CVD ALD sami franssilaaalto fi CVD ALD Chemical







- Slides: 62

CVD & ALD sami. franssila@aalto. fi

CVD & ALD Chemical Vapor Deposition, CVD Atomic Layer Deposition, ALD Alternatives to PVD, but only partially. Major uses: -optical fiber fabrication -films in microelectronics & MEMS -optical coatings -solar cells -a-Si and poly-Si for flat panel displays

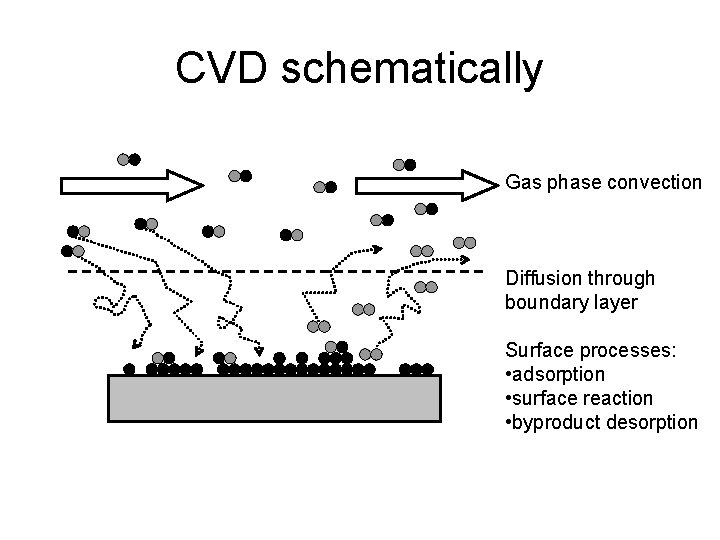
CVD schematically Gas phase convection Diffusion through boundary layer Surface processes: • adsorption • surface reaction • byproduct desorption

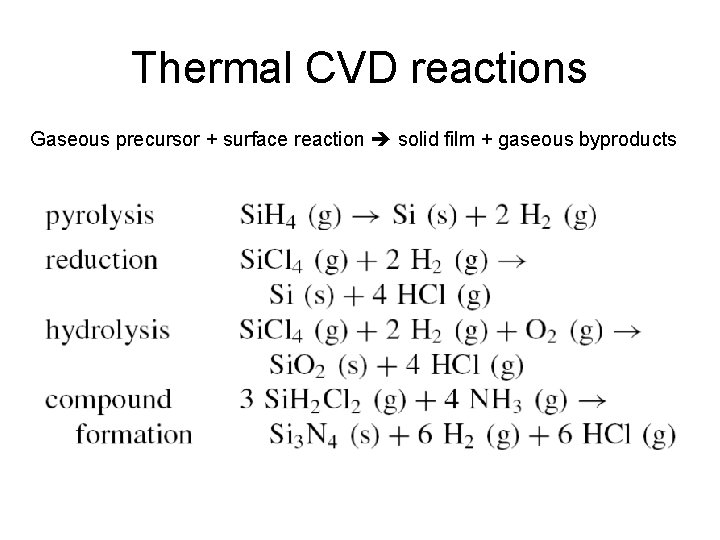
Thermal CVD reactions Gaseous precursor + surface reaction solid film + gaseous byproducts

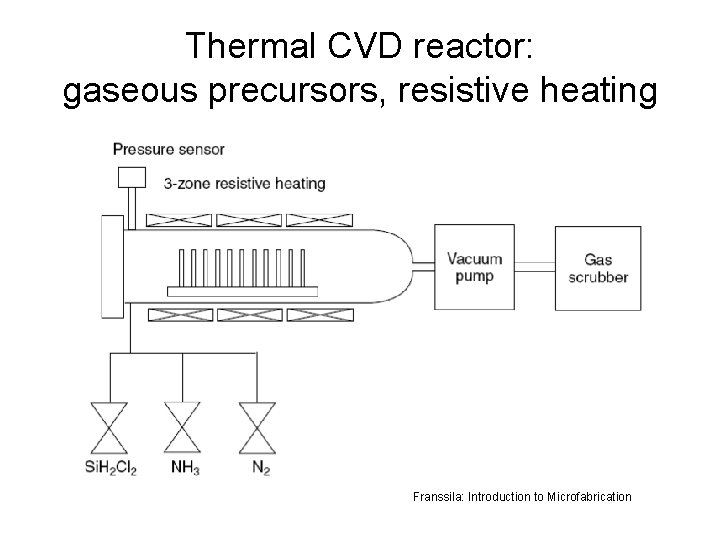
Thermal CVD reactor: gaseous precursors, resistive heating Franssila: Introduction to Microfabrication


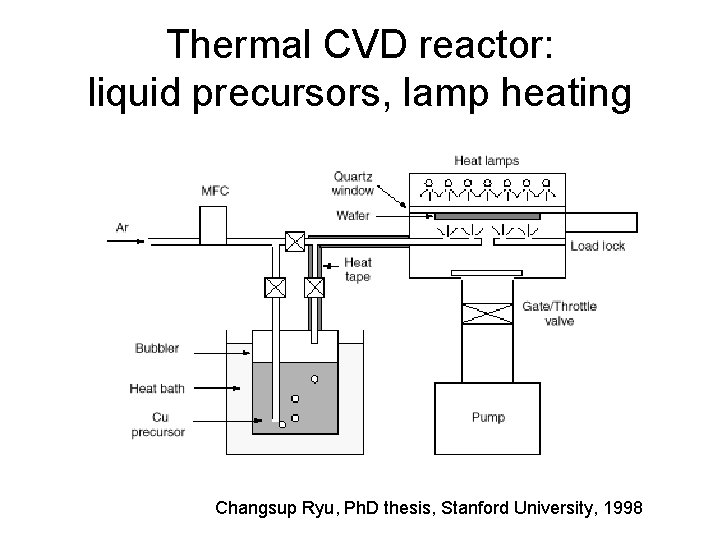
Thermal CVD reactor: liquid precursors, lamp heating Changsup Ryu, Ph. D thesis, Stanford University, 1998

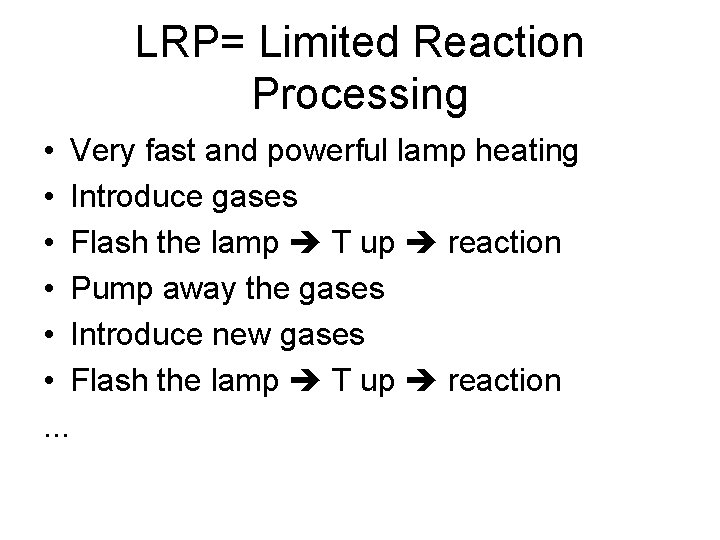
LRP= Limited Reaction Processing • Very fast and powerful lamp heating • Introduce gases • Flash the lamp T up reaction • Pump away the gases • Introduce new gases • Flash the lamp T up reaction. . .

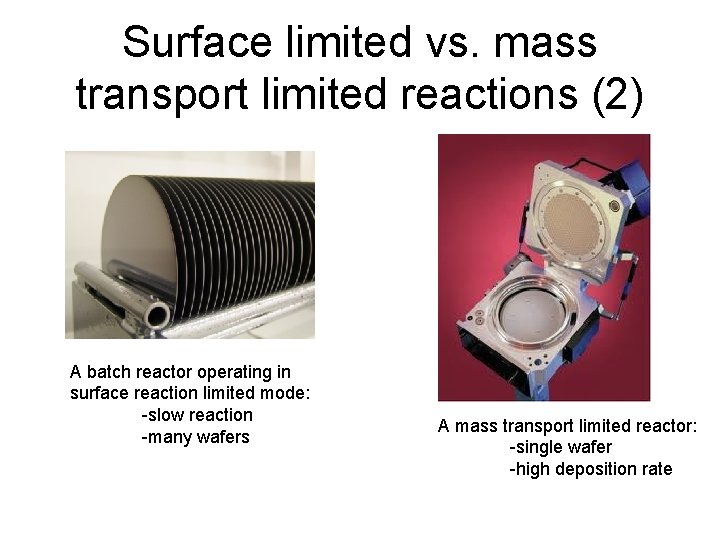
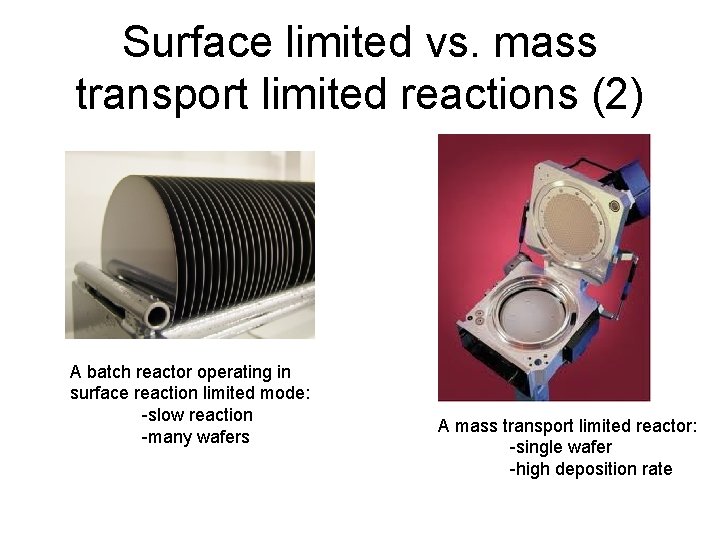
Surface limited vs. mass transport limited reactions (2) A batch reactor operating in surface reaction limited mode: -slow reaction -many wafers A mass transport limited reactor: -single wafer -high deposition rate

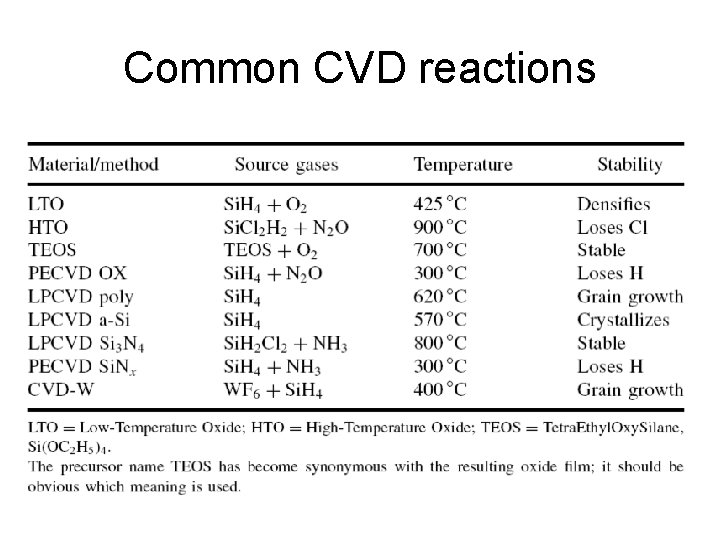
Common CVD reactions

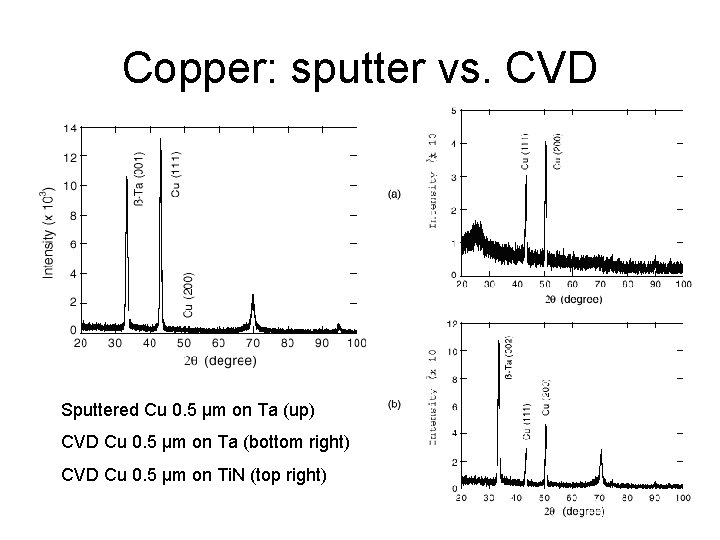
Copper: sputter vs. CVD Sputtered Cu 0. 5 µm on Ta (up) CVD Cu 0. 5 µm on Ta (bottom right) CVD Cu 0. 5 µm on Ti. N (top right)

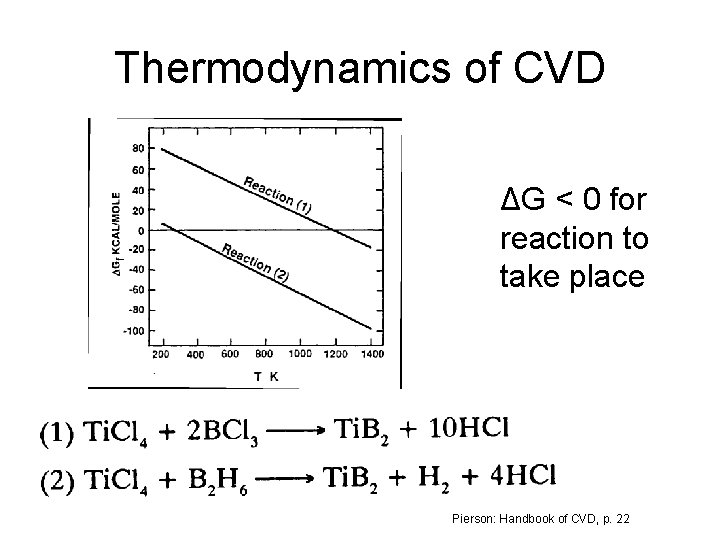
Thermodynamics of CVD ΔG < 0 for reaction to take place Pierson: Handbook of CVD, p. 22

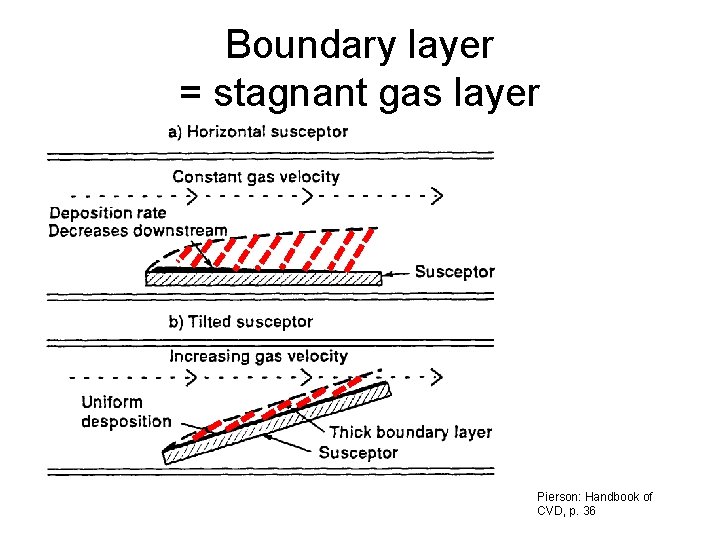
Boundary layer = stagnant gas layer Pierson: Handbook of CVD, p. 36

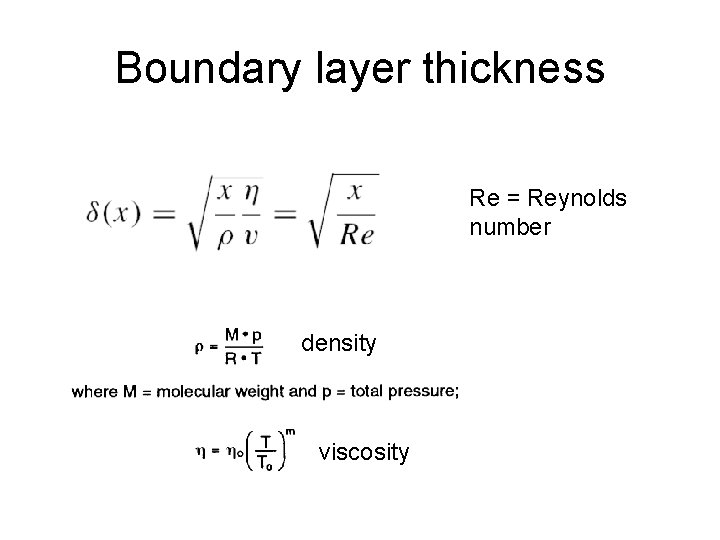
Boundary layer thickness Re = Reynolds number density viscosity

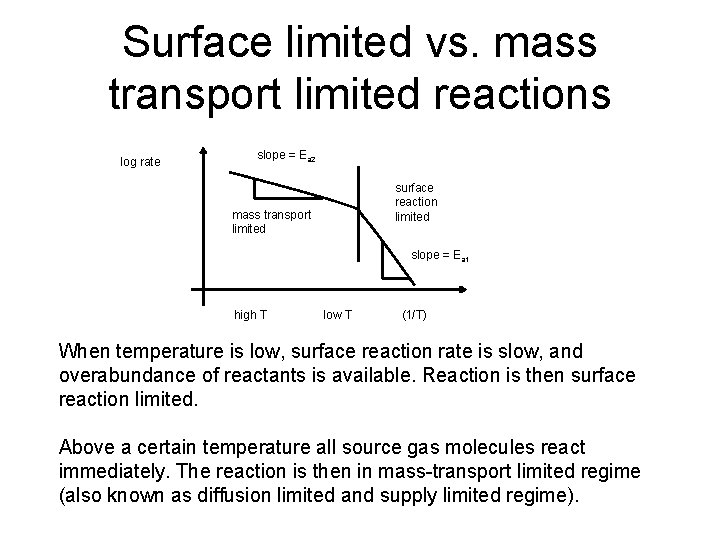
Surface limited vs. mass transport limited reactions log rate slope = Ea 2 mass transport limited surface reaction limited slope = Ea 1 high T low T (1/T) When temperature is low, surface reaction rate is slow, and overabundance of reactants is available. Reaction is then surface reaction limited. Above a certain temperature all source gas molecules react immediately. The reaction is then in mass-transport limited regime (also known as diffusion limited and supply limited regime).

Surface limited vs. mass transport limited reactions (2) A batch reactor operating in surface reaction limited mode: -slow reaction -many wafers A mass transport limited reactor: -single wafer -high deposition rate

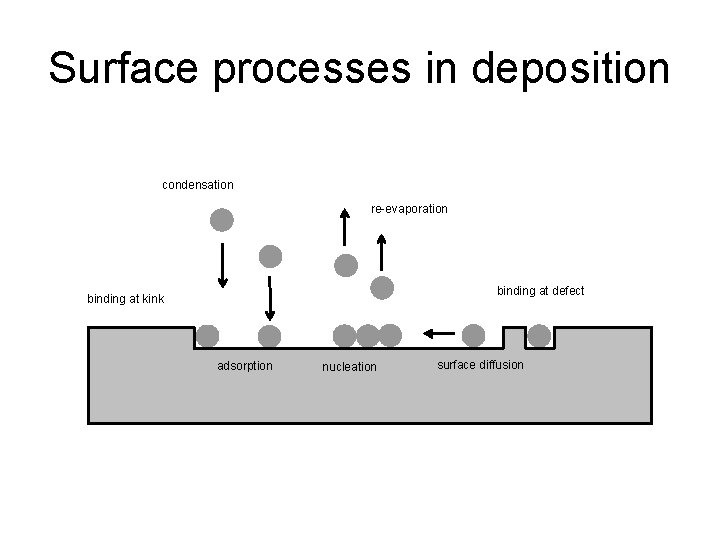
Surface processes in deposition condensation re-evaporation binding at defect binding at kink adsorption nucleation surface diffusion

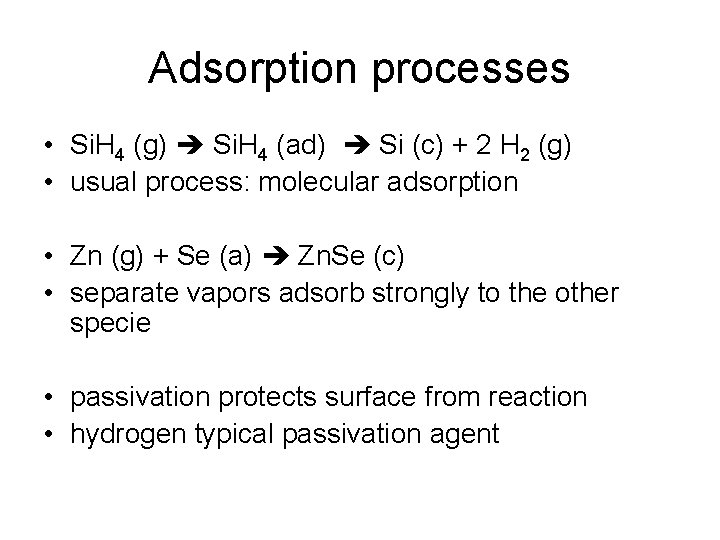
Adsorption processes • Si. H 4 (g) Si. H 4 (ad) Si (c) + 2 H 2 (g) • usual process: molecular adsorption • Zn (g) + Se (a) Zn. Se (c) • separate vapors adsorb strongly to the other specie • passivation protects surface from reaction • hydrogen typical passivation agent

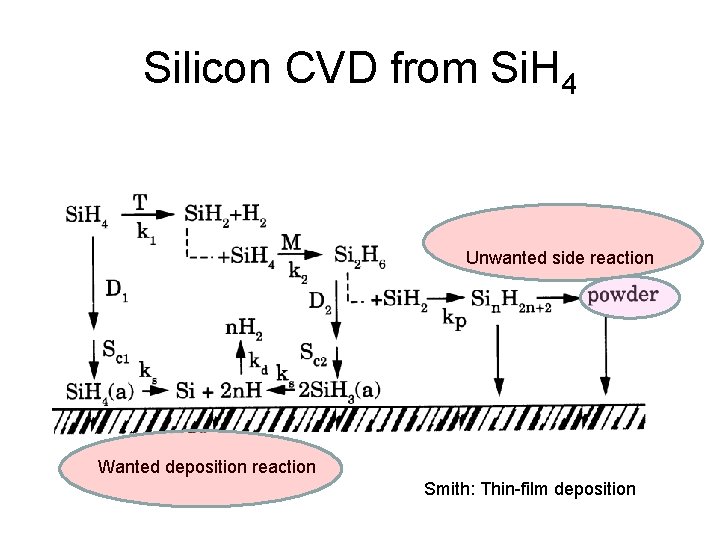
Silicon CVD from Si. H 4 Unwanted side reaction Wanted deposition reaction Smith: Thin-film deposition

Polysilicon • • • Si. H 4 (g) ==> Si (s) + 2 H 2 (g) Deposited by CVD at 625 o. C true poly Can be deposited at 575 o. C amorphous Anneal after deposition: a-Si poly ! Typical thickness 100 nm-2 µm


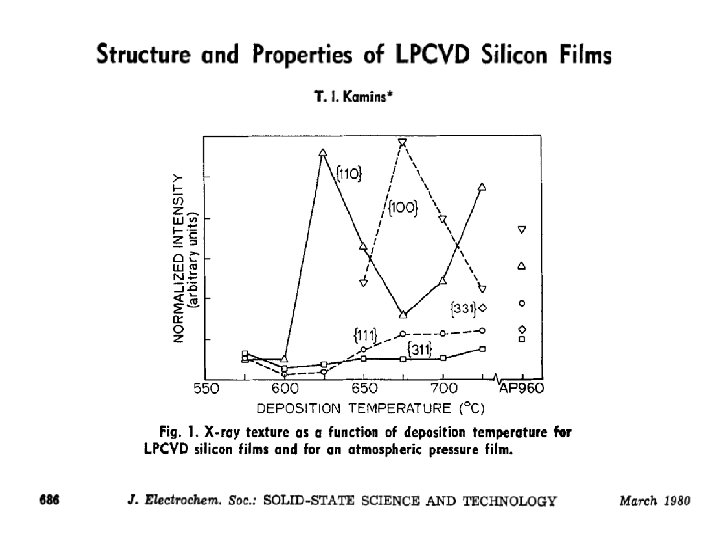
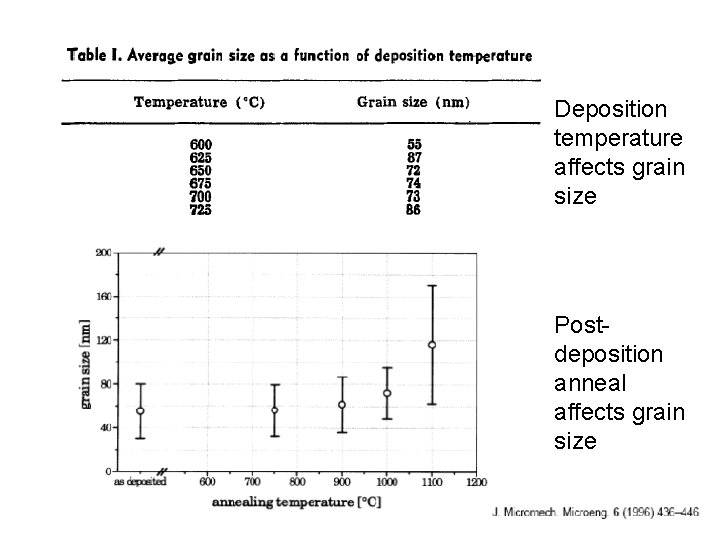
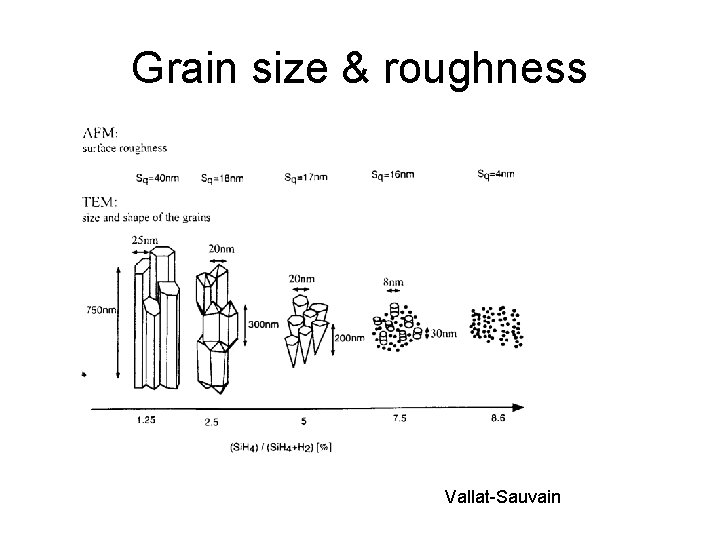
Deposition temperature affects grain size Post- deposition anneal affects grain size

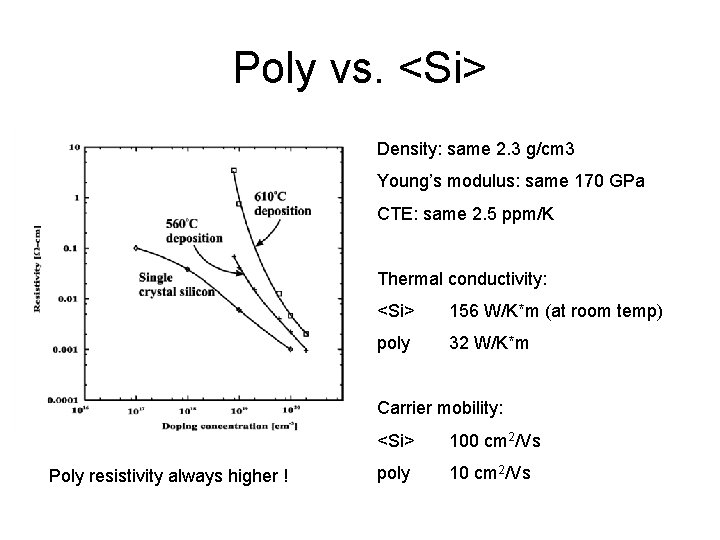
Poly vs. <Si> Density: same 2. 3 g/cm 3 Young’s modulus: same 170 GPa CTE: same 2. 5 ppm/K Thermal conductivity: <Si> 156 W/K*m (at room temp) poly 32 W/K*m Carrier mobility: Poly resistivity always higher ! <Si> 100 cm 2/Vs poly 10 cm 2/Vs

PECVD: Plasma Enhanced CVD • • Plasma aids in chemical reactions Can be done at low temperatures Wide deposition parameter range High rates (1 -10 nm/s) (thermal 10% of this)

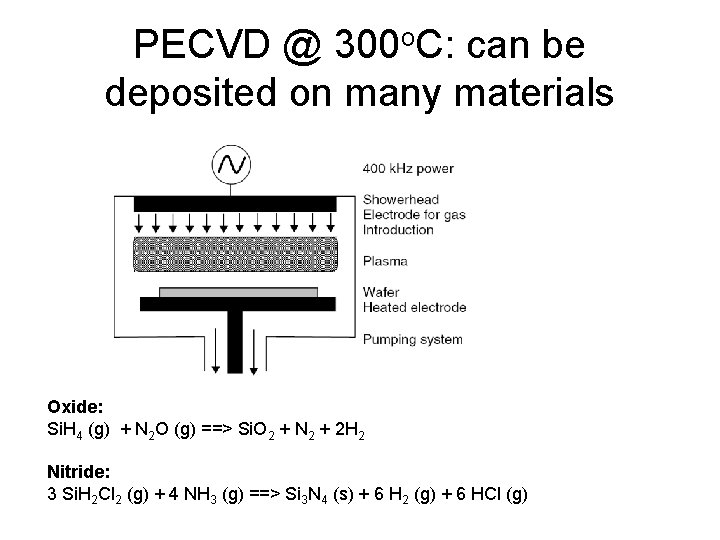
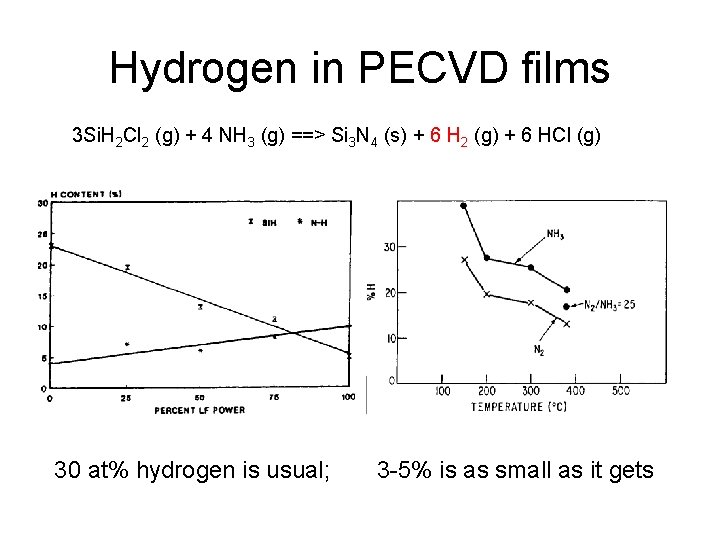
PECVD @ 300 o. C: can be deposited on many materials Oxide: Si. H 4 (g) + N 2 O (g) ==> Si. O 2 + N 2 + 2 H 2 Nitride: 3 Si. H 2 Cl 2 (g) + 4 NH 3 (g) ==> Si 3 N 4 (s) + 6 H 2 (g) + 6 HCl (g)

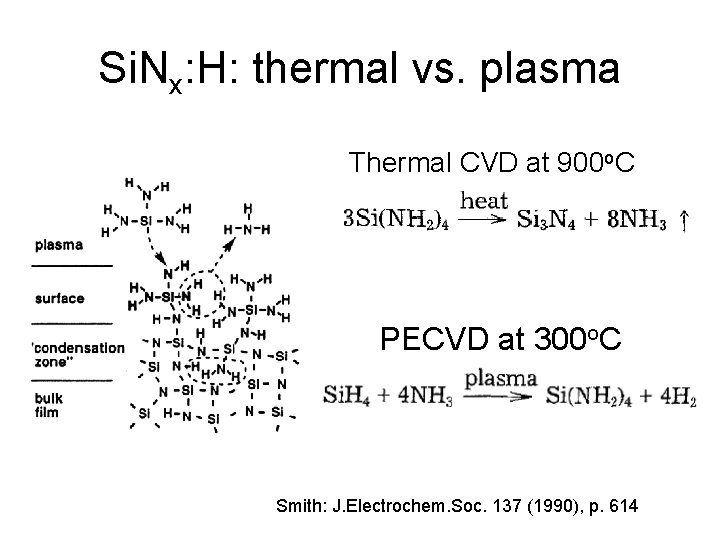
Si. Nx: H: thermal vs. plasma Thermal CVD at 900 o. C PECVD at 300 o. C Smith: J. Electrochem. Soc. 137 (1990), p. 614

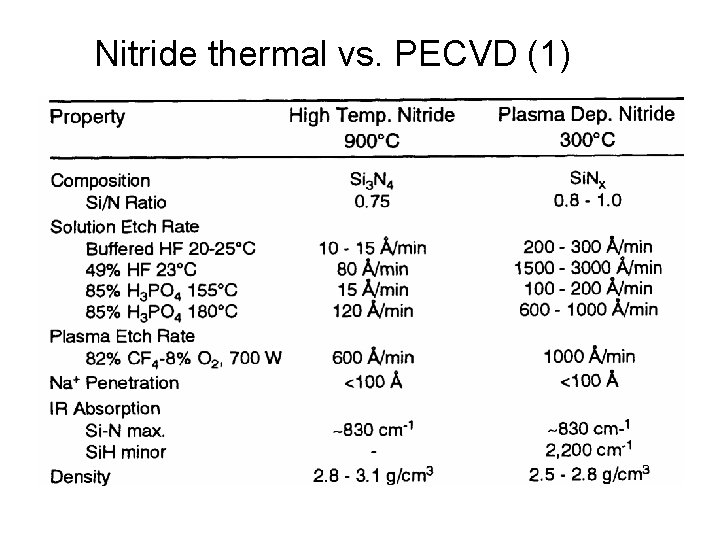
Nitride thermal vs. PECVD (1)

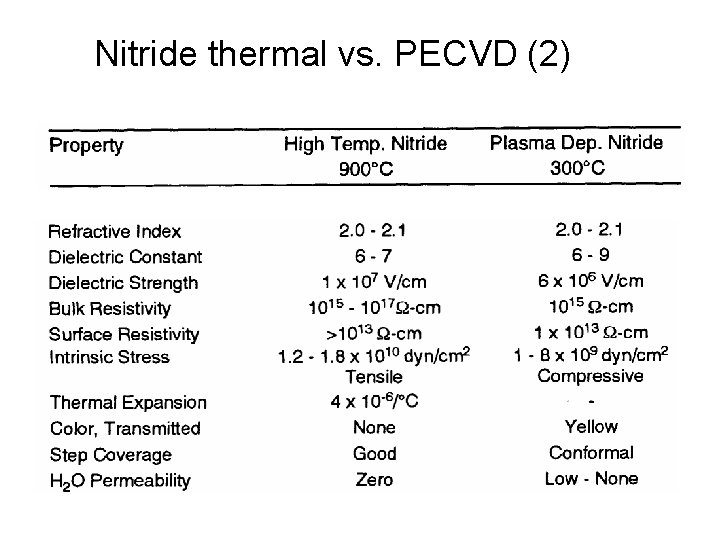
Nitride thermal vs. PECVD (2)

Half-time

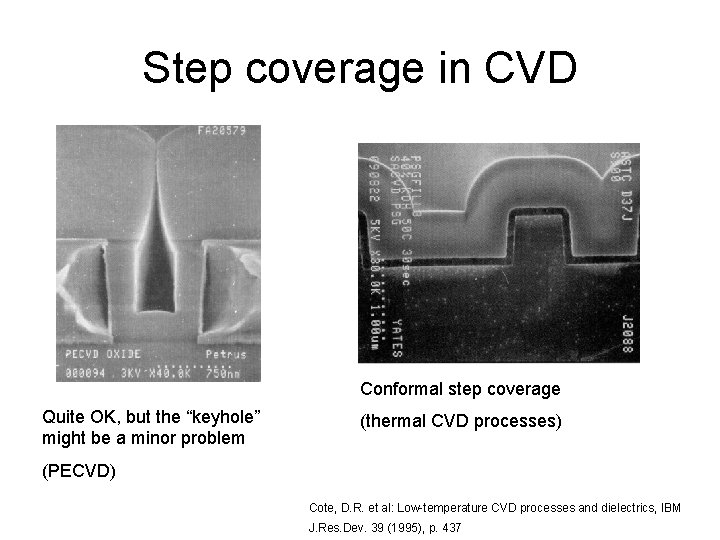
Step coverage in CVD Conformal step coverage Quite OK, but the “keyhole” might be a minor problem (thermal CVD processes) (PECVD) Cote, D. R. et al: Low-temperature CVD processes and dielectrics, IBM J. Res. Dev. 39 (1995), p. 437

Step coverage and surface limited reactions A batch reactor operating in surface reaction limited mode: -slow reaction -many wafers This is possible because gas diffusion is not critical: just wait enough time and gas diffuses between the wafers, and react at all surfaces good step coverage

Grain size & roughness Vallat-Sauvain

Hydrogen in PECVD films 3 Si. H 2 Cl 2 (g) + 4 NH 3 (g) ==> Si 3 N 4 (s) + 6 H 2 (g) + 6 HCl (g) 30 at% hydrogen is usual; 3 -5% is as small as it gets

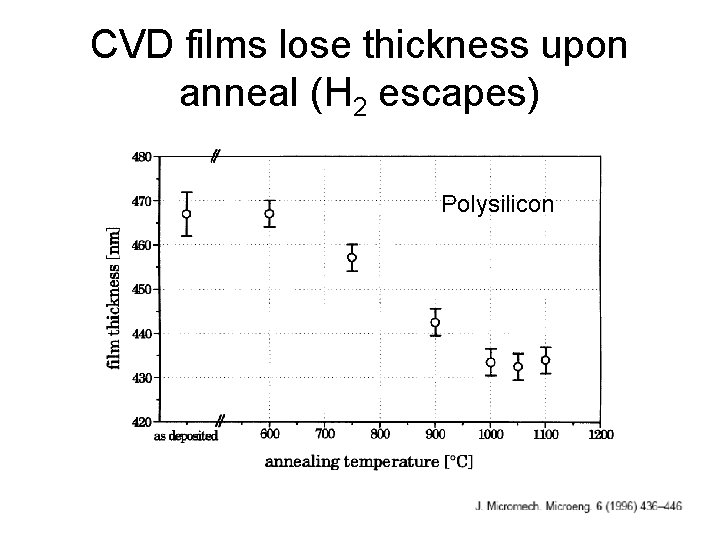
CVD films lose thickness upon anneal (H 2 escapes) Polysilicon

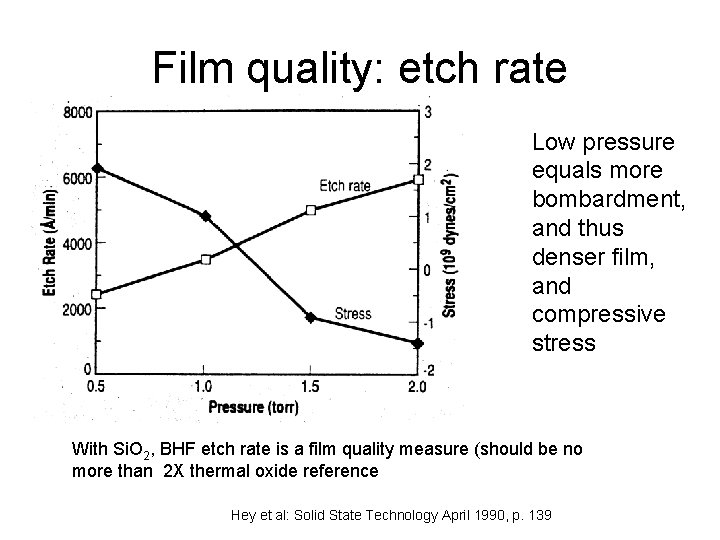
Film quality: etch rate Low pressure equals more bombardment, and thus denser film, and compressive stress With Si. O 2, BHF etch rate is a film quality measure (should be no more than 2 X thermal oxide reference Hey et al: Solid State Technology April 1990, p. 139

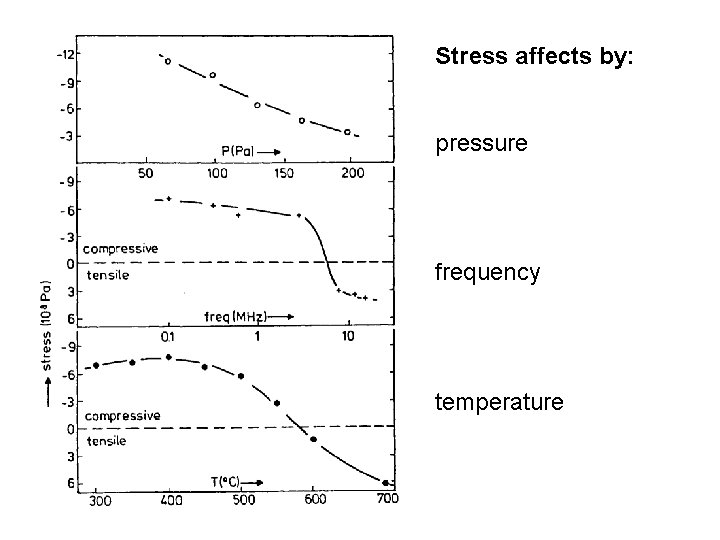
Stress affects by: pressure frequency temperature

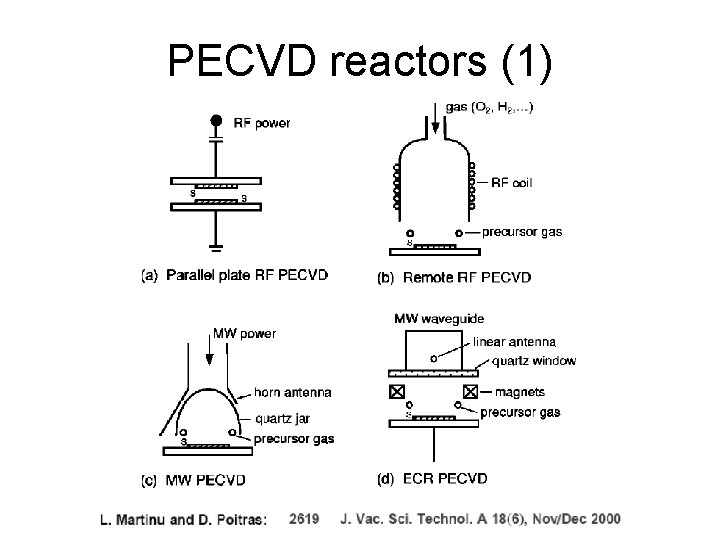
PECVD reactors (1)

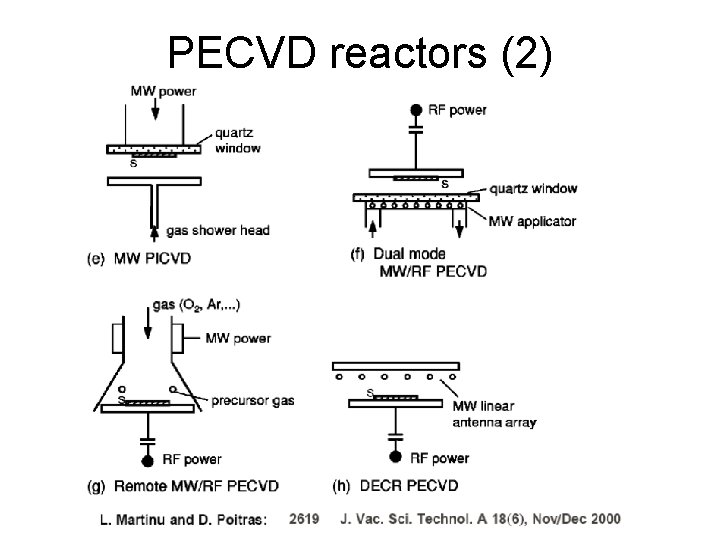
PECVD reactors (2)

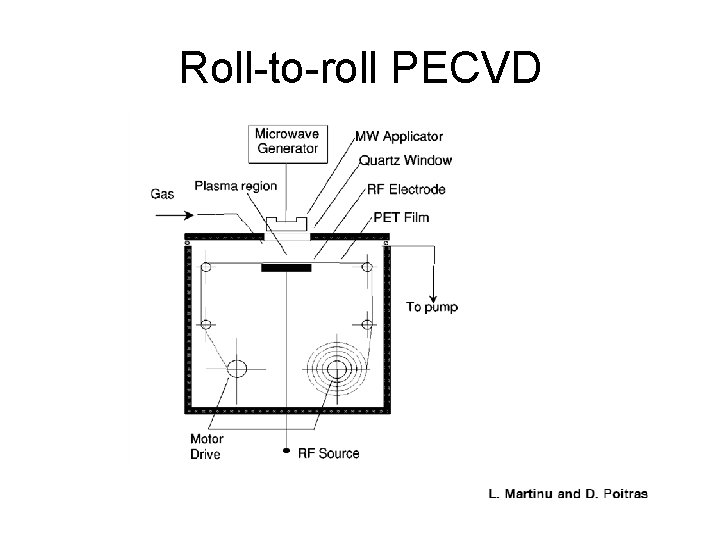
Roll-to-roll PECVD

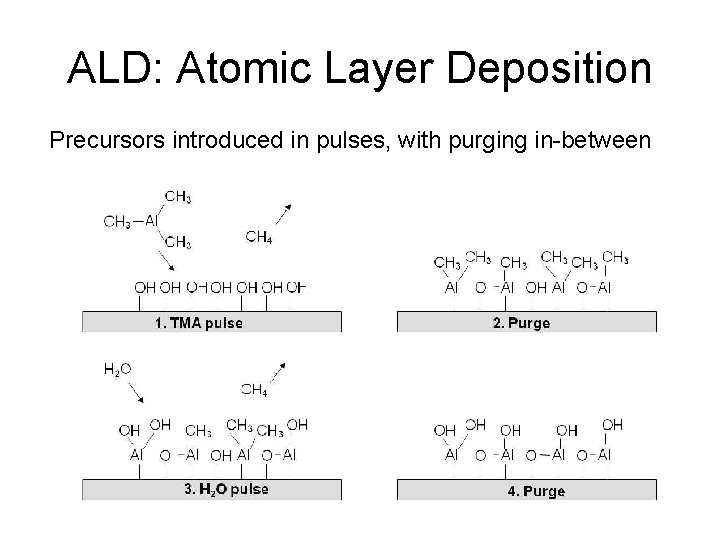
ALD: Atomic Layer Deposition Precursors introduced in pulses, with purging in-between

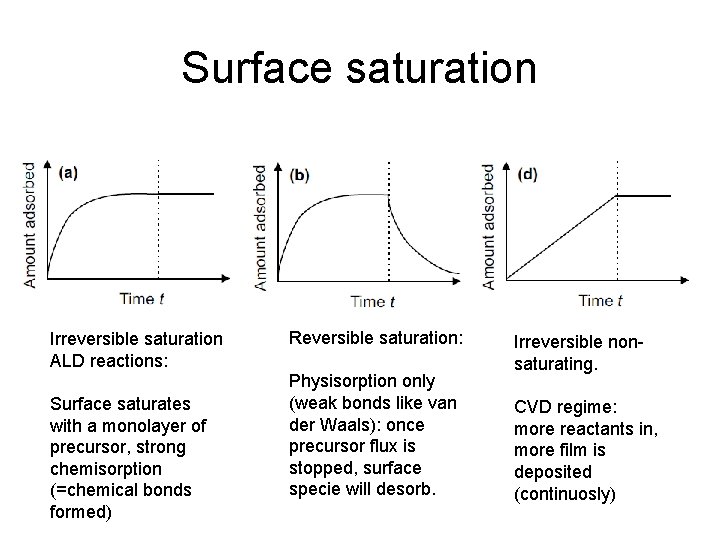
Surface saturation Irreversible saturation ALD reactions: Surface saturates with a monolayer of precursor, strong chemisorption (=chemical bonds formed) Reversible saturation: Physisorption only (weak bonds like van der Waals): once precursor flux is stopped, surface specie will desorb. Irreversible nonsaturating. CVD regime: more reactants in, more film is deposited (continuosly)

ALD process based on: conformal film

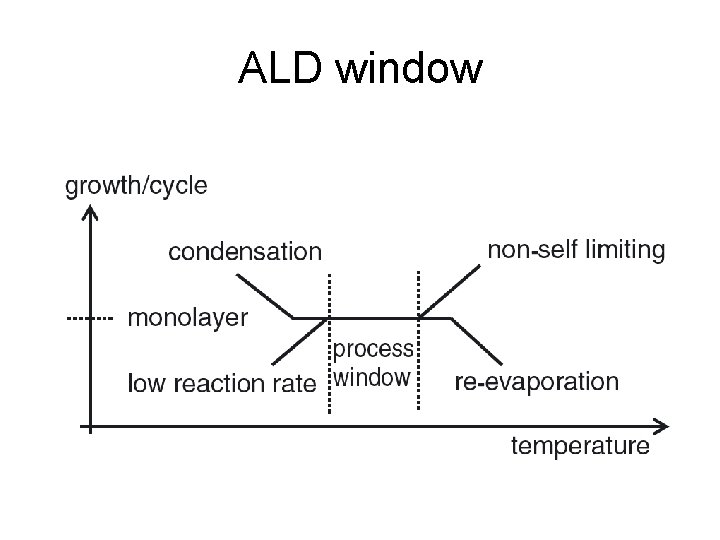
ALD window

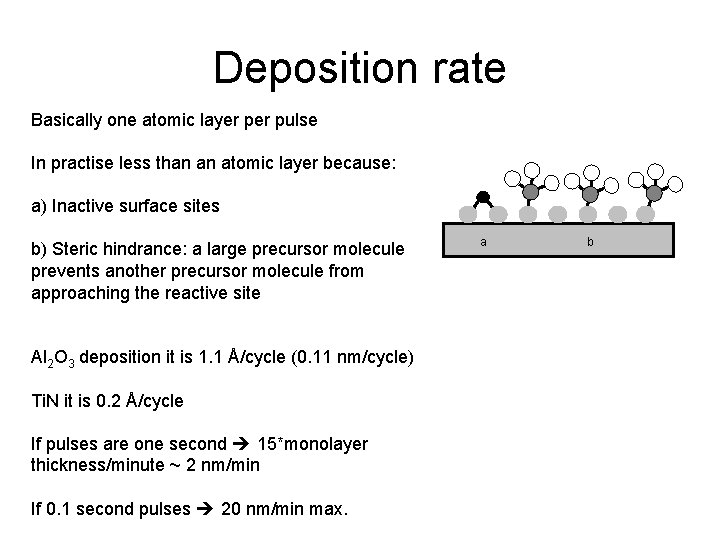
Deposition rate Basically one atomic layer pulse In practise less than an atomic layer because: a) Inactive surface sites b) Steric hindrance: a large precursor molecule prevents another precursor molecule from approaching the reactive site Al 2 O 3 deposition it is 1. 1 Å/cycle (0. 11 nm/cycle) Ti. N it is 0. 2 Å/cycle If pulses are one second 15*monolayer thickness/minute ~ 2 nm/min If 0. 1 second pulses 20 nm/min max. a b

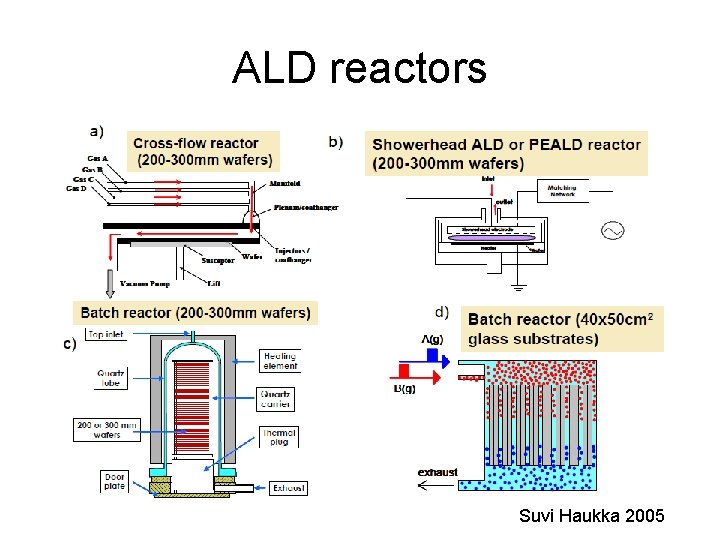
ALD reactors Suvi Haukka 2005

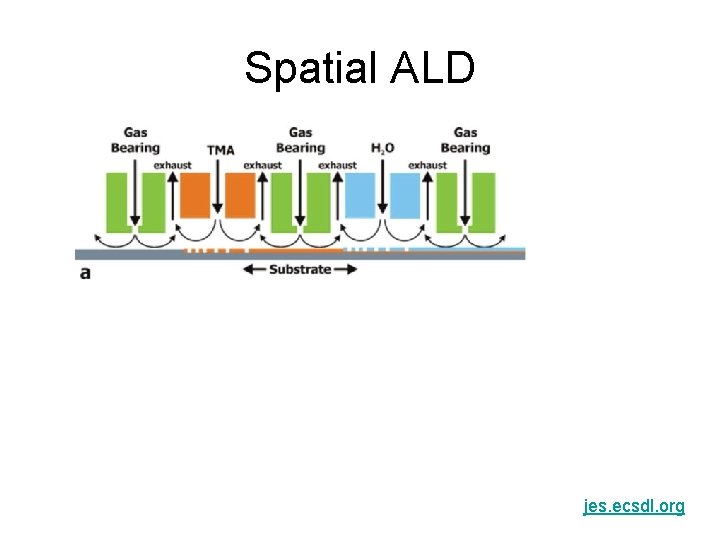
Spatial ALD jes. ecsdl. org

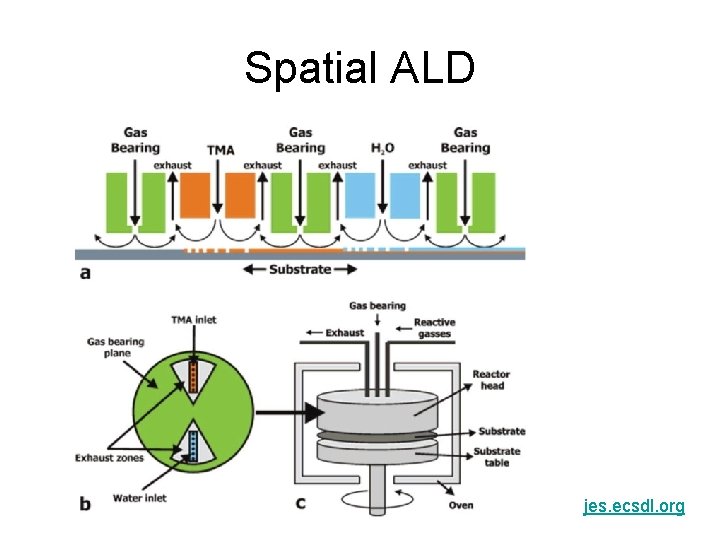
Spatial ALD jes. ecsdl. org

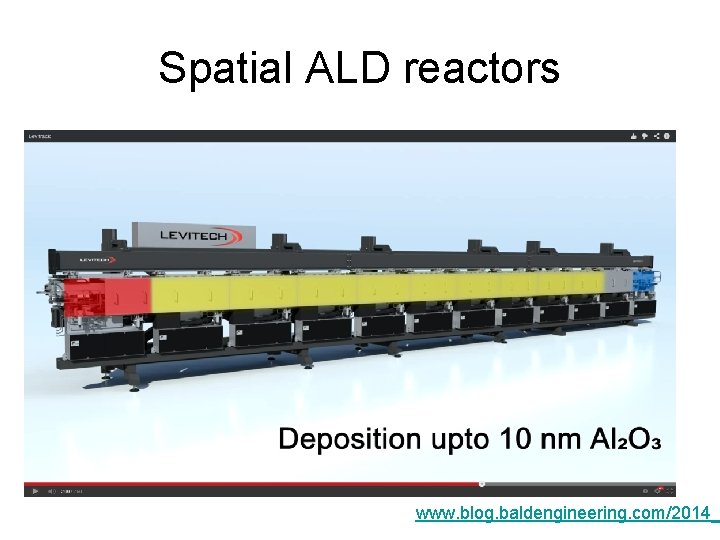
Spatial ALD reactors www. blog. baldengineering. com/2014_

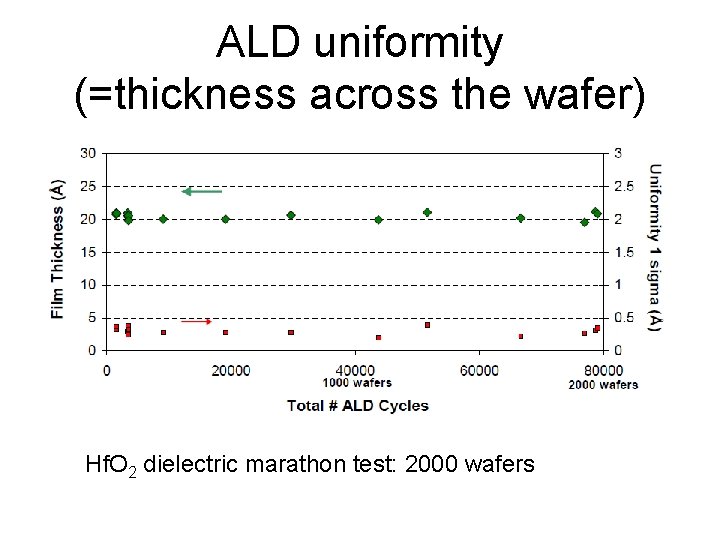
ALD uniformity (=thickness across the wafer) Hf. O 2 dielectric marathon test: 2000 wafers

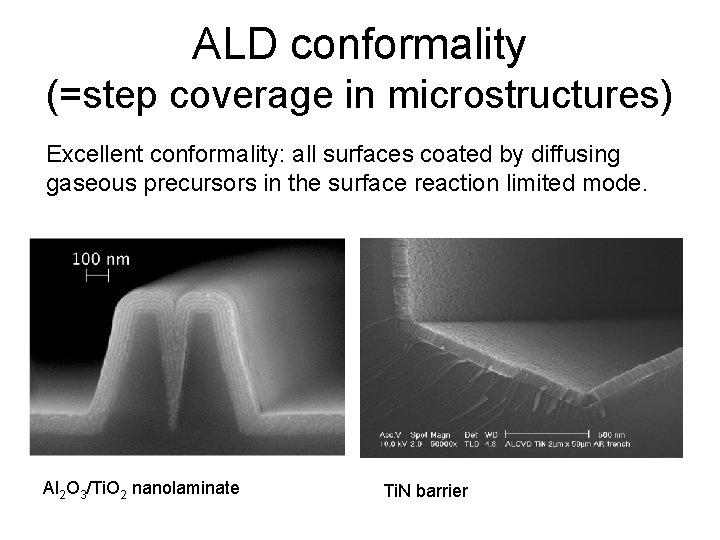
ALD conformality (=step coverage in microstructures) Excellent conformality: all surfaces coated by diffusing gaseous precursors in the surface reaction limited mode. Al 2 O 3/Ti. O 2 nanolaminate Ti. N barrier

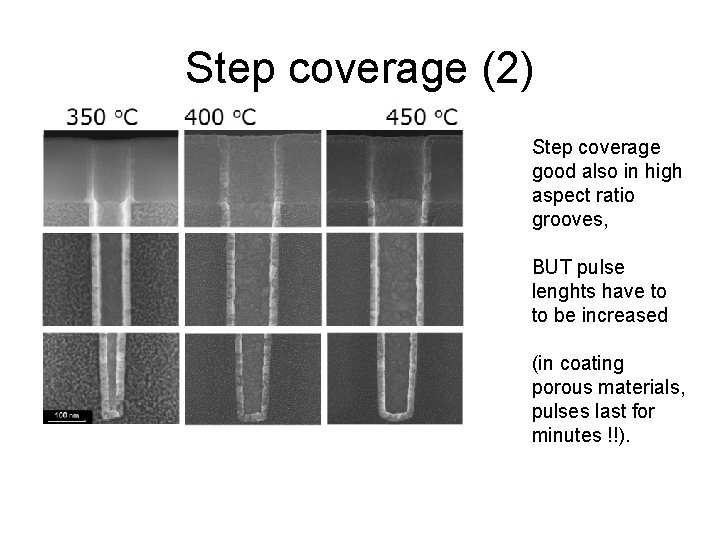
Step coverage (2) Step coverage good also in high aspect ratio grooves, BUT pulse lenghts have to to be increased (in coating porous materials, pulses last for minutes !!).

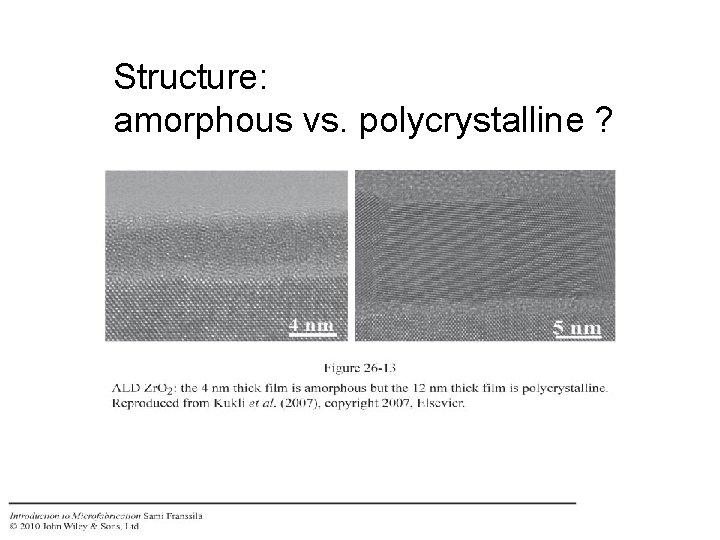
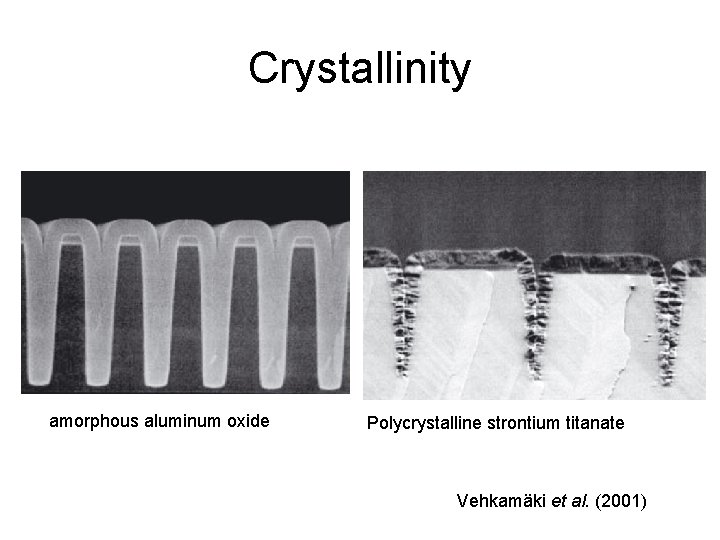
Structure: amorphous vs. polycrystalline ?

Crystallinity amorphous aluminum oxide Polycrystalline strontium titanate Vehkamäki et al. (2001)

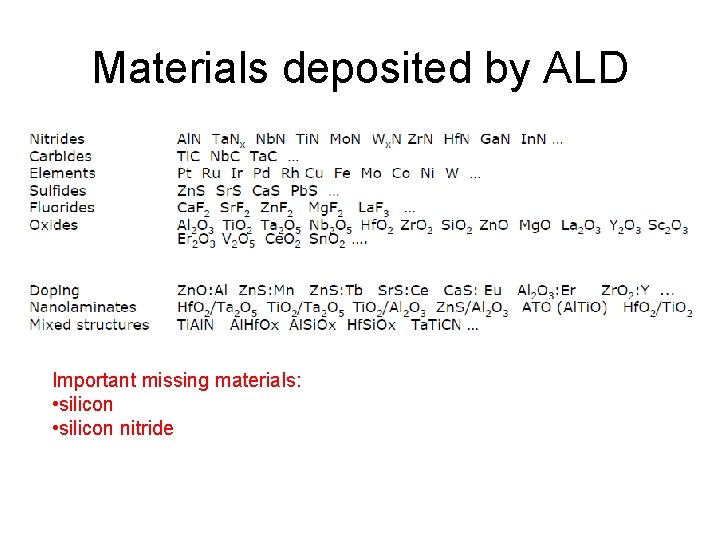
Materials deposited by ALD Important missing materials: • silicon nitride

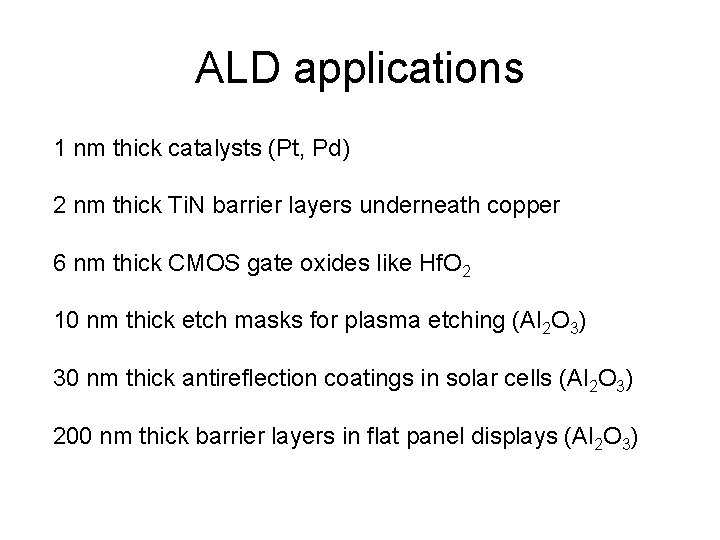
ALD applications 1 nm thick catalysts (Pt, Pd) 2 nm thick Ti. N barrier layers underneath copper 6 nm thick CMOS gate oxides like Hf. O 2 10 nm thick etch masks for plasma etching (Al 2 O 3) 30 nm thick antireflection coatings in solar cells (Al 2 O 3) 200 nm thick barrier layers in flat panel displays (Al 2 O 3)

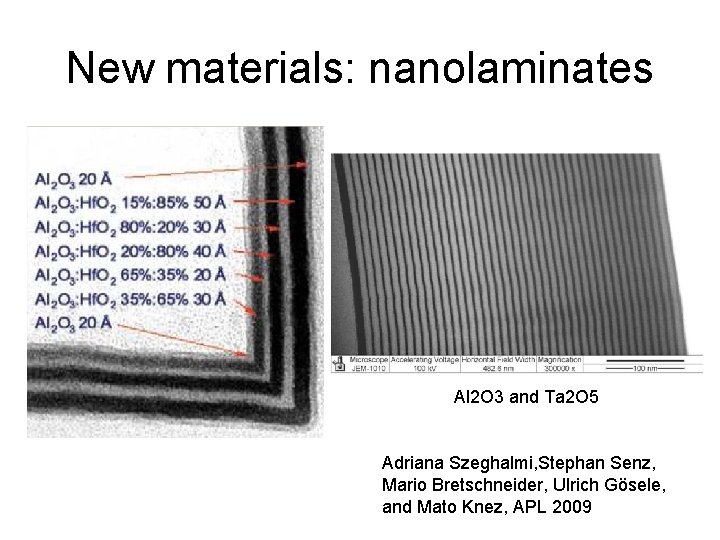
New materials: nanolaminates Al 2 O 3 and Ta 2 O 5 Adriana Szeghalmi, Stephan Senz, Mario Bretschneider, Ulrich Gösele, and Mato Knez, APL 2009

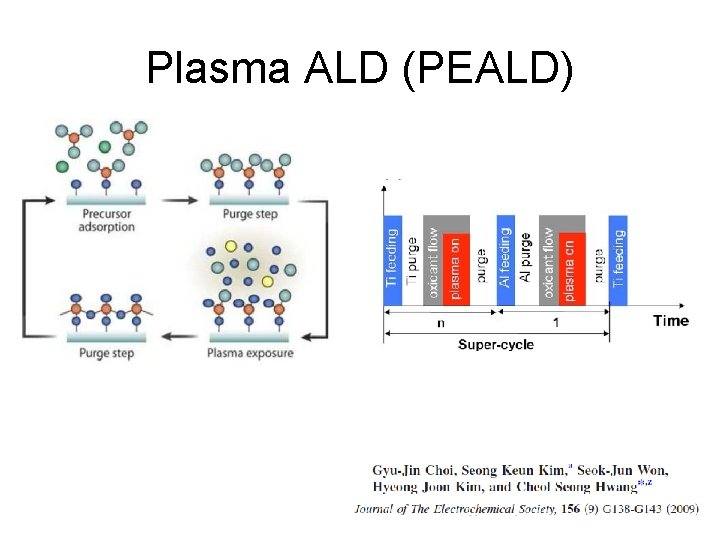
Plasma ALD (PEALD)

Plasma ALD benefits Plasma can break down precursors at lower temperature New precursors become available because plasma can break down precursors that could not be used in thermal ALD Ions can kick of loosely bound specie from surface, densifying the film

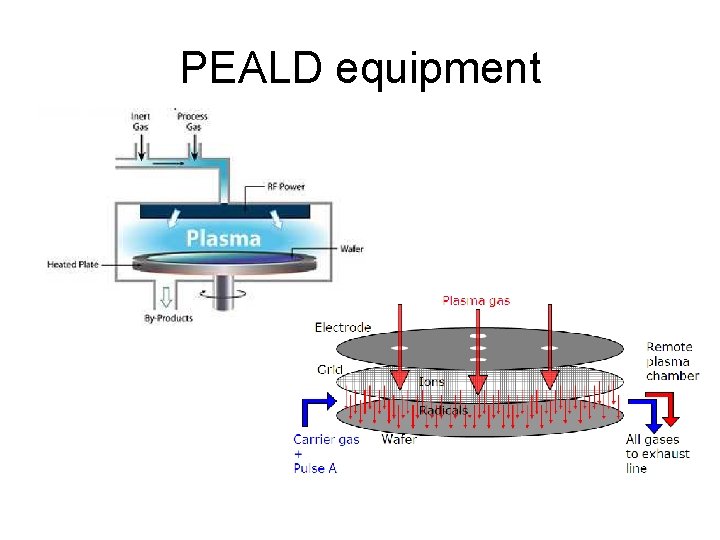
PEALD equipment

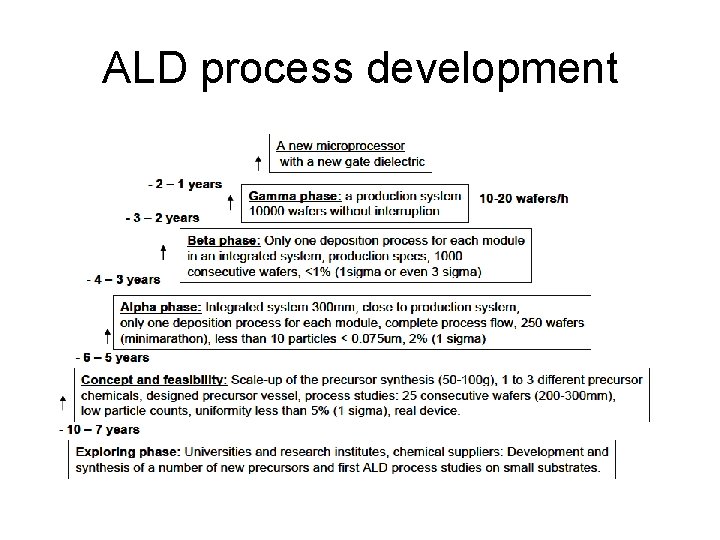
ALD process development

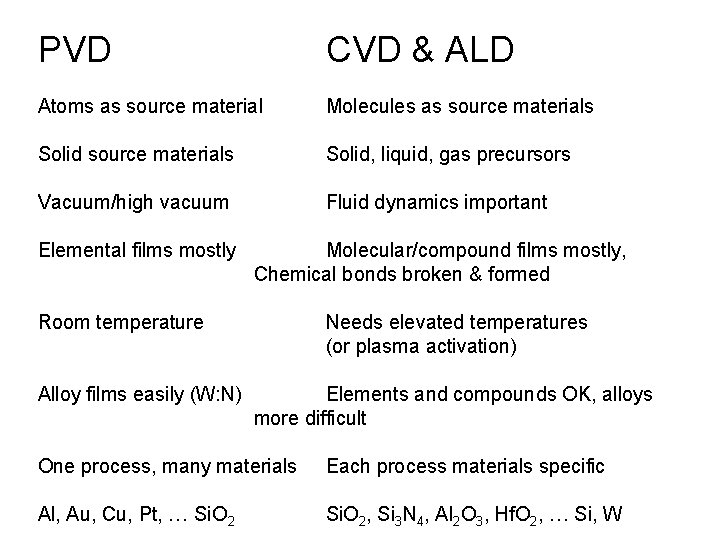
PVD CVD & ALD Atoms as source material Molecules as source materials Solid source materials Solid, liquid, gas precursors Vacuum/high vacuum Fluid dynamics important Elemental films mostly Molecular/compound films mostly, Chemical bonds broken & formed Room temperature Alloy films easily (W: N) Needs elevated temperatures (or plasma activation) Elements and compounds OK, alloys more difficult One process, many materials Each process materials specific Al, Au, Cu, Pt, … Si. O 2, Si 3 N 4, Al 2 O 3, Hf. O 2, … Si, W

Summary • Thermal CVD: excellent film quality • PECVD: reasonable film quality at low T • ALD: excellent film quality at low T • Thermal CVD: high temperature needed • PECVD: very high rate possible • ALD: best for very thin films
 Pvd cvd ald
Pvd cvd ald Commvault firewall ports
Commvault firewall ports Pvd vs cvd
Pvd vs cvd Pvd cvd coating
Pvd cvd coating Pvd, cvd
Pvd, cvd Ald 2life vetrina
Ald 2life vetrina Pec lextel napoli
Pec lextel napoli Mobilitysolutions.aldautomotive.it
Mobilitysolutions.aldautomotive.it Ald shirt
Ald shirt Schéma de wood ald
Schéma de wood ald Ald
Ald Ald automotive romania
Ald automotive romania Ald automotive permuta
Ald automotive permuta Kontrgaranti nedir
Kontrgaranti nedir Sami kulmala
Sami kulmala Left trochanteric fracture
Left trochanteric fracture Sami haims
Sami haims Sami sikstus
Sami sikstus Sami vibration switch
Sami vibration switch Bitta söderblom
Bitta söderblom Sami nikander
Sami nikander Sami zaatari
Sami zaatari Sami boutros
Sami boutros Gdyby wszyscy byli tacy sami twardowski
Gdyby wszyscy byli tacy sami twardowski Sami boutros
Sami boutros Sami khuri
Sami khuri Sami ramek
Sami ramek Attahiyat dua in english
Attahiyat dua in english Sami länkimäki
Sami länkimäki Acar f klavye
Acar f klavye Chapter 14 from randomness to probability
Chapter 14 from randomness to probability Sami khuri
Sami khuri Maher zain nationality
Maher zain nationality Perjuangan golongan sami buddha
Perjuangan golongan sami buddha Sami kivivasara
Sami kivivasara Sami liedes
Sami liedes Sami rollins
Sami rollins Sami napari
Sami napari Radiology case presentation
Radiology case presentation Sami franssila
Sami franssila Fafsav
Fafsav Sami people language
Sami people language Sami kryeziu
Sami kryeziu Sami sipahi ortaokulu
Sami sipahi ortaokulu Sami arpa
Sami arpa Vabcd is a solid pyramid
Vabcd is a solid pyramid Sami siiki
Sami siiki Sami kibria
Sami kibria Malingering disorder
Malingering disorder Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chopping wood chemical or physical change
Chopping wood chemical or physical change International chemical congress of pacific basin societies
International chemical congress of pacific basin societies Is dissolving salt in water chemical or physical change
Is dissolving salt in water chemical or physical change Spraggins fitness
Spraggins fitness Is the shine of chrome physical or chemical
Is the shine of chrome physical or chemical Combustion reaction
Combustion reaction How to calculate cod
How to calculate cod Tips for balancing chemical equations
Tips for balancing chemical equations