Spring Final Exam Stoichiometry Review Molar Mass the



- Slides: 16

Spring Final Exam Stoichiometry Review

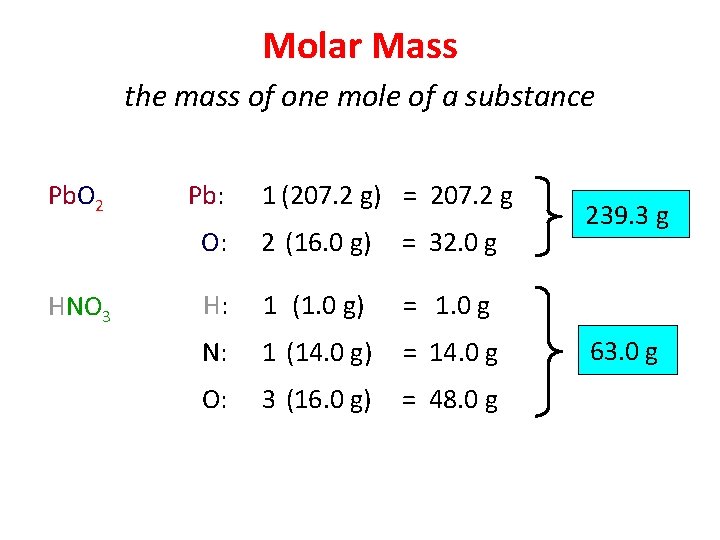
Molar Mass the mass of one mole of a substance Pb. O 2 HNO 3 Pb: 1 (207. 2 g) = 207. 2 g O: 2 (16. 0 g) = 32. 0 g H: 1 (1. 0 g) = 1. 0 g N: 1 (14. 0 g) = 14. 0 g O: 3 (16. 0 g) = 48. 0 g 239. 3 g 63. 0 g

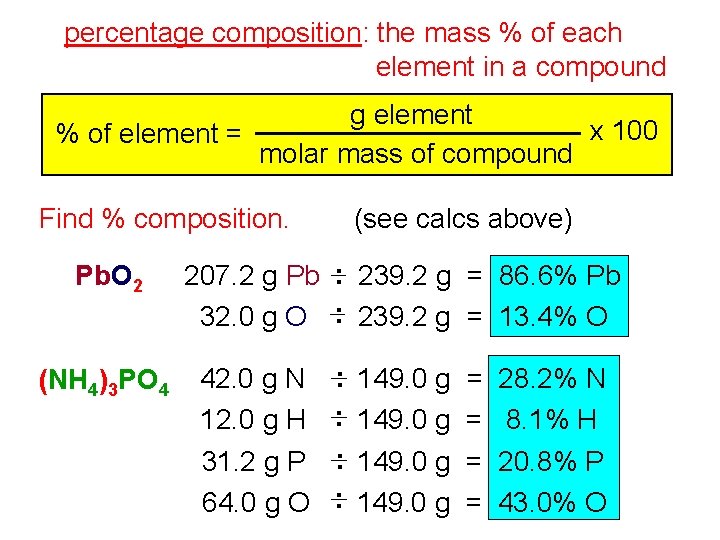
percentage composition: the mass % of each element in a compound g element x 100 % of element = molar mass of compound Find % composition. Pb. O 2 (NH 4)3 PO 4 (see calcs above) 207. 2 g Pb : 239. 2 g = 86. 6% Pb 32. 0 g O : 239. 2 g = 13. 4% O 42. 0 g N 12. 0 g H 31. 2 g P 64. 0 g O : 149. 0 g = = 28. 2% N 8. 1% H 20. 8% P 43. 0% O

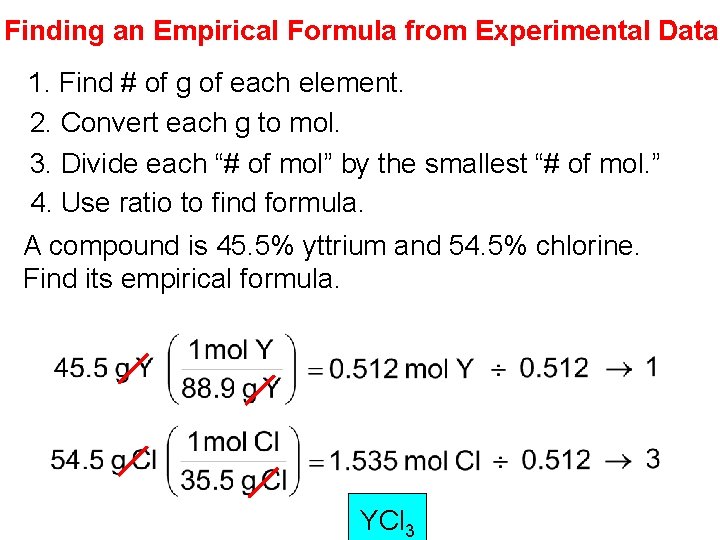
Finding an Empirical Formula from Experimental Data 1. Find # of g of each element. 2. Convert each g to mol. 3. Divide each “# of mol” by the smallest “# of mol. ” 4. Use ratio to find formula. A compound is 45. 5% yttrium and 54. 5% chlorine. Find its empirical formula. YCl 3

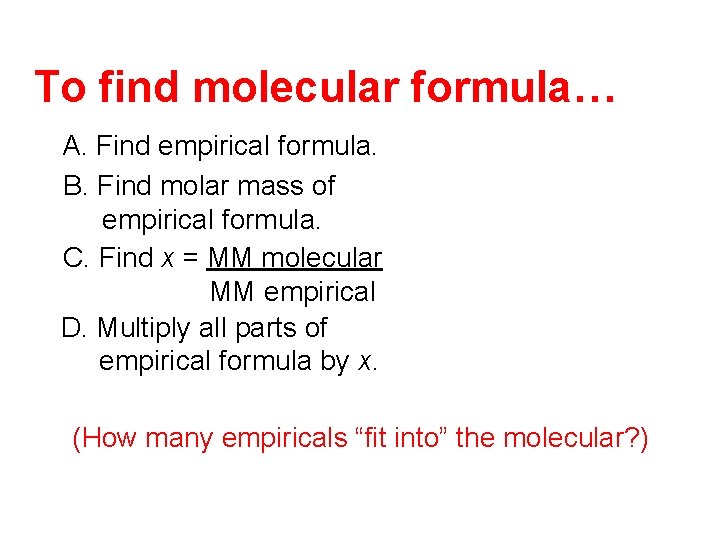
To find molecular formula… A. Find empirical formula. B. Find molar mass of empirical formula. C. Find x = MM molecular MM empirical D. Multiply all parts of empirical formula by x. (How many empiricals “fit into” the molecular? )

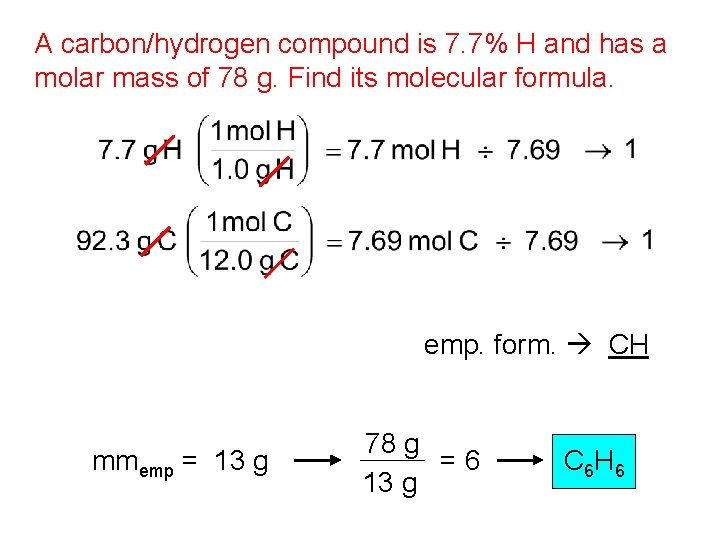
A carbon/hydrogen compound is 7. 7% H and has a molar mass of 78 g. Find its molecular formula. emp. form. CH mmemp = 13 g 78 g =6 13 g C 6 H 6

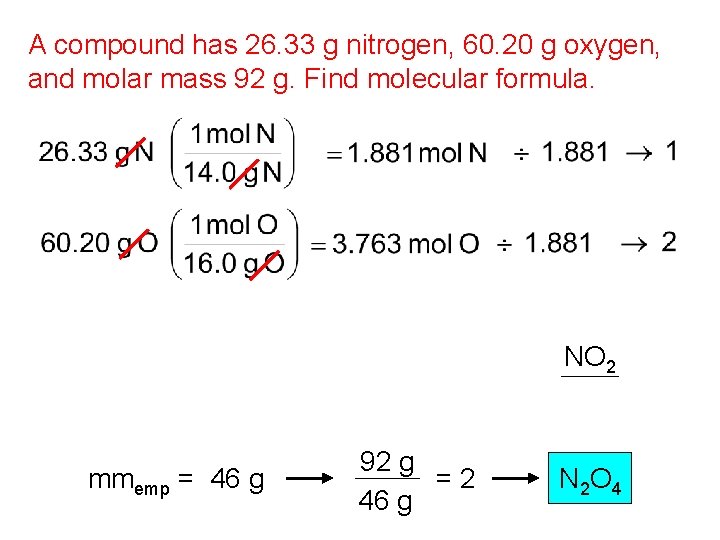
A compound has 26. 33 g nitrogen, 60. 20 g oxygen, and molar mass 92 g. Find molecular formula. NO 2 mmemp = 46 g 92 g =2 46 g N 2 O 4

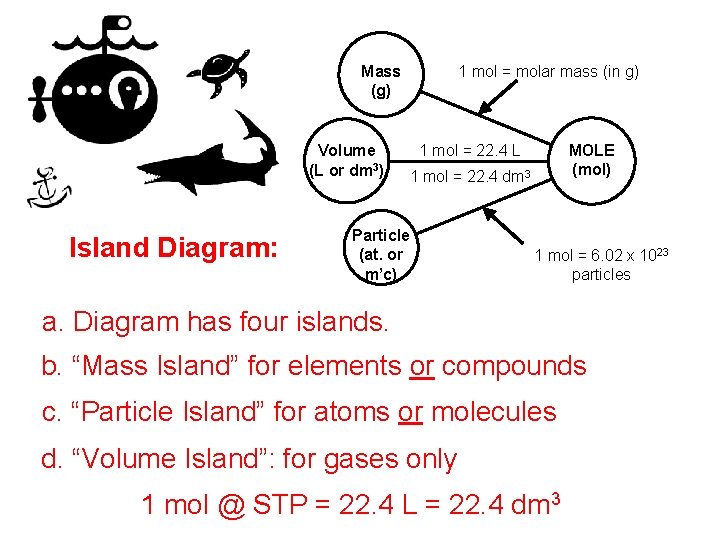
1 mol = molar mass (in g) Mass (g) Volume (L or dm 3) Island Diagram: 1 mol = 22. 4 L MOLE (mol) 1 mol = 22. 4 dm 3 Particle (at. or m’c) 1 mol = 6. 02 x 1023 particles a. Diagram has four islands. b. “Mass Island” for elements or compounds c. “Particle Island” for atoms or molecules d. “Volume Island”: for gases only 1 mol @ STP = 22. 4 L = 22. 4 dm 3

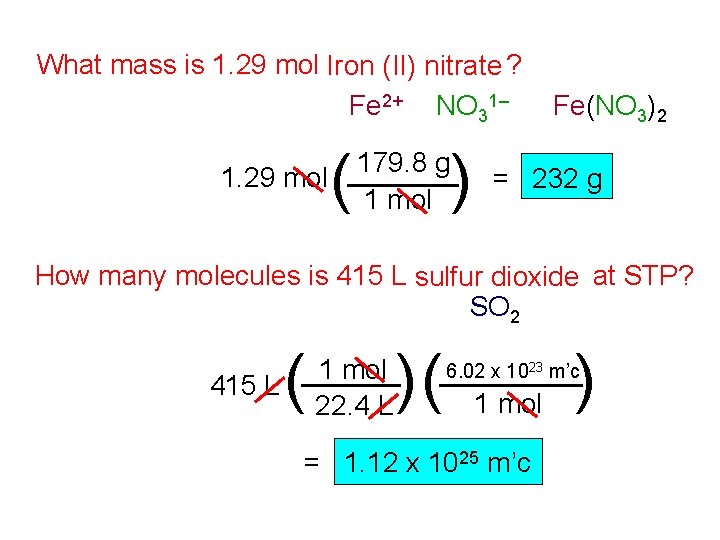
What mass is 1. 29 mol Iron (II) nitrate ? Fe 2+ NO 31– 1. 29 mol ( ) 179. 8 g 1 mol Fe(NO 3)2 = 232 g How many molecules is 415 L sulfur dioxide at STP? SO 2 415 L ( 1 mol 22. 4 L )( ) 6. 02 x 1023 m’c 1 mol = 1. 12 x 1025 m’c

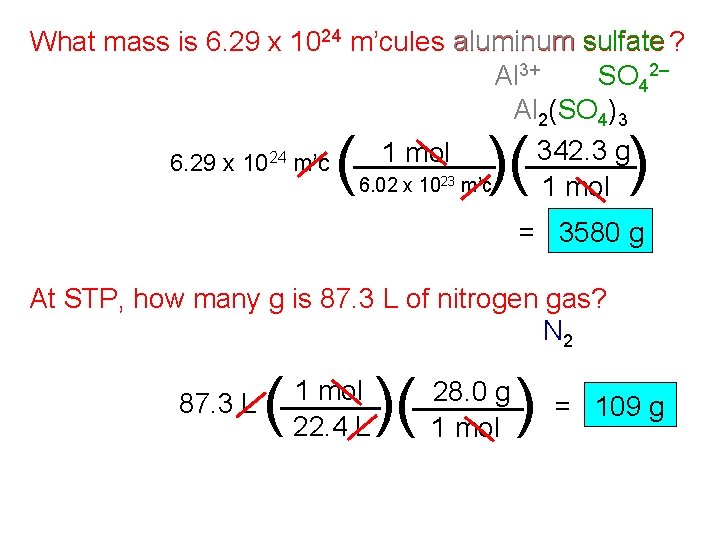
What mass is 6. 29 x 1024 m’cules aluminum sulfate ? Al 3+ SO 42– Al 2(SO 4)3 342. 3 g 1 mol 6. 29 x 1024 m’c 6. 02 x 1023 m’c 1 mol ( )( ) = 3580 g At STP, how many g is 87. 3 L of nitrogen gas? N 2 87. 3 L ( 1 mol 22. 4 L )( 28. 0 g 1 mol )= 109 g

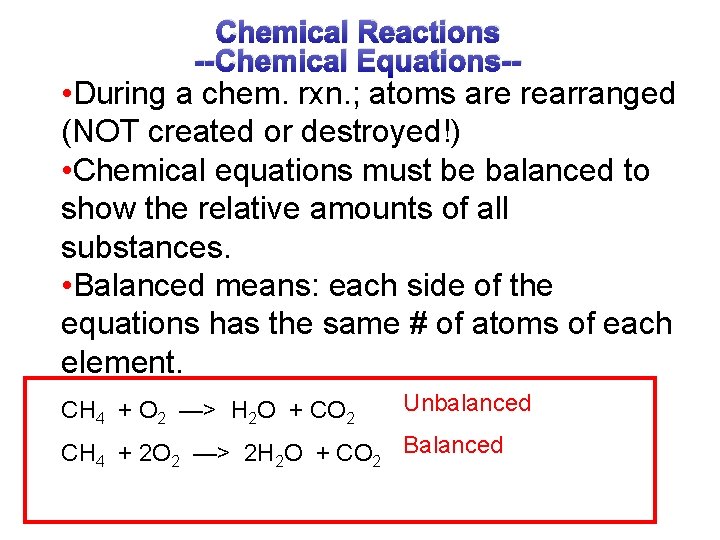
Chemical Reactions --Chemical Equations-- • During a chem. rxn. ; atoms are rearranged (NOT created or destroyed!) • Chemical equations must be balanced to show the relative amounts of all substances. • Balanced means: each side of the equations has the same # of atoms of each element. CH 4 + O 2 —> H 2 O + CO 2 Unbalanced CH 4 + 2 O 2 —> 2 H 2 O + CO 2 Balanced

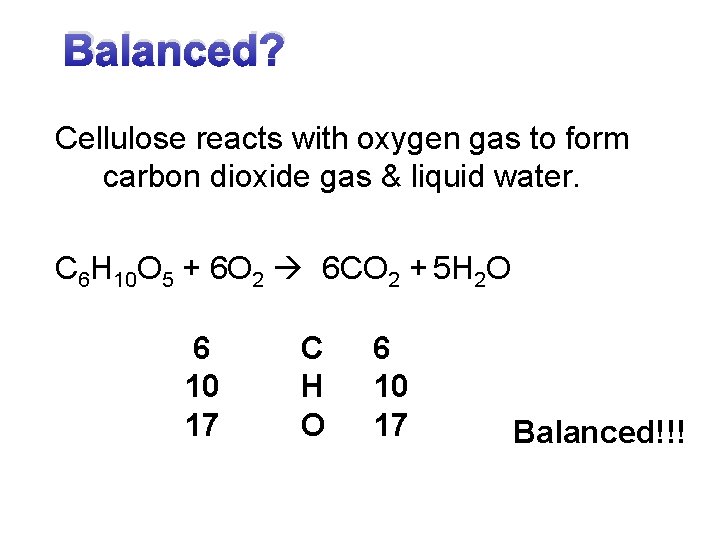
Balanced? Cellulose reacts with oxygen gas to form carbon dioxide gas & liquid water. C 6 H 10 O 5 + 6 O 2 6 CO 2 + 5 H 2 O 6 10 17 C H O 6 10 17 Balanced!!!

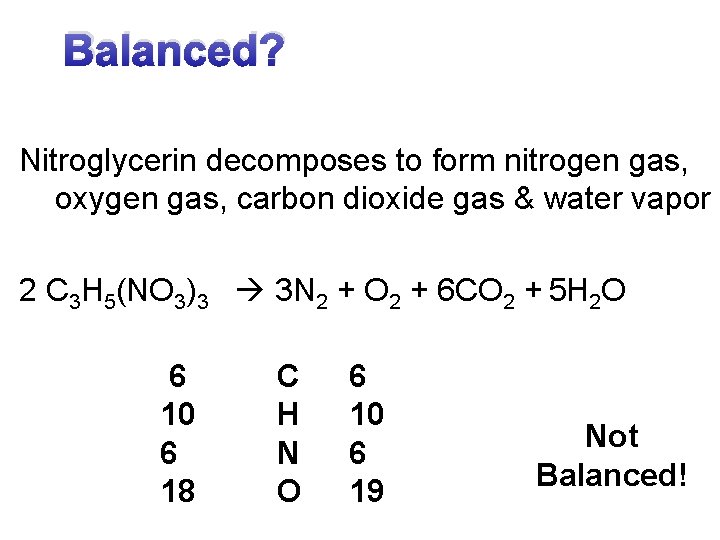
Balanced? Nitroglycerin decomposes to form nitrogen gas, oxygen gas, carbon dioxide gas & water vapor 2 C 3 H 5(NO 3)3 3 N 2 + O 2 + 6 CO 2 + 5 H 2 O 6 10 6 18 C H N O 6 10 6 19 Not Balanced!

• When balancing equations, you may change coefficients as much as you need to, but you may never change subscripts because you can’t change what substances are involved.

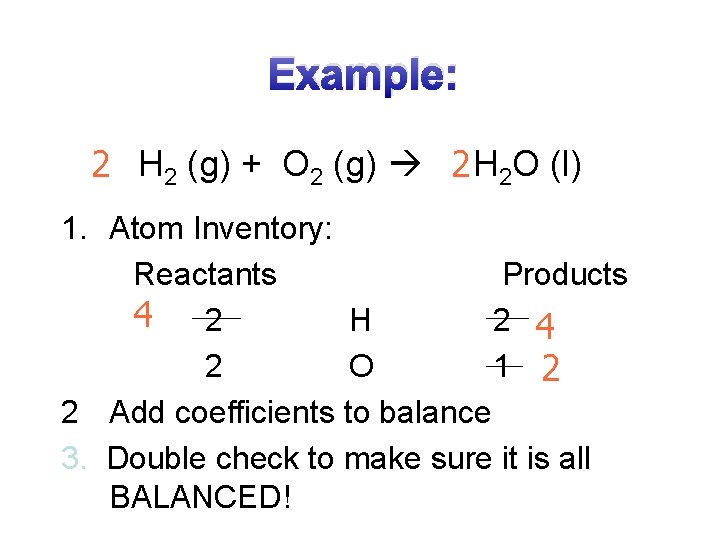
Example: 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 1. Atom Inventory: Reactants Products 4 2 H 2 4 2 O 1 2 2 Add coefficients to balance 3. Double check to make sure it is all BALANCED!

• Balancing Chemical Equations practice