Chpt 22 Some Families of Organic Compounds Organic





















































- Slides: 107

Chpt. 22: Some Families of Organic Compounds (Organic Chemistry)

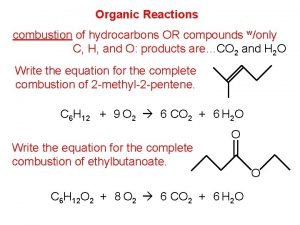
Previously studied organic families: Alkanes Alkenes Alkynes Aromatic Compounds This section involves the study of further organic families: Chloroalkanes Alcohols Aldehydes Ketones Carboxylic Acids Esters

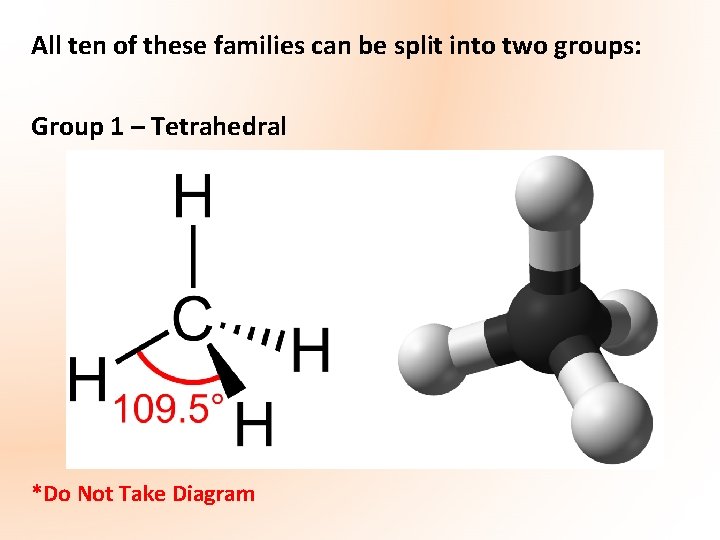
All ten of these families can be split into two groups: Group 1 – Tetrahedral *Do Not Take Diagram

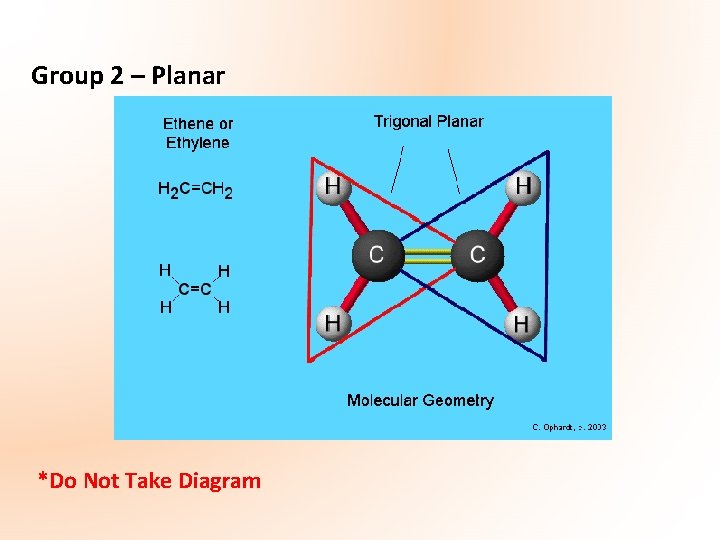
Group 2 – Planar *Do Not Take Diagram

Group 1: Tetrahedral Group 2: Planar Ø Alkanes Ø Alkenes Ø Chloroalkanes Ø Alcohols Ø Alkynes Ø Aldehydes Ø Ketones Ø Carboxylic Acids Ø Esters Ø Aromatic Conpounds

Tetrahedral Carbon Compounds In saturated organic compounds, ALL of the carbon atoms are tetrahedral CHLOROALKANES (Haloalkanes): Chloroalkanes are compounds in which one or more of the hydrogen atoms in an alkane molecule have been replaced by a chlorine atom The part of an alkane remaining after one hydrogen is removed is an alkyl group e. g. Methane CH 4 minus a hydrogen group leaves methyl -CH 3

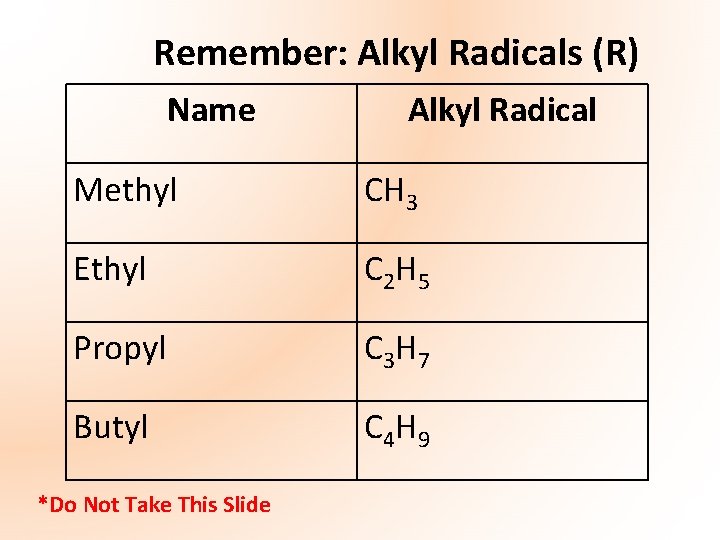
Remember: Alkyl Radicals (R) Name Alkyl Radical Methyl CH 3 Ethyl C 2 H 5 Propyl C 3 H 7 Butyl C 4 H 9 *Do Not Take This Slide

Chloroalkanes are named after the alkane from which they are derived with the prefix chloro- indicating the presence of chlorine.

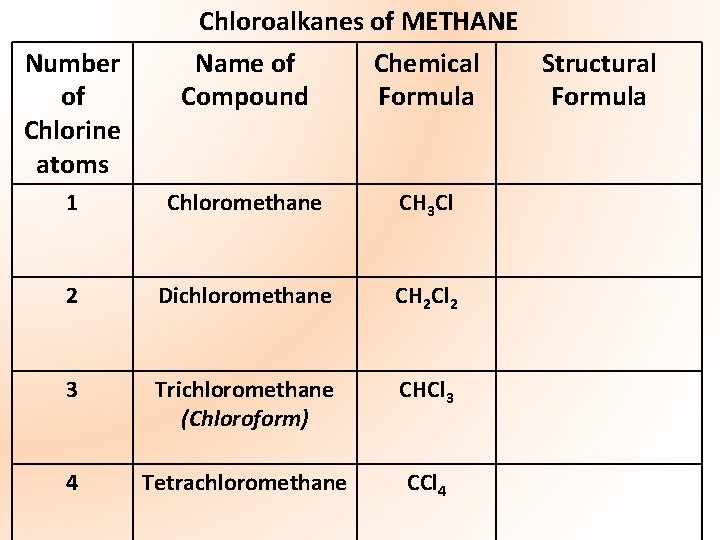
Number of Chlorine atoms Chloroalkanes of METHANE Name of Chemical Structural Compound Formula 1 Chloromethane CH 3 Cl 2 Dichloromethane CH 2 Cl 2 3 Trichloromethane (Chloroform) CHCl 3 4 Tetrachloromethane CCl 4

*Important Note* You must be able to name and draw the structure of all the chloroalkanes for the first four alkanes!!! (methane, propane, butane)

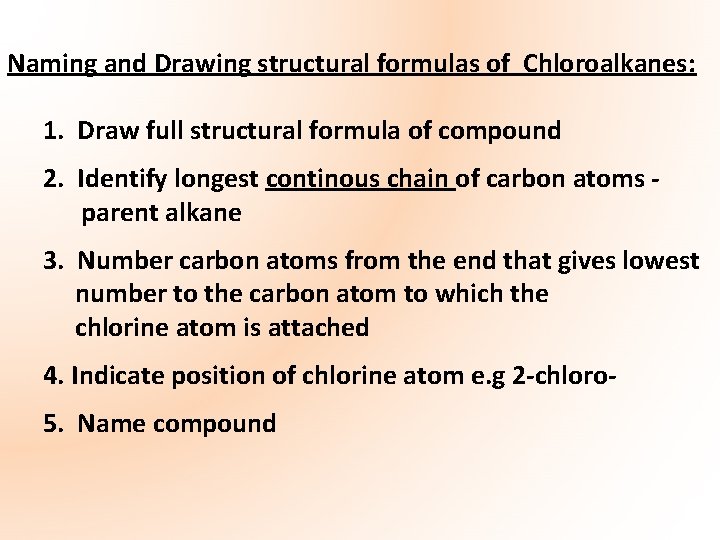
Naming and Drawing structural formulas of Chloroalkanes: 1. Draw full structural formula of compound 2. Identify longest continous chain of carbon atoms parent alkane 3. Number carbon atoms from the end that gives lowest number to the carbon atom to which the chlorine atom is attached 4. Indicate position of chlorine atom e. g 2 -chloro 5. Name compound

Example: Name the compounds: a) CH 3 CH 2 CHCl. CH 3 b) CH 3 CCl 2 CHCl. CH 3 c) CH 3 CCl(CH 3)CH 3 Student Questions: Workbook – pg 60 W 22. 1, W 22. 2, W 22. 3 Homework: Book – pg 360 22. 1, 22. 2, 22. 3

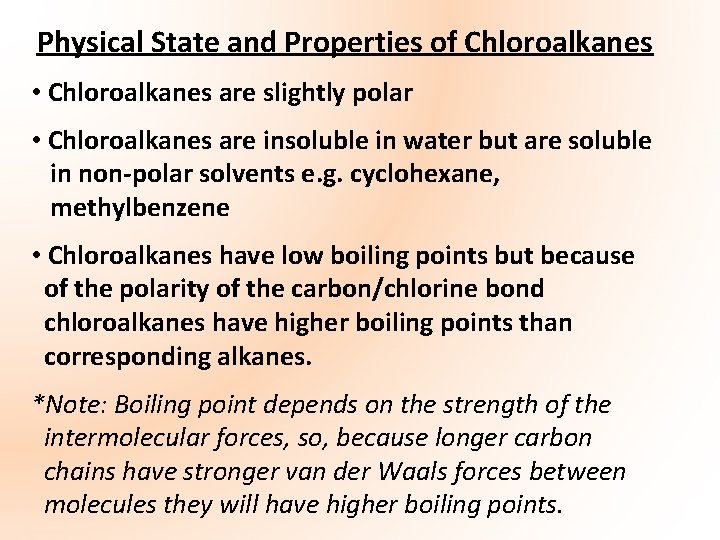
Physical State and Properties of Chloroalkanes • Chloroalkanes are slightly polar • Chloroalkanes are insoluble in water but are soluble in non-polar solvents e. g. cyclohexane, methylbenzene • Chloroalkanes have low boiling points but because of the polarity of the carbon/chlorine bond chloroalkanes have higher boiling points than corresponding alkanes. *Note: Boiling point depends on the strength of the intermolecular forces, so, because longer carbon chains have stronger van der Waals forces between molecules they will have higher boiling points.

• Most chloroalkanes liquid at room temperature, exception – chloromethane* and chloroethane are gaseous • Main use is as solvents: - for removing oil and grease machinery, dry cleaning - paint stripper (dichloromethane) - Tippex* *Ozone Layer

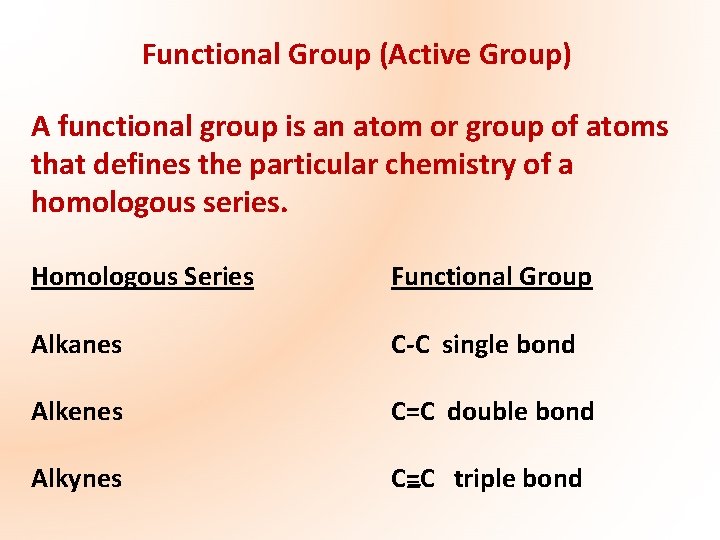
Functional Group (Active Group) A functional group is an atom or group of atoms that defines the particular chemistry of a homologous series. Homologous Series Functional Group Alkanes C-C single bond Alkenes C=C double bond Alkynes C=C triple bond

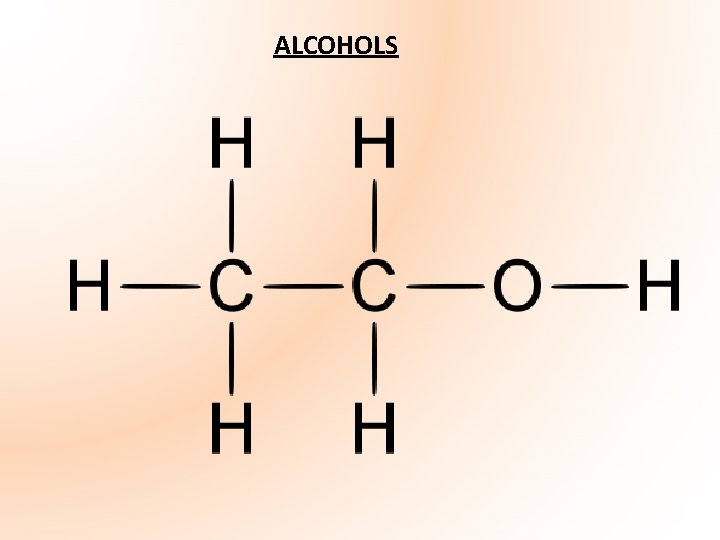
ALCOHOLS

Alcohols form a homologous series of compounds of formula: Cn. H 2 n + 1 OH • Functional group – OH group (V-shaped) called hydroxyl group Alc. OHol • Alcohols are formed when the hydrogen atom in an alkane is replaced by the hydroxyl group (OH) • Carbon atoms including that joined to the OH group are *TETRAHEDRAL* • Named by replacing -ane at end of corresponding alkane with -anol

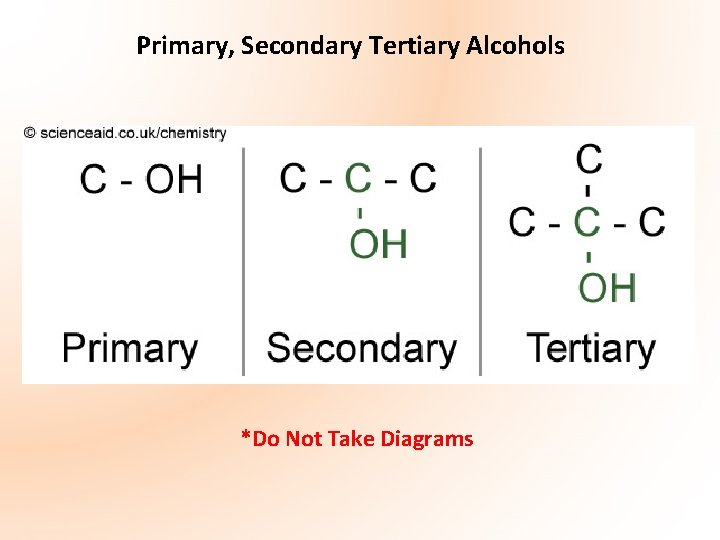
Primary, Secondary Tertiary Alcohols *Do Not Take Diagrams

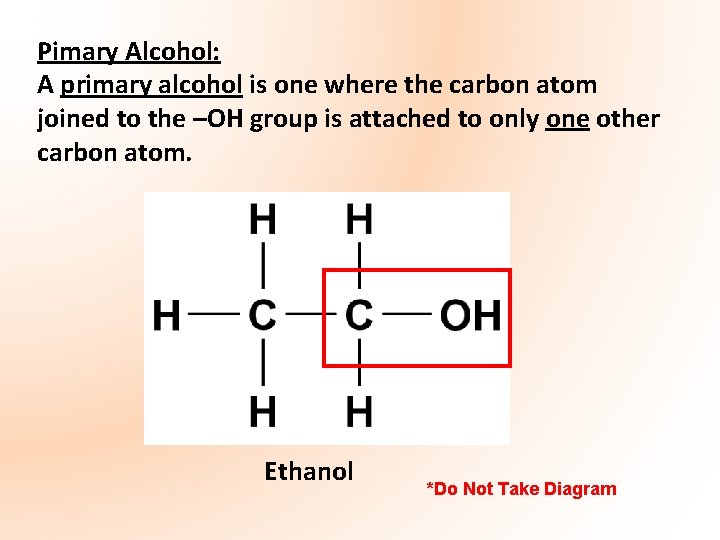
Pimary Alcohol: A primary alcohol is one where the carbon atom joined to the –OH group is attached to only one other carbon atom. Ethanol *Do Not Take Diagram

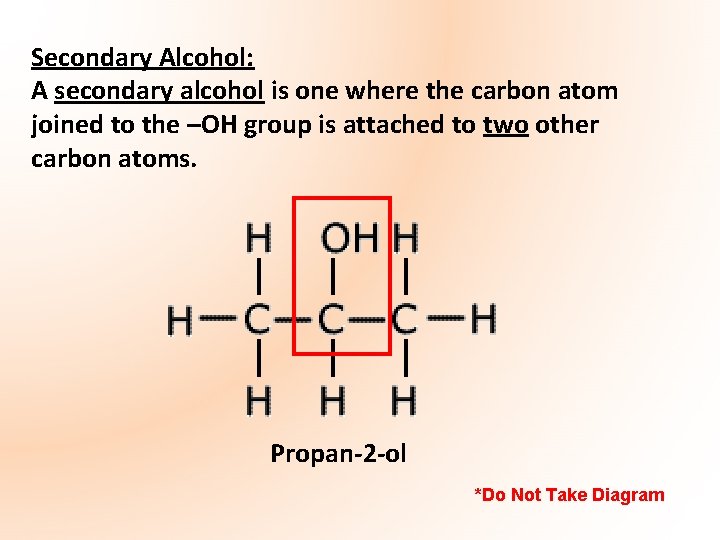
Secondary Alcohol: A secondary alcohol is one where the carbon atom joined to the –OH group is attached to two other carbon atoms. Propan-2 -ol *Do Not Take Diagram

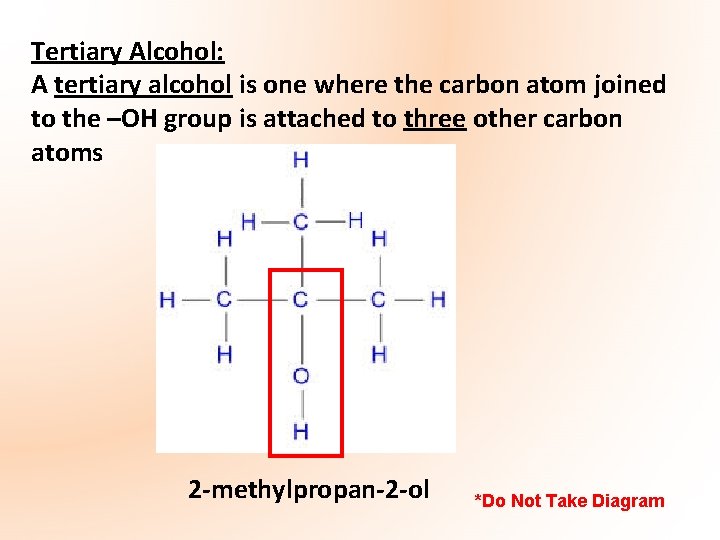
Tertiary Alcohol: A tertiary alcohol is one where the carbon atom joined to the –OH group is attached to three other carbon atoms 2 -methylpropan-2 -ol *Do Not Take Diagram

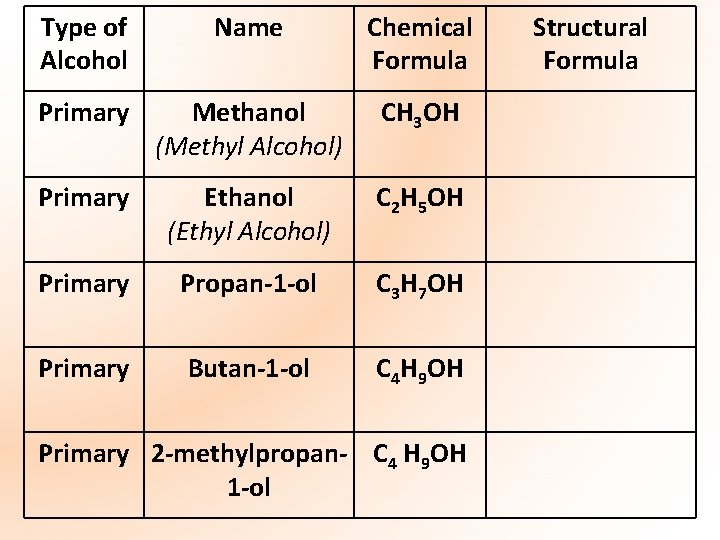
*Important Note* You must be able to name and draw the structure of all the alcohols, primary and secondary, up to C 4 (methanol, propanol, butanol)

Type of Alcohol Name Chemical Formula Primary Methanol (Methyl Alcohol) CH 3 OH Primary Ethanol (Ethyl Alcohol) C 2 H 5 OH Primary Propan-1 -ol C 3 H 7 OH Primary Butan-1 -ol C 4 H 9 OH Primary 2 -methylpropan- C 4 H 9 OH 1 -ol Structural Formula

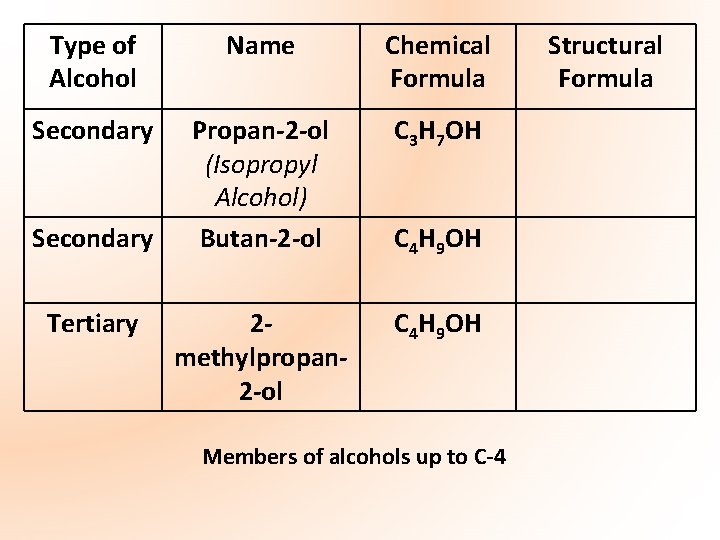
Type of Alcohol Name Chemical Formula Secondary Propan-2 -ol (Isopropyl Alcohol) Butan-2 -ol C 3 H 7 OH 2 methylpropan 2 -ol C 4 H 9 OH Secondary Tertiary C 4 H 9 OH Members of alcohols up to C-4 Structural Formula

Naming and Drawing structural formulas of Alcohols: 1. Draw full structural formula of compound 2. Identify longest continous chain of carbon atoms containing the carbon atom to which the –OH group is attached – parent alkane change ane to anol 3. Number carbon atoms from the end that gives lowest number to the carbon atom to which the -OH group is attached 4. Indicate position of –OH group e. g 2 -butanol 5. Name compound

Example: Name the compounds: a) CH 3 CH 2 CH(OH)CH 3 b) CH 3 CH 2 OH c) CH 3 CH(CH 3)CH 2 OH Student Questions: Book – pg 360 W 22. 4 Workbook – pg 60 W 22. 4

Physical State and Properties of Alcohols Boiling Points: Alcohols have much higher boiling points than alkanes of comparable relative molecular mass: First 4 members of alkanes – gases First 4 members of alcohols – liquids WHY? ? ?

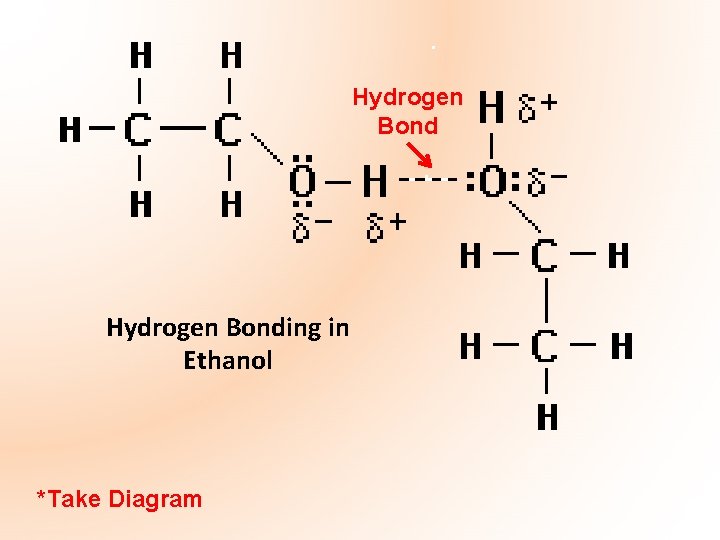
• An alcohol can be regarded as a compound similar to a water molecule with an alkyl group replacing a hydrogen atom • The higher boiling points are due to the fact that the highly polar –OH group gives rise to hydrogen bonding between the alcohol molecules. Oxygen, being more electronegative, has a partial negative charge, and hydrogen has a partial positive charge. The oxygen in the hydroxyl group in one molecule attracts the hydrogen in the hydroxyl group on a neighbouring molecule. • Because of this hydrogen bonding extra energy has to be supplied to break these bonds – hence higher boiling points than corresponding alkanes

Hydrogen Bonding in Ethanol *Take Diagram

Solubility: Methanol, ethanol and propan-1 -ol are completely miscible with water. They are said to be infinitely soluble in water i. e. miscible in all proportions. WHY? ? ? • This solubility is possible because hydrogen bonding occurs between the alcohol molecules and the water molecules. • It is impossible to separate ethanol from water by distillation gives a mixture called 95% ethanol thus to obtain an absolute alcohol sample water must be removed using a chemical drying agent e. g. calcium oxide

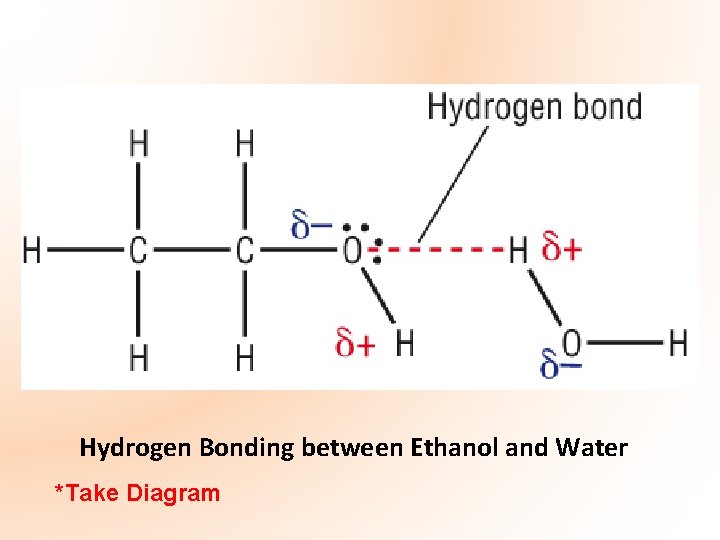
Hydrogen Bonding between Ethanol and Water *Take Diagram

• Solubility of alcohols decreases with length of carbon chain. • The tendency of the polar –OH group to make the alcohol soluble is counteracted by the insoluble alkyl (non-polar) portion of the molecule which becomes more significant as the carbon chain increases. • Thus while the lower members of the alcohols, C 1 – C 3, are completely soluble with water the higher alcohols have poor solubility in water and readily dissolve in solvents like cyclohexane

Occurences and Uses of Alcohols Ethanol is the most commonly known of all alcohols • Ethanol is the alcohol found in alcoholic drinks and is produced by a process known as fermentation. • Ethanol in alcoholic drinks is made by fermentation of sugars in fruits such as grapes – wine or apples – cider. This process involves a series of reactions brought about by enzymes (zymase) contained in yeast. Enzymes break down the sugar to give alcohol and CO 2

• Fermentation is used in brewing industry to produce beer (malted grain) and cider (apples) – 7 -8% v/v. • To produce drinks of higher alcohol concentration fermented liquids are distilled e. g. distillation of wine produces brandy. Other distilled products include: whiskey, gin, vodka, brandy – 40% v/v. • Ethanol (made by fermentation of sugar cane) mixed with petroleum products – used as a motor fuel instead of petrol. • Ethanol is a very good solvent ( solubility properties). It is widely used as a solvent for perfumes, aftershaves, lotions, deodorants, hair sprays.

• Methanol is TOXIC. Methanol along with a purple dye are added to industrial alcohol (ethanol) to prevent people drinking it. Methanol added in this way is called a denaturing agent. • Ethanol is the main component of methylated spirits which is burned in some types of camping stoves.

Planar Carbon Compounds Planar carbon atoms feature in a number of organic families – those whose compounds have a carbon (C=C) or carbon-oxygen (C=O) double bond. The atoms in the double bond are planar, while other carbon atoms in the molecule may be tetrahedral. Presence of double bond means these compounds are unsaturated.

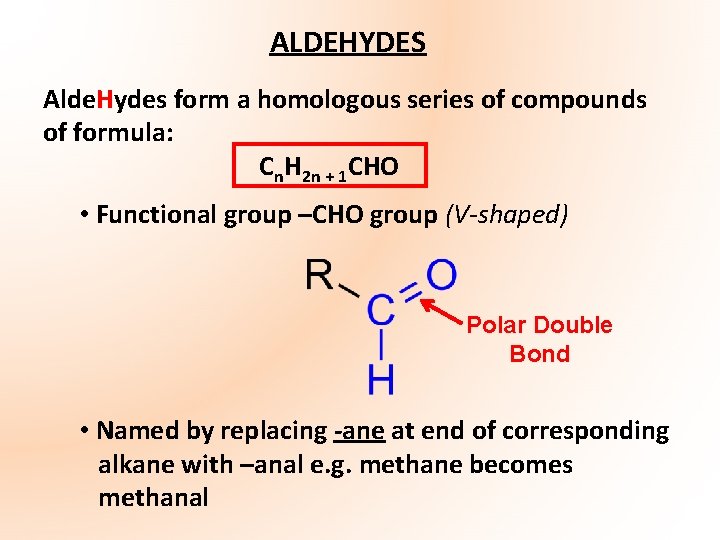
ALDEHYDES Alde. Hydes form a homologous series of compounds of formula: Cn. H 2 n + 1 CHO • Functional group –CHO group (V-shaped) Polar Double Bond • Named by replacing -ane at end of corresponding alkane with –anal e. g. methane becomes methanal

Important Note* You must be able to name and draw the structure of the aliphatc aldehydes up to C-4 (incl. isomers) (methanal, propanal, butanal)

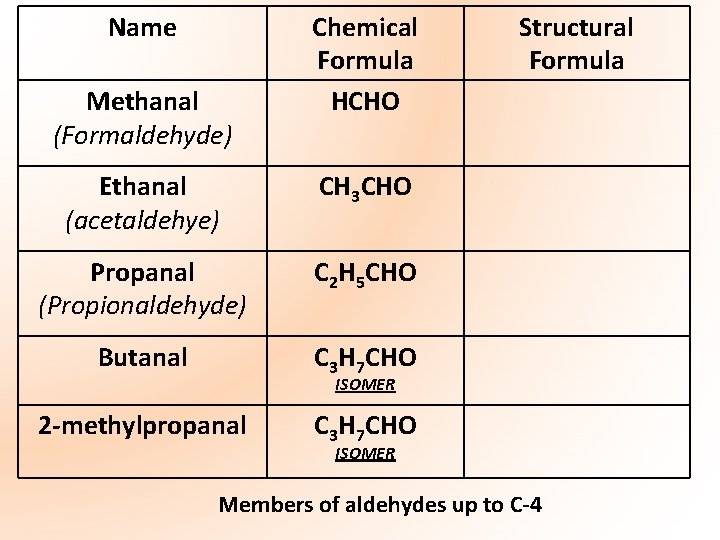
Name Methanal (Formaldehyde) Chemical Formula HCHO Ethanal (acetaldehye) CH 3 CHO Propanal (Propionaldehyde) C 2 H 5 CHO Butanal C 3 H 7 CHO 2 -methylpropanal C 3 H 7 CHO Structural Formula ISOMER Members of aldehydes up to C-4

Naming and Drawing structural formulas of Aldehydes: • Aldehyde functional group (-CHO) must always occur at the end of the carbon chain. • Therefore naming aldehydes is easier as there is no need to use a number to indicate the position of the functional group. • Molecular formula worked out by changing the last carbon in the chain of the alkane from being a part of a CH 3 group, to being part of the aldehyde –CHO group • Must use numbers to indicate position of substituents on carbon chain i. e. 2 -methylpropanal

Example: Write the name, chemical formula and structural formula of the aldehyde containing three carbon atoms.

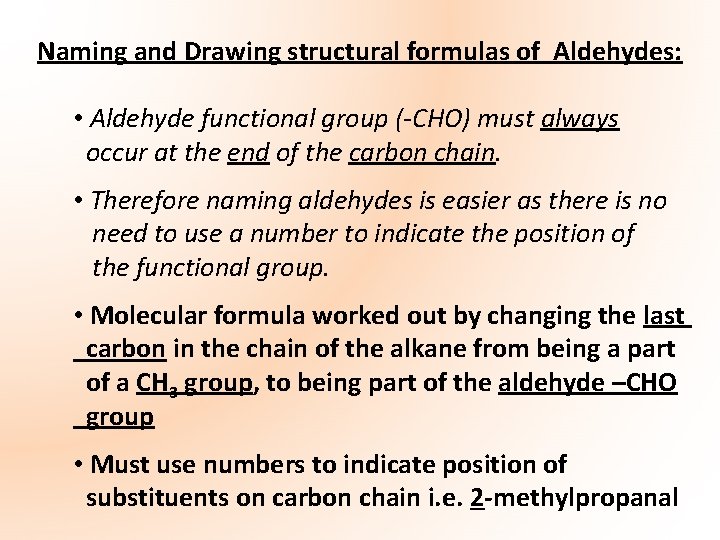
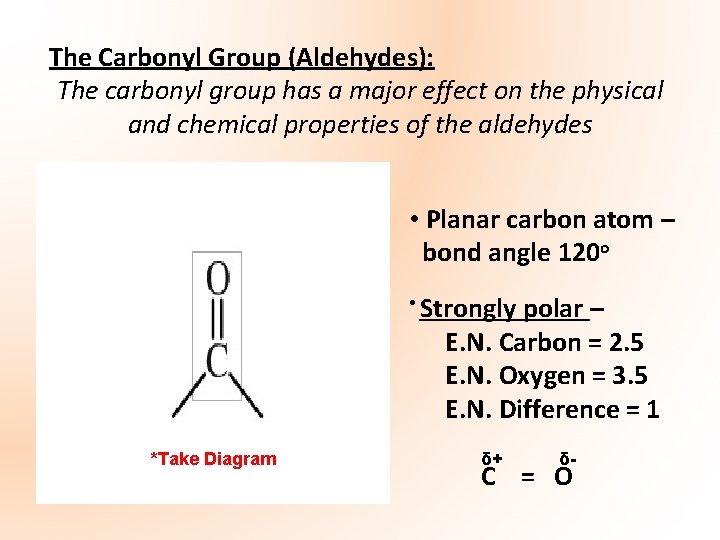
The Carbonyl Group (Aldehydes): The carbonyl group has a major effect on the physical and chemical properties of the aldehydes • Planar carbon atom – bond angle 120 o • Strongly polar – E. N. Carbon = 2. 5 E. N. Oxygen = 3. 5 E. N. Difference = 1 *Take Diagram δ+ δ- C = O

Physical State & Properties of Aldehydes Boiling Points • Due to the polarity of the carbonyl group (C=O) dipole- dipole attractions exist between adjacent aldehyde molecules. • Boiling points are higher than those of corresponding alkanes (because of dipole-dipole forces) but less than those of the corresponding alcohols (which contain H – bonding) – graph pg. 348 e. g. Ethanol – boiling pt = 78 OC Ethanal – boiling pt = 21 OC (volatile) • Methanal – gas at room temperature but other lower members of the series are liquids

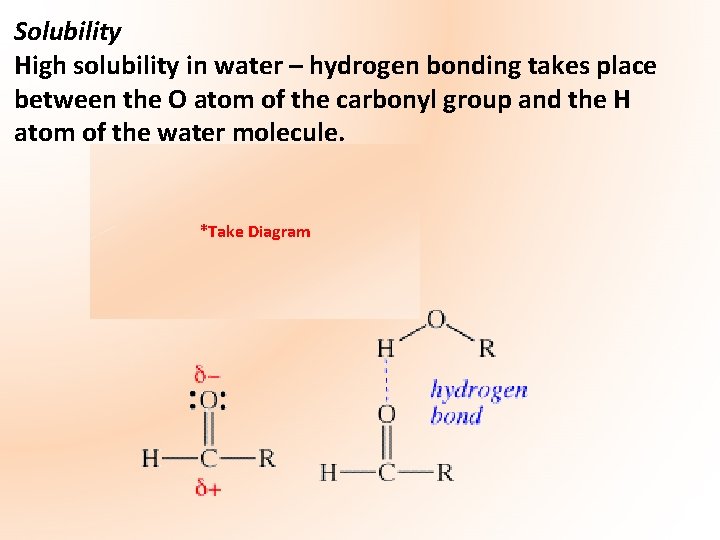
Solubility High solubility in water – hydrogen bonding takes place between the O atom of the carbonyl group and the H atom of the water molecule. *Take Diagram

• The lower members of the aldehydes are very soluble in water and, like the alcohols, will dissolve both polar and non-polar substances. • This solubility in water decreases with the length of the carbon chain i. e. the more non-polar the molecule becomes the less it dissolves • Aldehydes are soluble in non-polar solvents • Aromatic aldehyde: Benzaldehyde • found in almond kernels • manufactured to make almond essence for use in cooking *Do Not Take Diagram

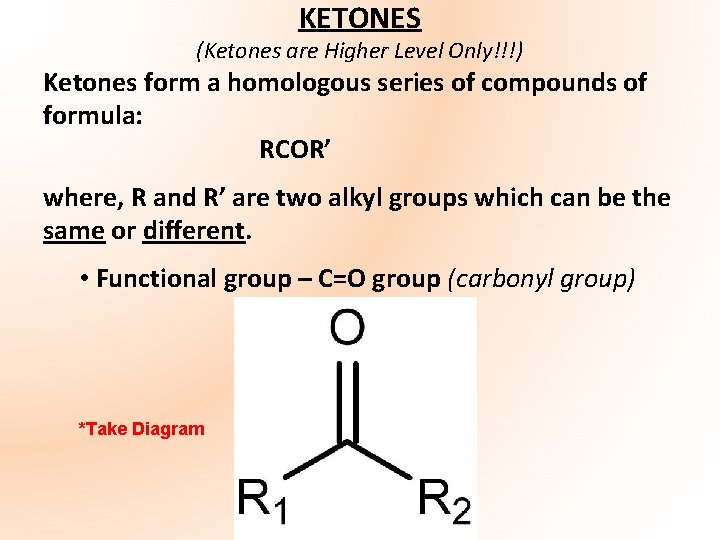
KETONES (Ketones are Higher Level Only!!!) Ketones form a homologous series of compounds of formula: RCOR’ where, R and R’ are two alkyl groups which can be the same or different. • Functional group – C=O group (carbonyl group) *Take Diagram

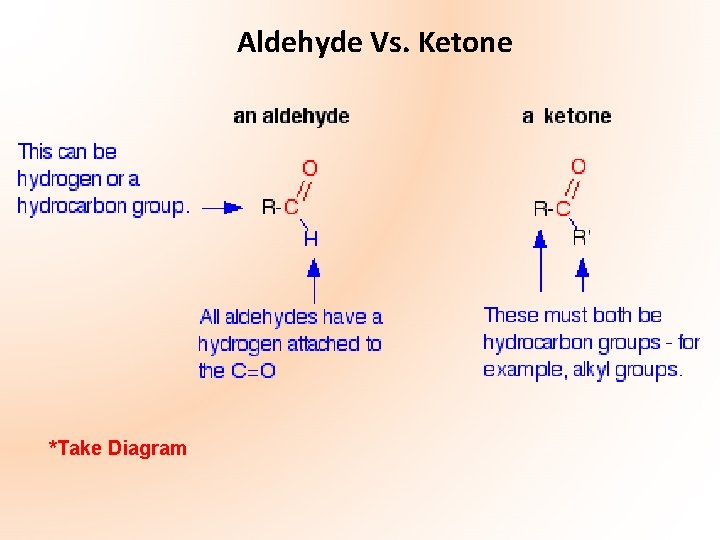
Aldehyde Vs. Ketone *Take Diagram

• Carbonyl carbon – planar carbon • Named by changing the final –e of the parent alkane to –one e. g. propane becomes propanone, butane becomes butanone • Since functional group in ketones has two alkyl groups attached it cannot be located at end of chain i. e. located in middle of chain so must be at least 3 carbon atoms present. • Most common ketone – propanone (acetone) – organic solvent – nail varnish remover

Important Note* You must be able to name and draw the structure of the Ketones up to C-4 i. e. first two members (propanone and butanone)

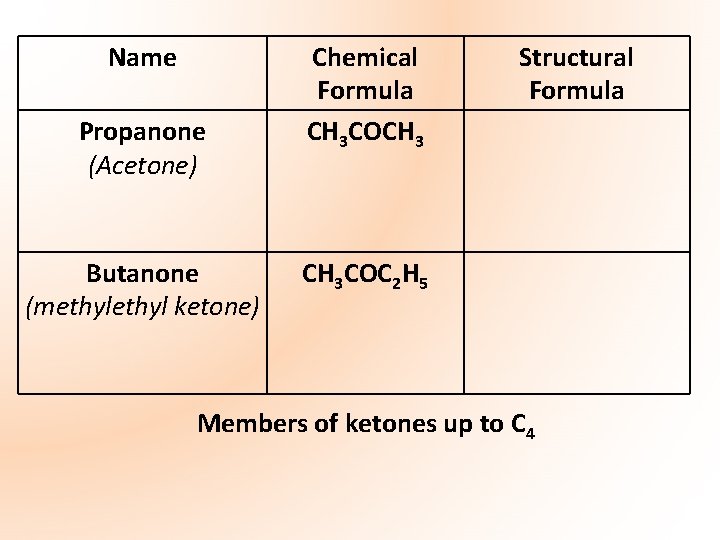
Name Propanone (Acetone) Butanone (methyl ketone) Chemical Formula CH 3 COCH 3 Structural Formula CH 3 COC 2 H 5 Members of ketones up to C 4

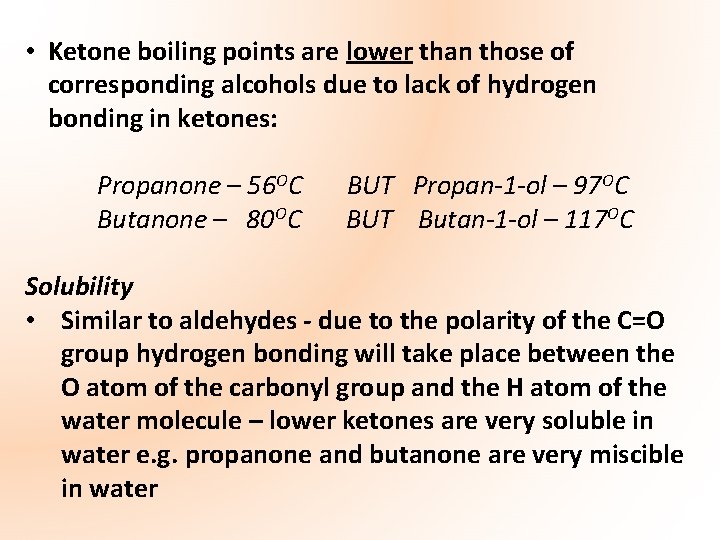
Physical State and Properties of Ketones Physical properties of ketones very similar to those of aldehydes – both contain carbonyl group Boiling Points • Lower ketones are liquids at room temperature • Due to the polarity of the carbonyl group (C=O) dipole-dipole attractions exist between adjacent ketone molecules • Ketone boiling points are higher than those of corresponding alkanes due to dipole-dipole forces: Propane – -42 OC Butane – -0. 5 OC BUT Propanone – 56 OC Butanone – 800 C

• Ketone boiling points are lower than those of corresponding alcohols due to lack of hydrogen bonding in ketones: Propanone – 56 OC Butanone – 80 OC BUT Propan-1 -ol – 97 OC BUT Butan-1 -ol – 117 OC Solubility • Similar to aldehydes - due to the polarity of the C=O group hydrogen bonding will take place between the O atom of the carbonyl group and the H atom of the water molecule – lower ketones are very soluble in water e. g. propanone and butanone are very miscible in water

• All ketones are soluble in organic solvents but since propanone and butanone can act as solvents for both polar and non-polar substances they are widely used as solvents in industry

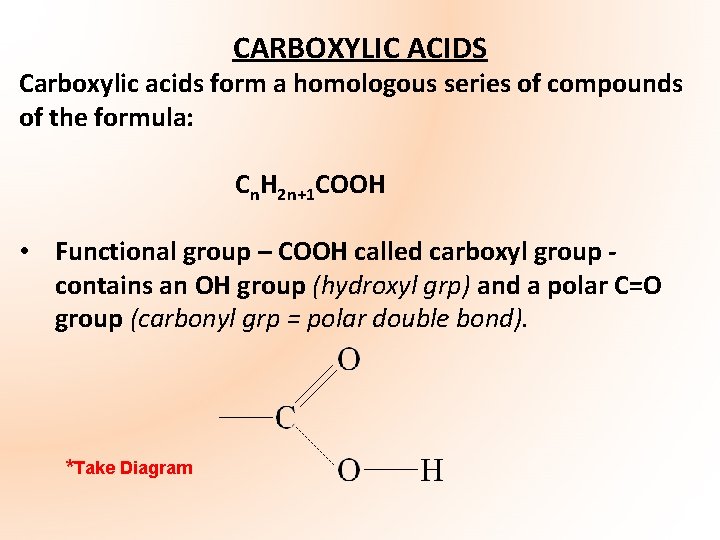
CARBOXYLIC ACIDS Carboxylic acids form a homologous series of compounds of the formula: Cn. H 2 n+1 COOH • Functional group – COOH called carboxyl group contains an OH group (hydroxyl grp) and a polar C=O group (carbonyl grp = polar double bond). *Take Diagram

• Carboxyl carbon atom – in effect is a carbonyl carbon i. e. it is a planar carbon • Carboxylic acids are formed when the last methyl group in the chain of the alkane is replaced with the carboxyl functional group • Carboxylic acids are named by replacing the final – e at the end of the corresponding alkane with –oic acid e. g. methane becomes methanoic acid, ethane becomes ethanoic acid

*Important Note You must be able to name and draw the structure of the carboxylic acids up to C-4 (incl. isomers)

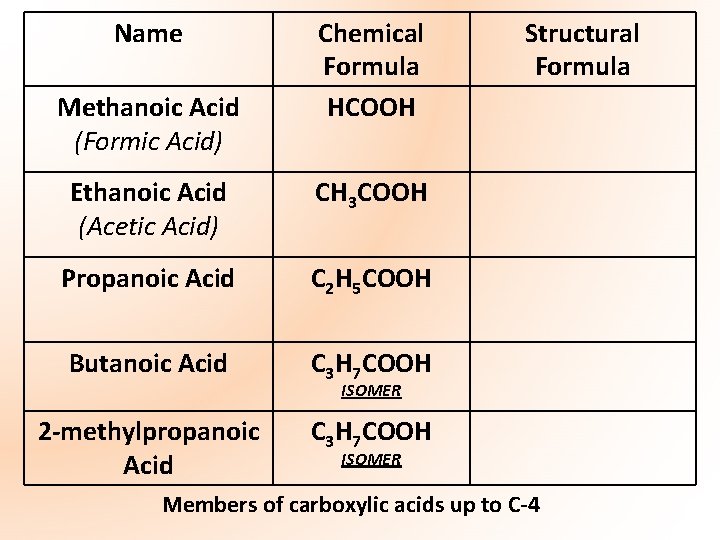
Name Methanoic Acid (Formic Acid) Chemical Formula HCOOH Ethanoic Acid (Acetic Acid) CH 3 COOH Propanoic Acid C 2 H 5 COOH Butanoic Acid C 3 H 7 COOH 2 -methylpropanoic Acid C 3 H 7 COOH Structural Formula ISOMER Members of carboxylic acids up to C-4

Naming and Drawing structural formulas of Carboxylic Acids: 1. Draw full structural formula of compound 2. Identify longest continuous chain of carbon atoms containing the COOH group – parent alkane change -e to -oic acid. 3. Number carbon atoms from the –COOH end. The carbon atom of the carbonyl group is always carbon atom 1. 4. Indicate position of substituents by a number 5. Name compound

Example: Name the compounds: a) CH 3 CH(CH 3)COOH b) CH 3 CCl 2 CH 2 COOH Student Questions – AFTER ESTERS!!!

Physical State and Properties Physical properties of carboxylic acids are governed by their ability to form hydrogen bonds!!! Melting and Boiling Points • Due to INCREASED hydrogen bonding the melting point of pure carboxylic acids is much higher than those of corresponding organic families Ethane Ethanal Ethanoic Acid -183 OC -124 OC -117 OC

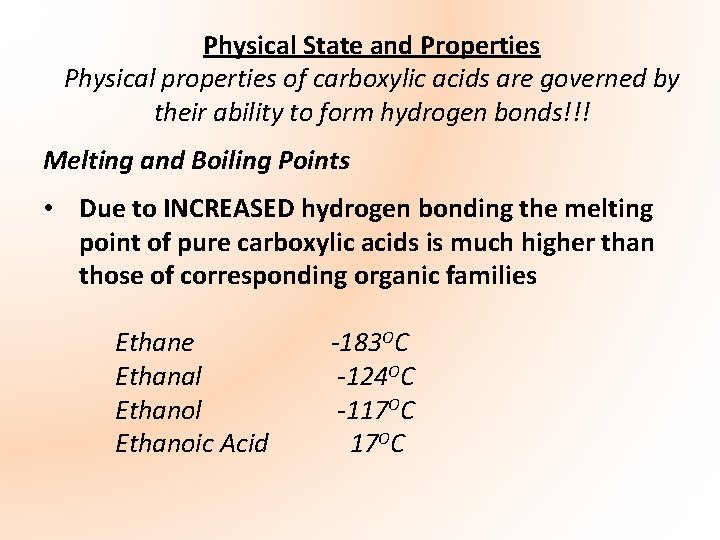
• These relatively high melting points result from the formation of dimers, where two carboxylic acid molecules are held together by two hydrogen bonds. These arise due to the polarity in the C=O bond and the O-H bond. *Note: Dimer – groups of two molecules joined together

• Each H atom (partial positive charge) forms a hydrogen bond with the carbonyl oxygen (partial negative charge) on the neighbouring acid molecule. The reverse also happens resulting in the formation of two hydrogen bonds *Take Diagram

• Pure ethanoic acid is a liquid at room temperature but if temperature drops below 17 OC the ethanoic acid turns into a solid (room now too cold to melt pure ethanoic acid). In its solid from ethanoic acid looks like ice and is known as glacial acetic acid. • Similarly due to INCREASED hydrogen bonding the boiling point of carboxylic acids is even higher than those of corresponding alcohols Ethanol Ethanoic Acid 78 OC 118 OC • Lower carboxylic acids – methanoic, propanoic, butanoic acid are all liquids at room temperature

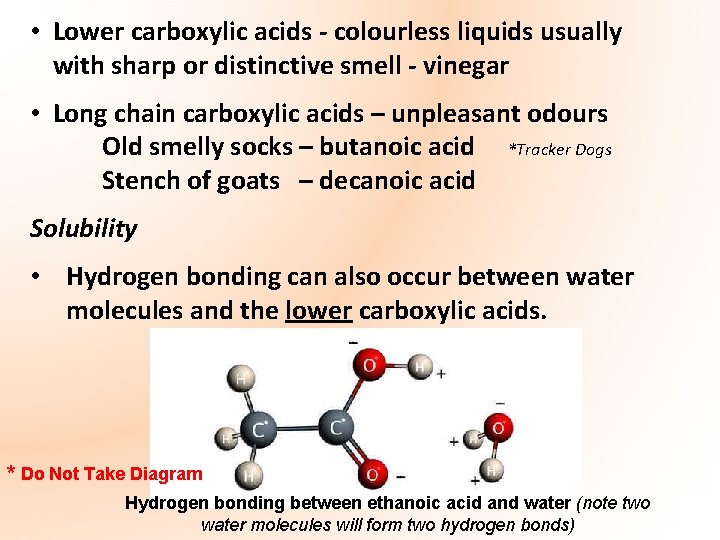
• Lower carboxylic acids - colourless liquids usually with sharp or distinctive smell - vinegar • Long chain carboxylic acids – unpleasant odours Old smelly socks – butanoic acid *Tracker Dogs Stench of goats – decanoic acid Solubility • Hydrogen bonding can also occur between water molecules and the lower carboxylic acids. * Do Not Take Diagram Hydrogen bonding between ethanoic acid and water (note two water molecules will form two hydrogen bonds)

• As a result of this hydrogen bonding acids containing up to four carbon atoms are highly soluble in water. However, as the carbon chain increases in length, the molecule becomes less polar and solubility in water decreases e. g. benzoic acid not very soluble in cold water • Higher carboxylic acid members become more soluble in organic solvents - cyclohexane

Occurrence and Uses of Carboxylic Acids • Methanoic acid is an irritant fluid emitted by ants and also found in nettle stings (Antidote – apply a weak base to affected area – sodium hydrogencarbonate/bread soda) • Ethanoic acid – best known carboxylic acid! Principal acid found in vinegar. It is usually made by the oxidation of ethanol by air. Also used to make cellulose acetate which is used in varnishes and photographic film (Undrinkable wine!!!!)

Higher Level Only • Propanoic acid, benzoic acid (C 6 H 5 COOH) and some of their salts (isodium benzoate) are widely used in the preservation of food

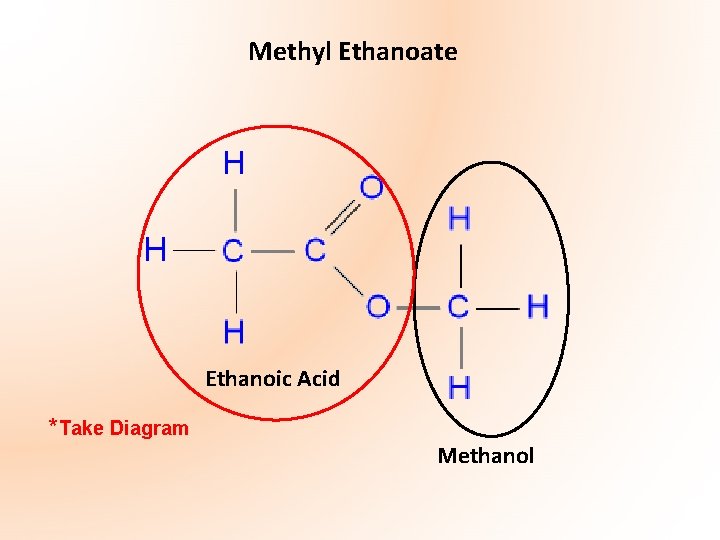
ESTERS (Esters are Higher Level Only!!!) Esters form a homologous series of compounds of formula: RCOOR’ where, R could be a hydrogen atom or an alkyl group and R’ is an alkyl group. • Functional group – -COO- group (contains planar carbon in the form C=0) • Consist of two parts – R’ alkyl group derived from an alcohol - RCOO- derived from a carboxylic acid

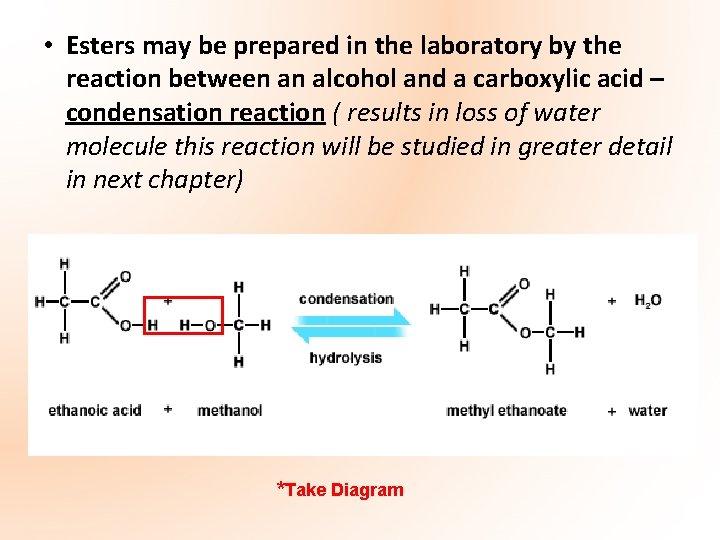
Methyl Ethanoate Ethanoic Acid *Take Diagram Methanol

• Esters may be prepared in the laboratory by the reaction between an alcohol and a carboxylic acid – condensation reaction ( results in loss of water molecule this reaction will be studied in greater detail in next chapter) *Take Diagram

Naming Esters 2 parts to the name: - first part is alkyl group derived from the alcohol - second part is the name of the acid, with the ending –oic replaced with -oate

*Important Note You must be able to name and draw the structure of the carboxylic acids up to C-4

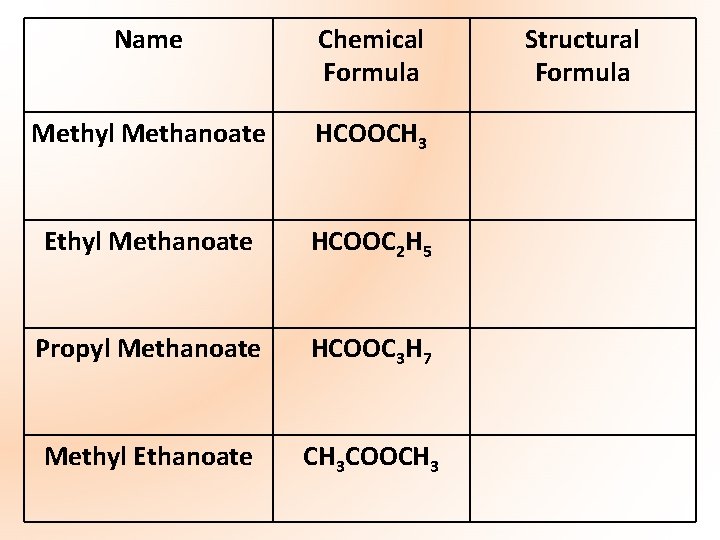
Name Chemical Formula Methyl Methanoate HCOOCH 3 Ethyl Methanoate HCOOC 2 H 5 Propyl Methanoate HCOOC 3 H 7 Methyl Ethanoate CH 3 COOCH 3 Structural Formula

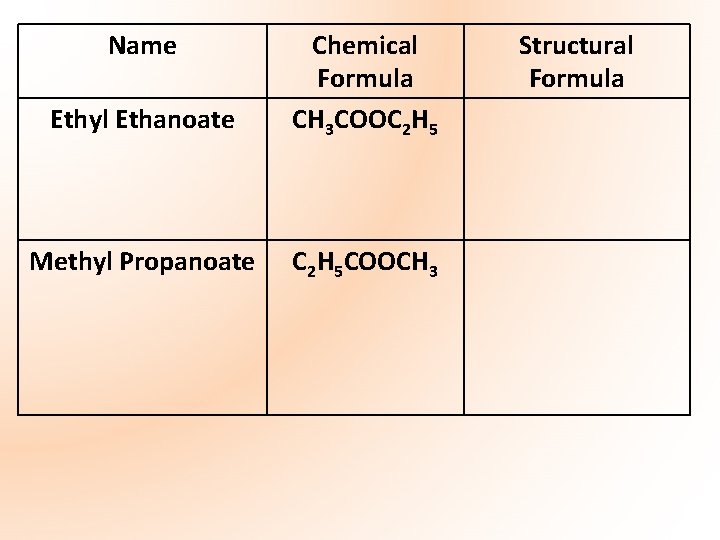
Name Ethyl Ethanoate Chemical Formula CH 3 COOC 2 H 5 Methyl Propanoate C 2 H 5 COOCH 3 Structural Formula

Naming and Drawing structural formulas of Esters: 1. Draw full structural formula of ester 2. Divide structural formula into two parts: - alcohol part - carboxylic acid part 3. Write down name of alkyl group attached to bridging oxygen atom 4. Identify carboxylic acid and change -oic ending to -oate 5. Name compound putting name of alkyl group first.

Example: Name the compound: a) HCOOCH 2 CH 3 Student Questions: Book – pg 360 22. 6 Workbook – pg 61 W 22. 6

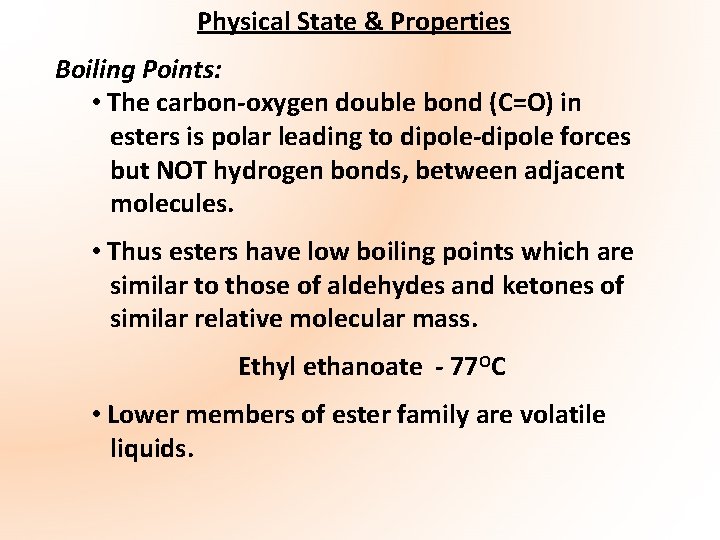
Physical State & Properties Boiling Points: • The carbon-oxygen double bond (C=O) in esters is polar leading to dipole-dipole forces but NOT hydrogen bonds, between adjacent molecules. • Thus esters have low boiling points which are similar to those of aldehydes and ketones of similar relative molecular mass. Ethyl ethanoate - 77 OC • Lower members of ester family are volatile liquids.

Solubility: • The polarity of the C=O group allows hydrogen bonding to occur with water molecules. • The lower members of the ester family (up to C-5) are fairly soluble in water. • However, the solubility of the esters decreases as the length of the carbon chain increases. • Esters are soluble in organic solvents (non-polar)

Occurence and Uses of Esters • Members of the ester family have strong and often pleasant, fruity smells. Many esters occur naturally and are responsible for the flavour in fruits and the smells of flowers • Polyester – refers to a type of fabric used in clothing etc. – consists of millions of esters linked together. • Fats and oils are naturally occurring esters. They are used by plants and animals for storing energy. Fat - solid ester Oil - liquid ester

• Fats and oils are esters which are insoluble in water but soluble in organic solvents. They usually consist of an alcohol named glycerol and various long chain carboxylic acids (fatty acids) e. g. Ester - Glycerl Tristearate Alcohol - Glycerol Fatty Acid – Stearic Acid (3) * Note: Glycerol is the only alcohol with more than one OH group on this course • Fats and oils – used in manufacturing soap

• Ethyl Ethanoate- used as a solvent for printing inks and paints. Short Experiment: Test – Tube Preparation of Esters

Aromatic Compounds In chapter 21 we learned that any compound containing a benzene ring is called an aromatic compound, with benzene itself being the most important. Benzene

Bonding in Benzene (Brief): Although it was expected that benzene would have three double bonds its lack of reactivity suggested otherwise. It was discovered that the bonds are actually intermediate between single and double bonds. When bond lengths were measured it was expected that: C-C - 0. 154 nm C=C - 0. 13 nm However, all carbon –carbon bond lengths were the same measuring 0. 139 nm i. e. between that of a double and single bond. Hence, it was suggested that the 6 valence electrons, one from each carbon atom, belonged to the whole molecule (delocalised) rather than being localised in 3 double bonds.

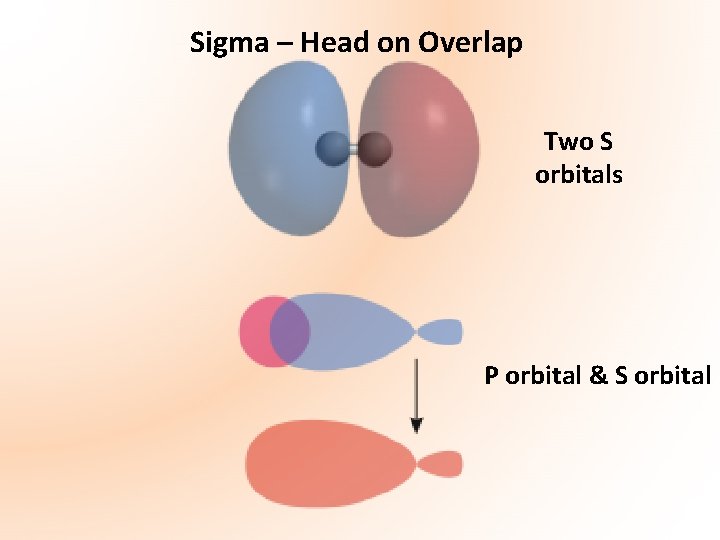
Remember: Sigma Bond – head on overlap of two atomic orbital's Pi Bond – sideways overlap of two atomic orbital's *Do Not Take Diagrams

Bonding in Benzene – Detail: The benzene molecule consists of six carbon atoms joined to form a hexagonal planar ring. Each carbon atom (1 s 2, 2 p 2) has four electrons in its outer shell: 1 e-- used to bond with hydrogen - sigma 1 e- - used to bond carbon and its adjacent carbon (left) – sigma 1 e- - used to bond carbon and its adjacent carbon (right) - sigma

Sigma – Head on Overlap Two S orbitals P orbital & S orbital

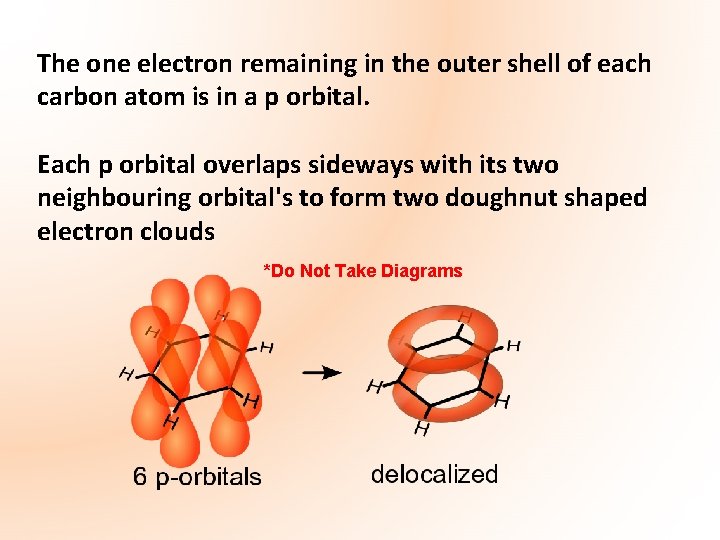
The one electron remaining in the outer shell of each carbon atom is in a p orbital. Each p orbital overlaps sideways with its two neighbouring orbital's to form two doughnut shaped electron clouds *Do Not Take Diagrams

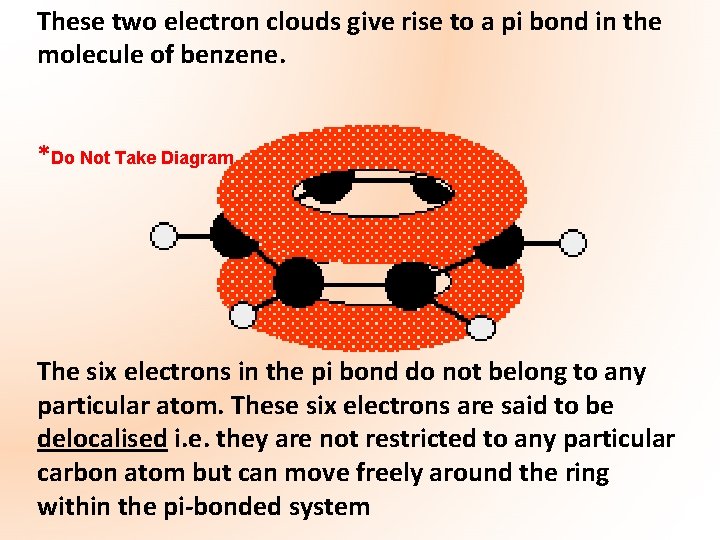
These two electron clouds give rise to a pi bond in the molecule of benzene. *Do Not Take Diagram The six electrons in the pi bond do not belong to any particular atom. These six electrons are said to be delocalised i. e. they are not restricted to any particular carbon atom but can move freely around the ring within the pi-bonded system

Physical Properties: Benzene is a planar, non-polar molecule. Benzene is a carcinogenic. Methylbenzene (benzene substitute) is widely used: - as an industrial solvent - in manufacture of plastics and explosives - added to petrol to improve octane rating While benzene is a carcinogenic there are many aromatic compounds that are not and which play an important role in our everyday lives

Aromatic Compounds and their Uses: Aromatic compounds are in widespread use in a range of different applications. They are used in manufacture of: - medicine - painkillers - dyes - detergents - insecticides - herbicides - disinfectants - acid-base indicators – methyl orange - phenolphthalein

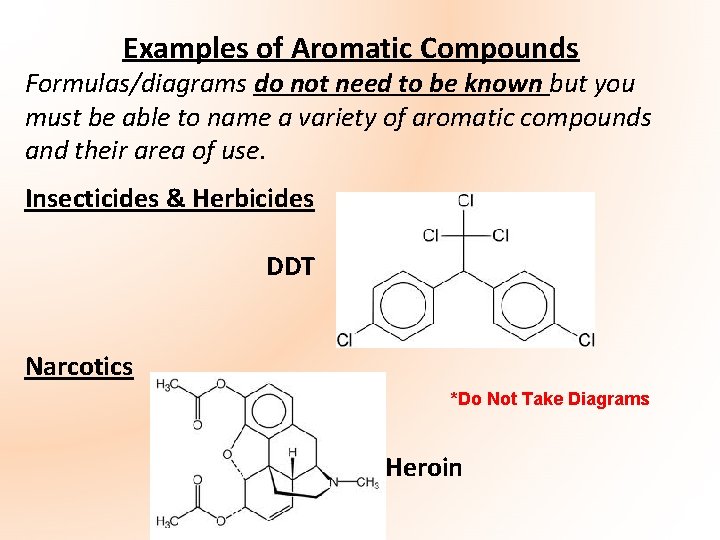
Examples of Aromatic Compounds Formulas/diagrams do not need to be known but you must be able to name a variety of aromatic compounds and their area of use. Insecticides & Herbicides DDT Narcotics *Do Not Take Diagrams Heroin

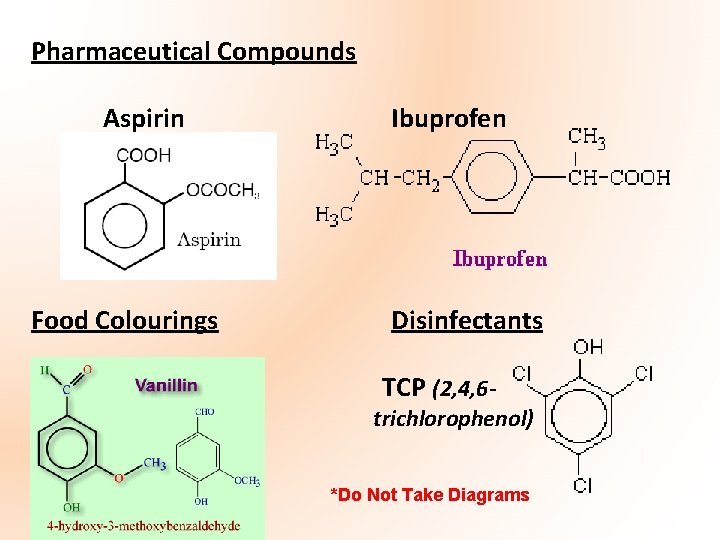
Pharmaceutical Compounds Aspirin Food Colourings ` Ibuprofen Disinfectants TCP (2, 4, 6 - trichlorophenol) *Do Not Take Diagrams

Demonstration: To Investigate the Solubility of Organic Compounds

Organic Natural Products (Know 2 examples) A natural product is any chemical produced in nature, either by plants or by animals Examples: - Benzaldehyde – almomds - Caffeine – tea/coffee - Nicotine – tobacco - Opium – poppies - Limonene – orange peel - Quinine – bark of cinchona trees - Penicillin – bread mould

Many of these natural products were isolated for their use as valuable medicines: - quinine (malaria) - morphine - paracetamol While others were isolated for their poisonous properties: - strychnine (rat poison) Natural products may also be isolated for their fragrant oils which can be used in perfume making

At the initial time of discovery of many of these substances it was not possible to determine the structure of their molecules and therefore they could not be synthesised in the lab. With modern developments in analytical techniques it is now possible to determine the molecular structure and to synthesise these compounds in the laboratory Synthesis of these natural products is important as they may be difficult to obtain otherwise due to seasonal nature of plants, weather conditions etc. and the fact that their medicinal properties may be improved upon.

Remember: Natural products may also be isolated for their fragrant oils which can be used in perfume making.

Steam Distillation Definition Steam Distillation: is a separation process used to isolate compounds at temperatures below their decomposition temperatures. It is carried out by bubbling steam through the material and distilling off the immiscible liquids e. g. – extracting oil from roses, lavender, cloves (eugenol) This technique allows organic substances to be isolated that, if heated on their own to higher temperatures, might partially decompose.

Principle of Steam Distillation: is that a mixture of two immiscible liquids boils at a temperature that is below that of the boiling point of each of the individual liquids. The hot mixture of water vapour (from steam) and oil (from plant) is passed through a condenser and the distillate that is collected contains a mixture of water and oil.

Vapor Pressure & Boiling Point • Vapor pressure is the pressure exhibited by vapor present above its liquid surface. • Boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas (atmospheric gases) above it i. e. normal boiling point of a liquid is at atmospheric pressure - 1 atm or 760 Torr • If you increase the vapor pressure of your liquid, you will hit atm. pressure faster and less temperature will be needed for boiling to occur. Thus, boiling point will decrease as vapor pressure increases

Steam Distillation – How it Works? ? ? • If two immiscible liquids A & B are kept stirred, the vapour pressure above the mixture is the total of the two separate vapour pressures i. e. Total Vapour = Vapour Pressure + Vapour Pressure of A of B • When this mixture is heated the vapour pressure of both liquids (total vapour pressure) will increase and when the total vapour pressure reaches atmospheric pressure the mixture will boil.

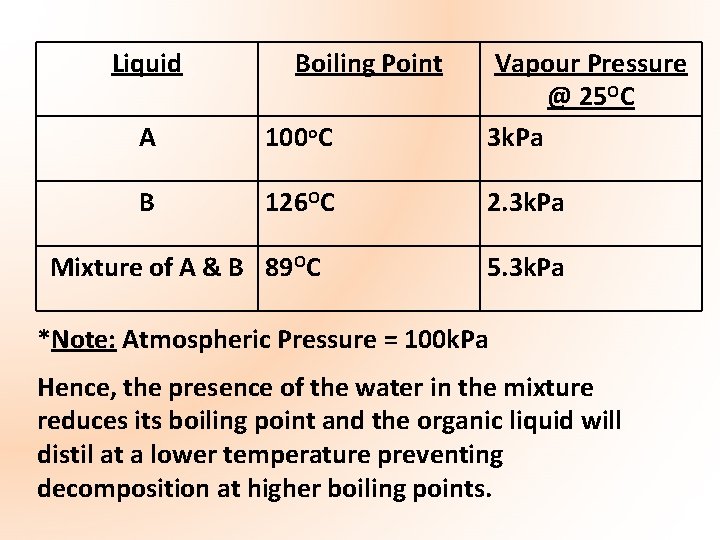
Liquid Boiling Point A 100 o. C Vapour Pressure @ 25 OC 3 k. Pa B 126 OC 2. 3 k. Pa Mixture of A & B 89 OC 5. 3 k. Pa *Note: Atmospheric Pressure = 100 k. Pa Hence, the presence of the water in the mixture reduces its boiling point and the organic liquid will distil at a lower temperature preventing decomposition at higher boiling points.

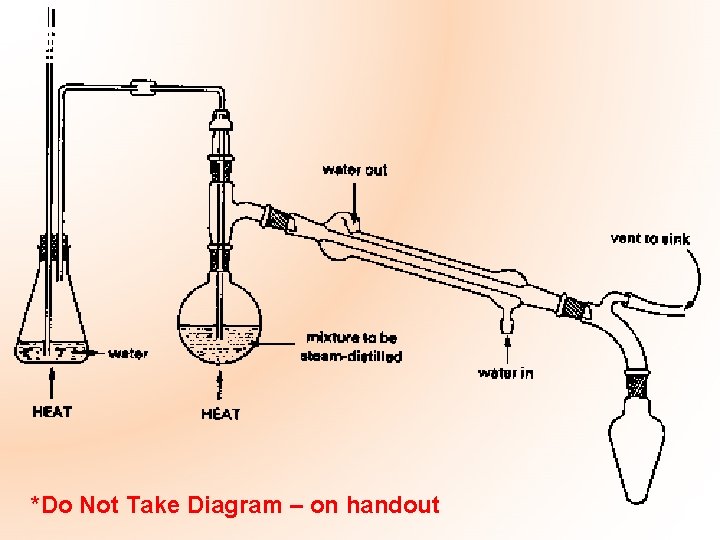
*Do Not Take Diagram – on handout

*Do Not Take Diagram – on handout

• The distillate produced is a mixture of the isolated organic component A and liquid B (water). • The mixture obtained is called an emulsion. An emulsion is a dispersion of small droplets of one liquid in another liquid in which it is not soluble • To obtain a pure sample of the organic component A, a technique called solvent extraction is used

Liquid-liquid extraction (Solvent extraction) • Process: this is a process in which two immiscible liquids (e. g. clove oil and water) are separated using a solvent (e. g. cyclohexane) in which one of the components of the mixture (e. g. oil of cloves) has a higher solubility than the other (e. g. water), i. e. the organic component is removed from the emulsion by dissolving this organic component in an organic solvent. • Principle: this extraction technique is based on the principle of solubility. Oil of cloves is non-polar and easily dissolves in the non-polar solvent, cyclohexane, unlike water which is polar - insoluble

Mandatory Experiment: The extraction & isolation of clove oil from cloves by steam distillation. Please see handout re: important points to note!!!!
 Families of organic compounds
Families of organic compounds Chpt er
Chpt er Chpt er
Chpt er Questionnaire design process chpt 11
Questionnaire design process chpt 11 Questionnaire design process chpt 11
Questionnaire design process chpt 11 Big families vs small families
Big families vs small families Ionic vs covalent vs metallic
Ionic vs covalent vs metallic Thanksgiving facts
Thanksgiving facts Meaning of the word organic
Meaning of the word organic Vitamins are organic compounds
Vitamins are organic compounds Four types of organic molecules
Four types of organic molecules Quantitative analysis of organic compounds ppt
Quantitative analysis of organic compounds ppt Organic vs inorganic compounds
Organic vs inorganic compounds All organic compounds must contain the element
All organic compounds must contain the element Is hagfish slime a lipid
Is hagfish slime a lipid Vitamins are organic compounds that
Vitamins are organic compounds that Pyranoses
Pyranoses Organic vs inorganic compounds
Organic vs inorganic compounds Biochemistry
Biochemistry Type of organic compounds
Type of organic compounds Decomposition of organic compounds
Decomposition of organic compounds What is the classification of organic compounds
What is the classification of organic compounds Organic compounds must contain:
Organic compounds must contain: Organic compounds
Organic compounds Organic compounds grade 10 life science
Organic compounds grade 10 life science Stamatitis
Stamatitis Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Dumas method
Dumas method Which group is carbon in
Which group is carbon in Priority of functional groups in iupac nomenclature
Priority of functional groups in iupac nomenclature Condensed structural formula
Condensed structural formula Organic halogen compounds
Organic halogen compounds Are vitamins organic compounds
Are vitamins organic compounds All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________. Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Combustion of organic substances
Combustion of organic substances Classification of hydrocarbons
Classification of hydrocarbons Principle of distillation
Principle of distillation Intro to organic chemistry
Intro to organic chemistry Some, any, no exercises
Some, any, no exercises No compounds
No compounds Some trust in horses
Some trust in horses They say it only takes a little faith
They say it only takes a little faith Force and motion
Force and motion Fire and ice diamante poem
Fire and ice diamante poem God when you choose to leave mountains unmovable
God when you choose to leave mountains unmovable Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Pear countable or uncountable
Pear countable or uncountable Linguistic refuge area
Linguistic refuge area Out of 800 families with 4
Out of 800 families with 4 Successful marriages and families
Successful marriages and families Military families
Military families Old world language families
Old world language families The simpsons text
The simpsons text Safe families database
Safe families database Orchestra seating chart
Orchestra seating chart Families professionals and exceptionality
Families professionals and exceptionality Families can be together forever song
Families can be together forever song Logic families
Logic families Dc healthy families
Dc healthy families What are the characteristics of a strong family
What are the characteristics of a strong family The alto in the family of woodwind instruments
The alto in the family of woodwind instruments Family solutions essex
Family solutions essex Logic families
Logic families Romeo and juliet two households
Romeo and juliet two households Compare and contrast the bach and marsalis families
Compare and contrast the bach and marsalis families Destroy the family
Destroy the family Effective support for families in essex
Effective support for families in essex Connecting families bath
Connecting families bath Families of elements
Families of elements Chapter 10 western musical instruments
Chapter 10 western musical instruments Periodic table families
Periodic table families Kinetic letters families
Kinetic letters families Compare and contrast the bach and marsalis families
Compare and contrast the bach and marsalis families Families on the periodic table
Families on the periodic table Southwest airlines
Southwest airlines Job framework redesign
Job framework redesign Designing product families
Designing product families Healthy families new york
Healthy families new york Word families examples
Word families examples Dugdale
Dugdale 3-2 families of graphs answers
3-2 families of graphs answers The eight families
The eight families Families professionals and exceptionality
Families professionals and exceptionality Csefel positive solutions for families
Csefel positive solutions for families Edsby blyth
Edsby blyth Work and families act 2006
Work and families act 2006 Periodic table families project
Periodic table families project Georgia department of children and families
Georgia department of children and families 74 logic family
74 logic family Nucleus periodic table
Nucleus periodic table Building respectful families
Building respectful families Effective support for children and families in essex
Effective support for children and families in essex Action for prisoners families
Action for prisoners families Personal facet
Personal facet Glumaceae
Glumaceae Serving individuals and families
Serving individuals and families Connecting families
Connecting families Germanic romance slavic
Germanic romance slavic Marriages and families changes choices and constraints
Marriages and families changes choices and constraints Families of graphs
Families of graphs Periodic table of elements families
Periodic table of elements families Circuit families in vlsi
Circuit families in vlsi Social family model
Social family model Carbon family
Carbon family Kinetic letters families
Kinetic letters families