Do Now 7 A physical change occurs when















- Slides: 19

Do Now #7 A physical change occurs when a substance changes form, but stays the same. A chemical change occurs when a substance changes into a new, different substance. 1. In which Station Activities did you observe physical changes? 2. In which Station Activities did you observe chemical changes?

Physical vs. Chemical Physical Property characteristics observed without changing the identity of the substance Characteristics such as color, density, odor Chemical Property describes the ability of a substance to undergo changes in identity

Physical vs. Chemical Lets think about the penny. What is the penny made out of? Please tell me you didn’t say just copper! Lets take a look at what the penny has done over the years. Composition? Why would the penny change (think of physical properties, as well as chemical properties?

B. Physical vs. Chemical Examples: melting point physical flammable chemical density physical magnetic physical tarnishes in air chemical

Classify Each of the following as Physical or Chemical Properties ¬ The boiling point of ethyl alcohol is 78°C. Physical property – describes inherent characteristic of alcohol – boiling point Diamond is very hard. Physical property – describes inherent characteristic of diamond – hardness ® Sugar ferments to form ethyl alcohol. Chemical property – describes behavior of sugar – forming a new substance (ethyl alcohol)

Changes in Matter Physical Changes are changes to matter that do not result in a change of the fundamental components that make that substance (CHANGE IN CONDITION) State Changes – boiling, melting, condensing Chemical Changes involve a change in the fundamental components of the substance (CHANGE IN MATERIAL) Produce a new substance Chemical reaction Reactants Products

It’s a physical change if It changes shape or size It dissolves.

• It’s a physical change if. . . It changes phase (freezes, boils, evaporates, condenses)

It’s a chemical change if…. It burns Temperature changes without heating/cooling

It’s a chemical change if. . . It bubbles (makes a gas)

It’s a chemical change if. . . It changes color It forms a precipitate

What kind of change is it if someone. . . Tears up paper? Physical change Mixes salt and water? Physical change

What kind of change is it if someone. . . Burns paper? Chemical change Evaporates salt water? Physical change

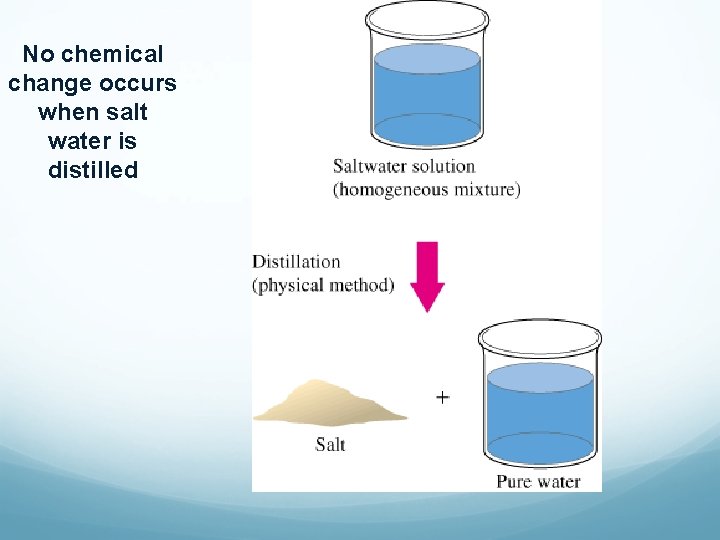
Table salt is stirred into water (left), forming a homogeneous mixture called a solution (right)

No chemical change occurs when salt water is distilled

What kind of change is it if someone. . . Mixes vinegar and baking soda? Chemical change

Physical vs. Chemical Examples: rusting iron chemical dissolving in water physical burning a log chemical melting ice physical grinding spices physical

Classify Each of the following as Physical or Chemical Changes ¬ Iron is melted. Physical change – describes a state change, but the material is still iron Iron combines with oxygen to form rust. Chemical change – describes how iron and oxygen react to make a new substance, rust ® Sugar ferments to form ethyl alcohol. Chemical change – describes how sugar forms a new substance (ethyl alcohol)

Properties of Matter PHYSICAL CHANGE PROPERTIES CHEMICAL New form of old substance. No new substances formed. Old substance destroyed. New substance formed. Description by senses – shape, color, odor, etc. Measurable properties – density, boiling point, etc. List of chemical changes possible.