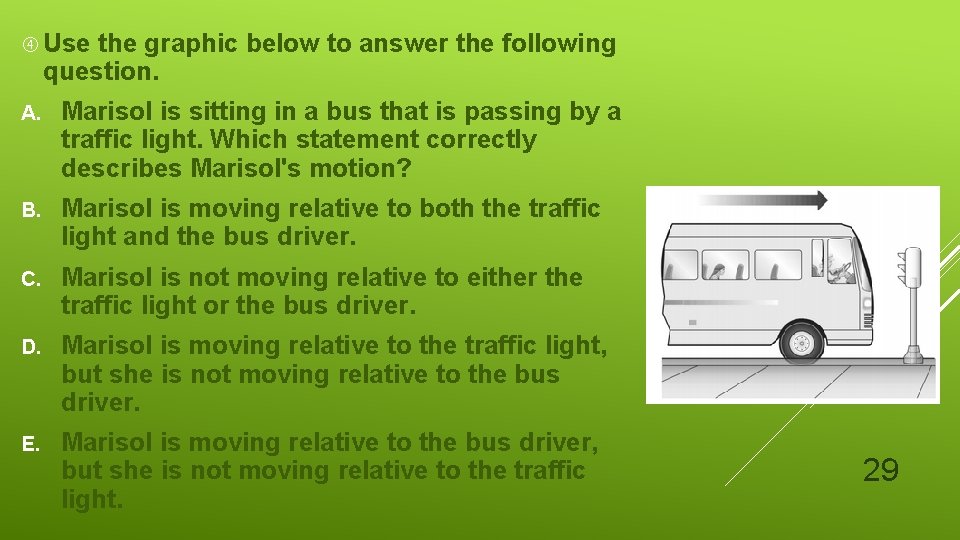
Use the graphic below to answer the following question. A. Marisol is sitting in a bus that is passing by a traffic light. Which statement correctly describes Marisol's motion? B. Marisol is moving relative to both the traffic light and the bus driver. C. Marisol is not moving relative to either the traffic light or the bus driver. D. Marisol is moving relative to the traffic light, but she is not moving relative to the bus driver. E. Marisol is moving relative to the bus driver, but she is not moving relative to the traffic light. 29

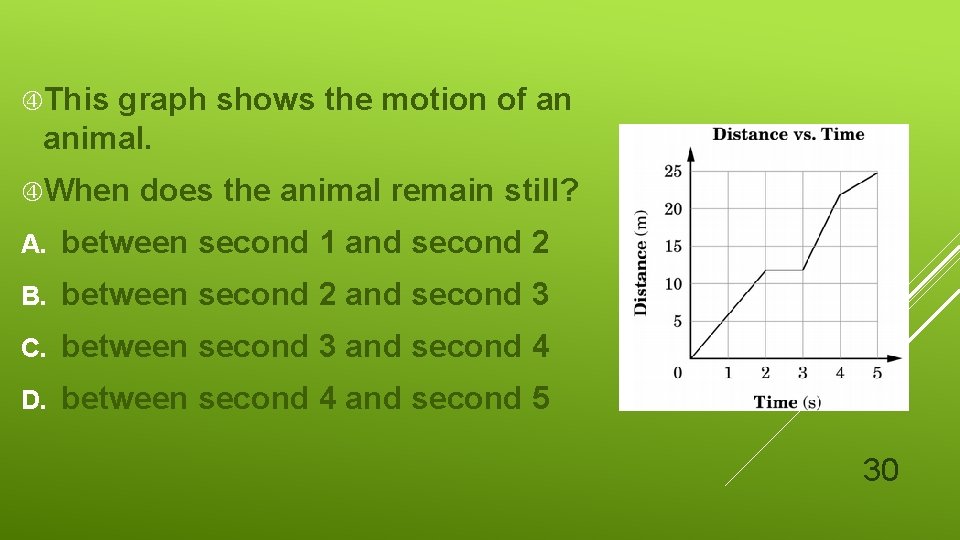
This graph shows the motion of an animal. When does the animal remain still? A. between second 1 and second 2 B. between second 2 and second 3 C. between second 3 and second 4 D. between second 4 and second 5 30

A rollerblader is blading along the sidewalk. Which forms of measurement would be the best to use to determine the rollerblader's speed? A. time and distance B. volume and time C. density and mass D. mass and distance 31

What is the mass of an asteroid with a speed of 200 m/s and a momentum of 2, 000 kg m/s? A. 0 kg B. 1, 800 kg C. 2, 200 kg D. 400, 000 kg 32

An airplane went from 120 m/s to 180 m/s in 4. 0 seconds. What was its acceleration? A. 15 m/s 2 B. 30. m/s 2 C. 45 m/s 2 D. 60. m/s 2 33

Noah carried a skateboard up a hill and then rode the skateboard down the hill. When Noah reached the bottom of the hill, he rolled to a stop. When did Noah have the most potential energy? A. while carrying the skateboard up the hill B. while standing on the skateboard at the top of the hill C. while riding the skateboard down the hill D. while standing on the skateboard at the 34 bottom of the hill

A student moves a box across the floor by exerting 23. 3 N of force and doing 47. 2 J of work on the box. How far does the student move the box? A. 0. 49 m B. 2. 03 m C. 23. 9 m D. 1, 099. 8 m 35

How much power is used to lift a box that weighs 50 newtons 10 meters in 10 seconds? A. 5 watts B. 50 watts C. 500 watts D. 5, 000 watts 36

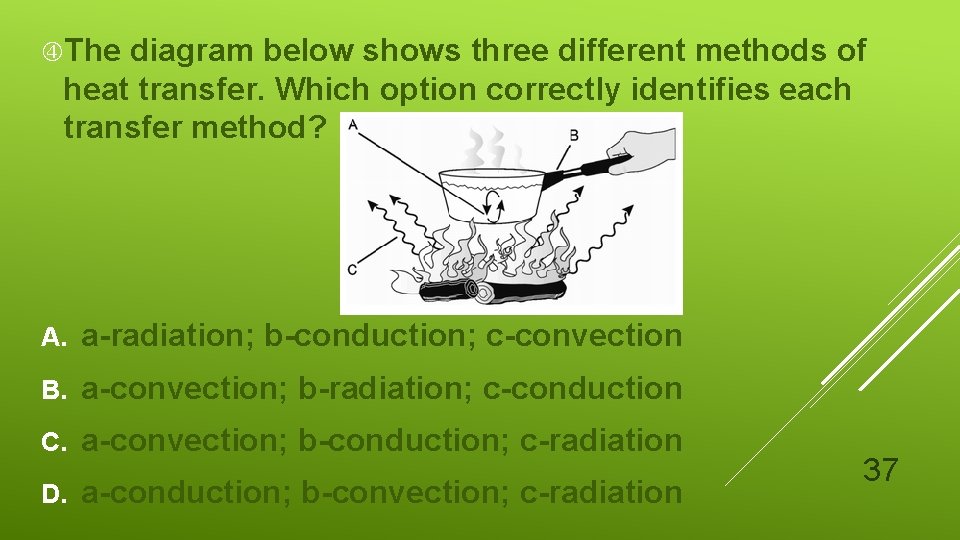
The diagram below shows three different methods of heat transfer. Which option correctly identifies each transfer method? A. a-radiation; b-conduction; c-convection B. a-convection; b-radiation; c-conduction C. a-convection; b-conduction; c-radiation D. a-conduction; b-convection; c-radiation 37

When electric current flows through a metal filament of a light bulb, electrical energy is converted to _____. A. heat energy only B. heat and light energy C. light and kinetic energy D. light energy only 38

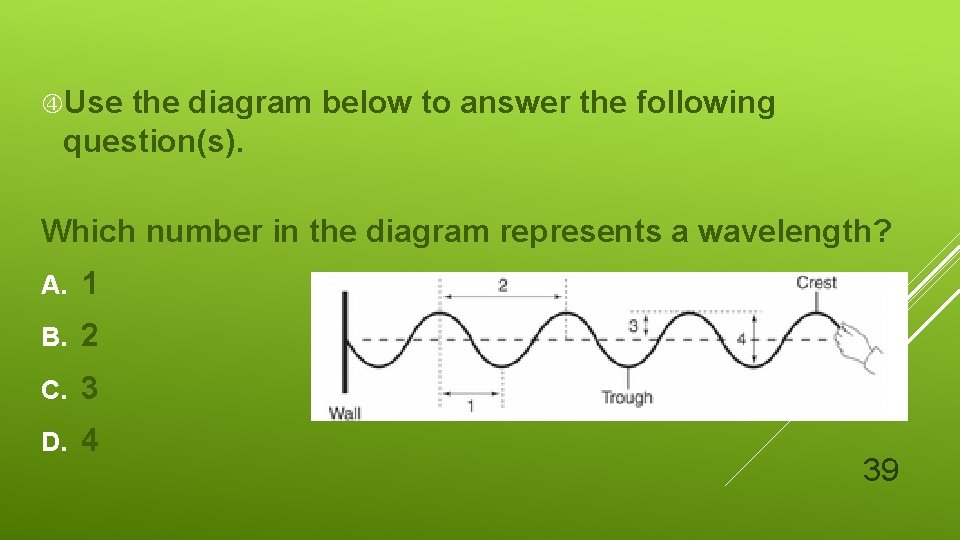
Use the diagram below to answer the following question(s). Which number in the diagram represents a wavelength? A. 1 B. 2 C. 3 D. 4 39

The diagram below represents a wave pattern. Which type of wave is represented? A. longitudinal wave B. transverse wave C. sound wave D. primary wave 40

A student shakes the end of a rope with a frequency of 1. 5 Hz, causing waves with a wavelength of 0. 8 m to travel along the rope. What is the velocity of the waves? A. 1. 9 m/s B. 1. 6 m/s C. 1. 2 m/s D. 0. 53 m/s 41

A light bulb with a voltage difference of 120 V across it carries a current of 1. 5 A. Which of the following is the power consumption of the light bulb? A. 0. 013 W B. 80 W C. 120 W D. 180 W 42

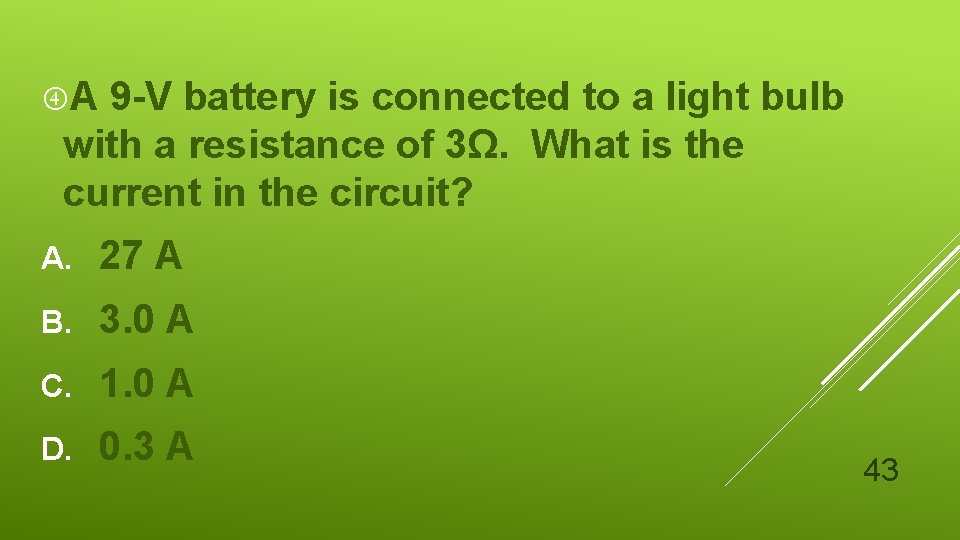
A 9 -V battery is connected to a light bulb with a resistance of 3Ω. What is the current in the circuit? A. 27 A B. 3. 0 A C. 1. 0 A D. 0. 3 A 43

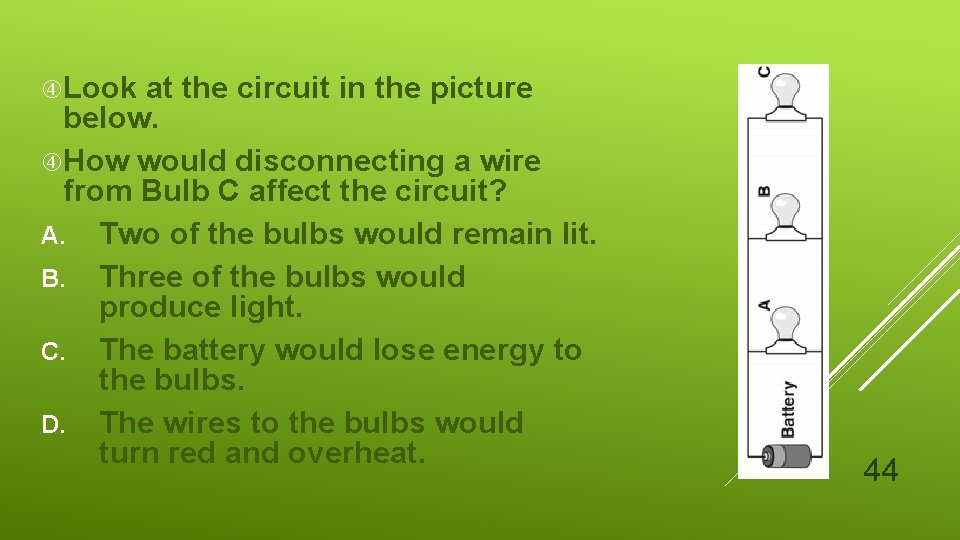
Look at the circuit in the picture below. How would disconnecting a wire from Bulb C affect the circuit? A. Two of the bulbs would remain lit. B. Three of the bulbs would produce light. C. The battery would lose energy to the bulbs. D. The wires to the bulbs would turn red and overheat. 44

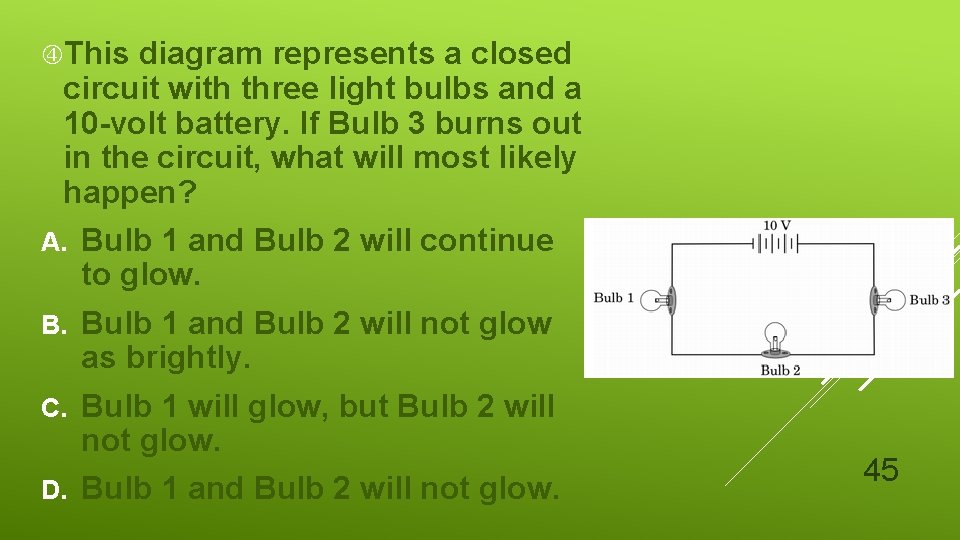
This diagram represents a closed circuit with three light bulbs and a 10 -volt battery. If Bulb 3 burns out in the circuit, what will most likely happen? A. Bulb 1 and Bulb 2 will continue to glow. Bulb 1 and Bulb 2 will not glow as brightly. C. Bulb 1 will glow, but Bulb 2 will not glow. D. Bulb 1 and Bulb 2 will not glow. 45

Most electrical wires are covered with plastic or rubber. The wires are covered with plastic or rubber because those materials _____. A. are conductors of electricity B. make complete electric circuits C. are not conductors of electricity D. make the electricity move quickly 46

FINAL JEOPARDY Total your points and determine how many you want to wager. Write on whiteboard to be recorded. 47

FINAL JEOPARDY QUESTIONS #1 A light bulb with a voltage difference of 120 V across it carries a current of 1. 5 A. Which of the following is the power consumption of the light bulb? Number & Unit 48

FINAL JEOPARDY QUESTIONS #2 500 grams of sugar occupies a volume of 0. 315 L. What is the density of the sugar in g/m. L? Number (2 decimal places) & Unit 49
 Physical science final exam review
Physical science final exam review Ap gov final review
Ap gov final review Environmental science final exam
Environmental science final exam Earth science final exam review
Earth science final exam review World history spring final exam review answers
World history spring final exam review answers Spanish 1 answer key
Spanish 1 answer key Pltw human body systems final exam
Pltw human body systems final exam Poe practice test kinematics answers
Poe practice test kinematics answers Ied final exam review
Ied final exam review World history semester exam
World history semester exam Principles of business final exam answer key
Principles of business final exam answer key Bm3final
Bm3final Ap world history final exam
Ap world history final exam Us history final semester 2 review
Us history final semester 2 review English 11 first semester exam
English 11 first semester exam Physics fall final exam review
Physics fall final exam review Mat1033c
Mat1033c Fe exam statics review
Fe exam statics review Zoology semester 1 exam review answers
Zoology semester 1 exam review answers Final exam review algebra 1
Final exam review algebra 1 Financial accounting final exam multiple choice
Financial accounting final exam multiple choice Personal finance final exam review
Personal finance final exam review Spanish 2a final exam
Spanish 2a final exam Psyc 1504 final exam
Psyc 1504 final exam Animal science final exam
Animal science final exam Earth science final exam answers
Earth science final exam answers Periodic table jeopardy
Periodic table jeopardy Chapter 15 review physical science
Chapter 15 review physical science Glencoe physical science chapter 2 review answers
Glencoe physical science chapter 2 review answers Physical science eoc study guide
Physical science eoc study guide Physical science eoc review
Physical science eoc review Chapter 4 work and energy section 1 work and machines
Chapter 4 work and energy section 1 work and machines Physical science chapter 14 test
Physical science chapter 14 test Chapter 4 review physical science
Chapter 4 review physical science Chapter 14 review physical science
Chapter 14 review physical science Physical science chapter 5 review
Physical science chapter 5 review As your room gets messier day by day, entropy is
As your room gets messier day by day, entropy is Chapter 16 review physical science
Chapter 16 review physical science Six branches of science
Six branches of science Natural and physical science
Natural and physical science What's your subject
What's your subject World history final exam study guide
World history final exam study guide Web design final exam
Web design final exam U.s. history semester 1 final exam
U.s. history semester 1 final exam Sbu final exam schedule
Sbu final exam schedule National latin exam results
National latin exam results National latin exam practice quiz
National latin exam practice quiz Street law final exam
Street law final exam Pointing
Pointing Realidades 2 final exam
Realidades 2 final exam