Organic Compounds Chapter 24 Organic Compounds n n




























- Slides: 62

Organic Compounds Chapter 24

Organic Compounds n n Section 1 - Simple Organic Compounds slides 3 -20 Section 2 - Other Organic Compounds slides 21 -32 Section 3 - Petroleum- A Source of Carbon Compounds slides 33 -44 Section 4 - Biological Compounds slides 45 -62

Section 1 Simple Organic Compounds n n What You’ll Learn: About organic and inorganic carbon compounds Difference between saturated and unsaturated hydrocarbons Identify isomers

1 Simple Organic Compounds n n n Most compounds that contain the element carbon are organic compounds made by living organisms or synthesized in laboratories. More than 90 % of carbon compounds are organic. Others like carbon dioxide and carbonates are inorganic compounds.

Why does carbon form so many organic compounds? n n n With 4 electrons in its outer energy level, carbon can form one covalent bond with each of these electrons. There are many C compounds because C can form so many bonds. Some are small like the ones used as fuel while some are complex like those in medicine and plastics.

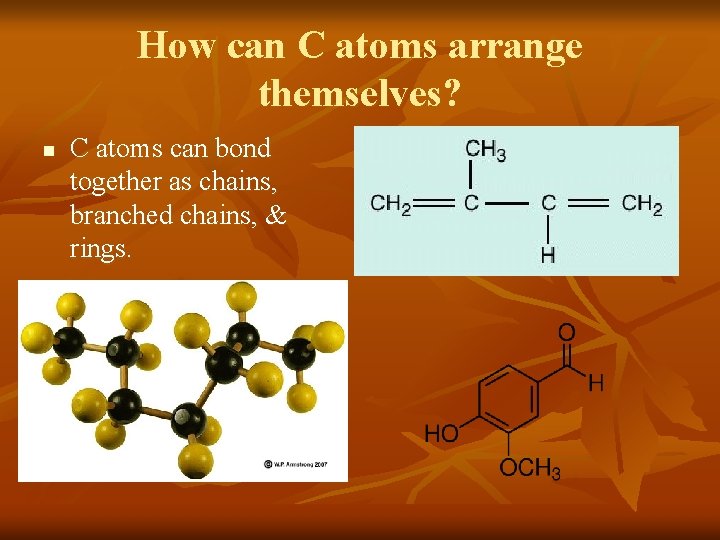
How can C atoms arrange themselves? n C atoms can bond together as chains, branched chains, & rings.

How can C atoms arrange themselves? n n The first structure shows carbon bonded in a straight chain as heptane, an organic compound in gasoline. The second structure, a branched chain, shows isoprene, an organic compound in natural rubber. The third structure, a cyclic ring or chain, is vanillin from vanilla flavoring. Also forms single, double or triple covalent bonds.

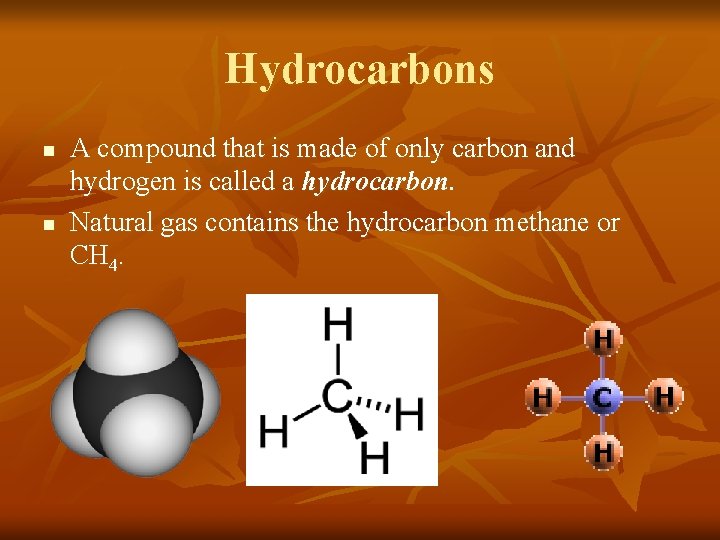
Hydrocarbons n n A compound that is made of only carbon and hydrogen is called a hydrocarbon. Natural gas contains the hydrocarbon methane or CH 4.

Single Bonds n Hydrocarbons with only single-bonded C atoms are called saturated hydrocarbons. It is saturated because each C is bonded to as many H as possible.

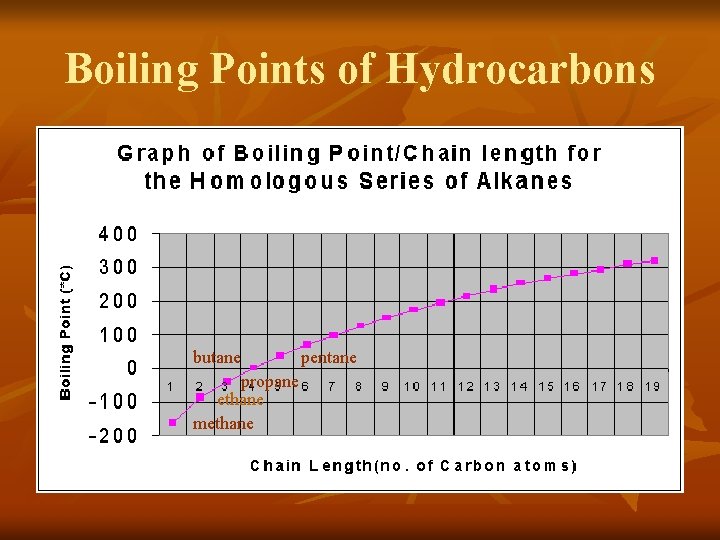
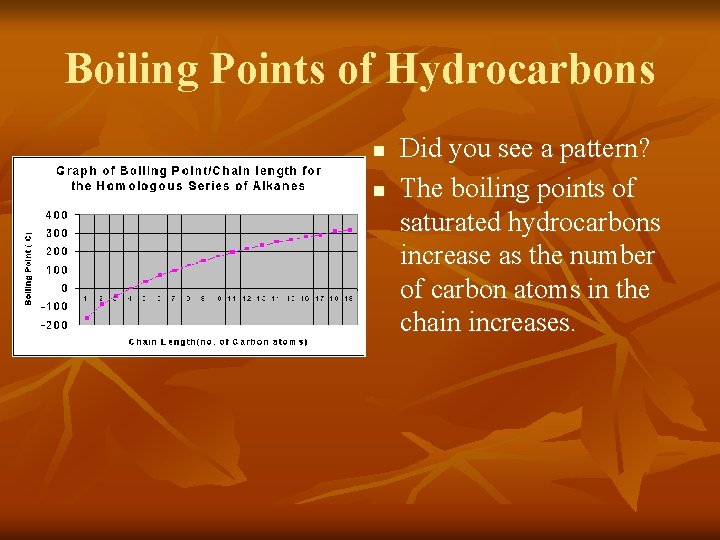
Boiling Points of Hydrocarbons butane propane ethane methane pentane

Boiling Points of Hydrocarbons n n Did you see a pattern? The boiling points of saturated hydrocarbons increase as the number of carbon atoms in the chain increases.

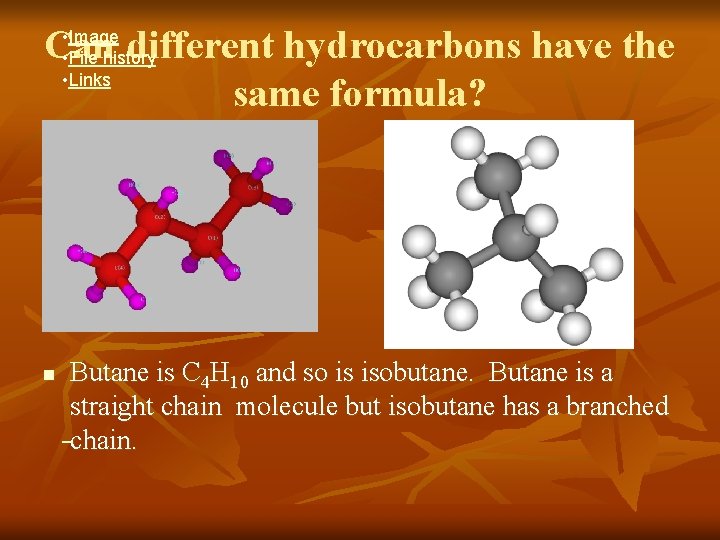
Can different hydrocarbons have the same formula? • Image • File history • Links n Butane is C 4 H 10 and so is isobutane. Butane is a straight chain molecule but isobutane has a branched chain.

What are isomers? n n n Isomers are compounds that have the same chemical formula, but have different molecular structures & shapes. Thousands of hydrocarbons are isomers. Butane & isobutane are two of them.

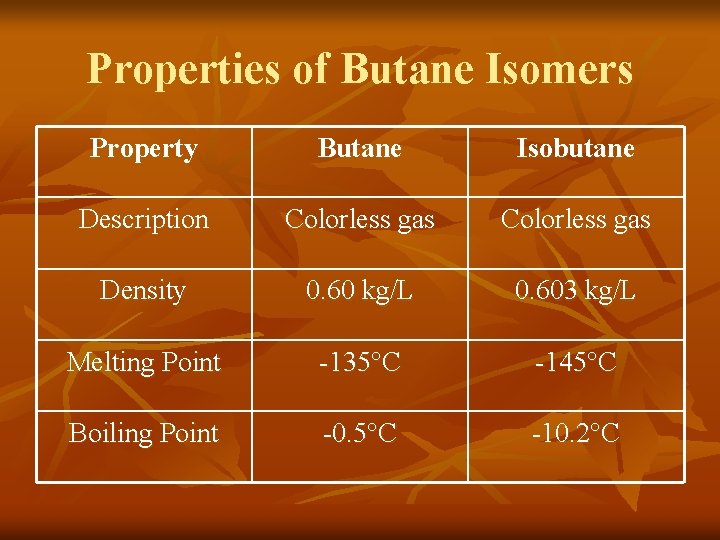
Properties of Butane Isomers Property Butane Isobutane Description Colorless gas Density 0. 60 kg/L 0. 603 kg/L Melting Point -135°C -145°C Boiling Point -0. 5°C -10. 2°C

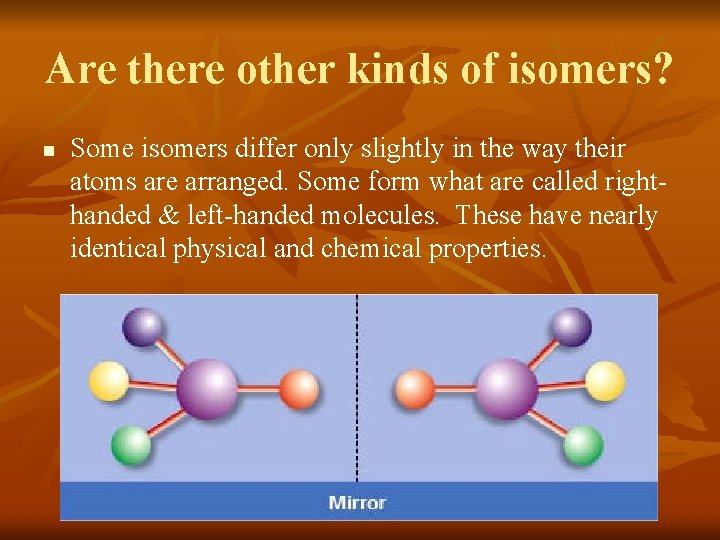
Are there other kinds of isomers? n Some isomers differ only slightly in the way their atoms are arranged. Some form what are called righthanded & left-handed molecules. These have nearly identical physical and chemical properties.

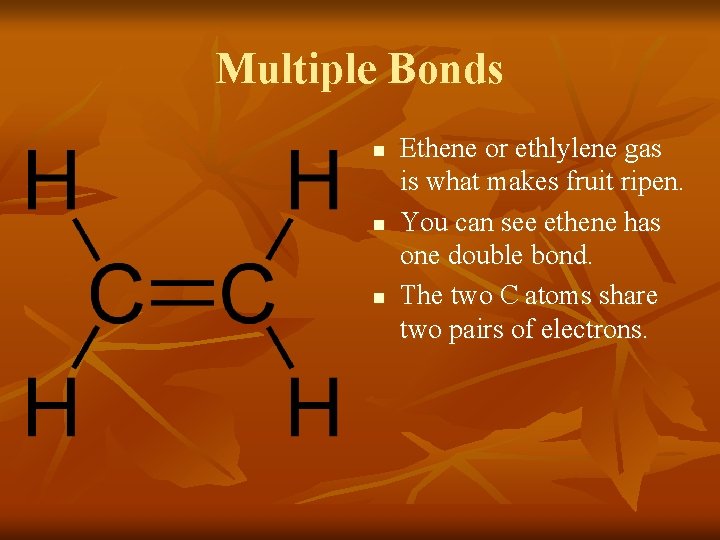
Multiple Bonds n n n Ethene or ethlylene gas is what makes fruit ripen. You can see ethene has one double bond. The two C atoms share two pairs of electrons.

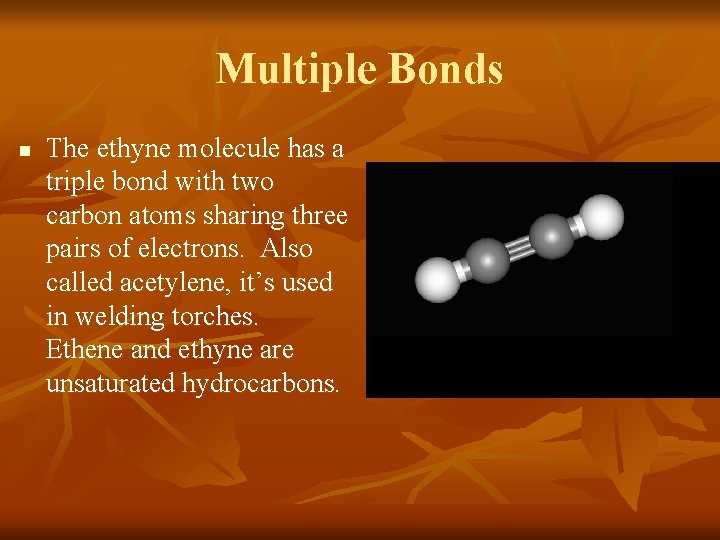
Multiple Bonds n The ethyne molecule has a triple bond with two carbon atoms sharing three pairs of electrons. Also called acetylene, it’s used in welding torches. Ethene and ethyne are unsaturated hydrocarbons.

Multiple Bonds n n An unsaturated hydrocarbon is one that has at least one double bond or triple bond. The compounds are unsaturated because each carbon atom is not bonded to as many hydrogens as possible. The last three letters tell what type of bond is in the molecule. Compounds ending in –ane have only single bonds. Those with –ene have at least one double bond and –yne signifies at least one triple bond.

Flow Chart Organic compounds Contain only C & H are called Straight chains _________ chains Bond as Single bonds ______ bonds

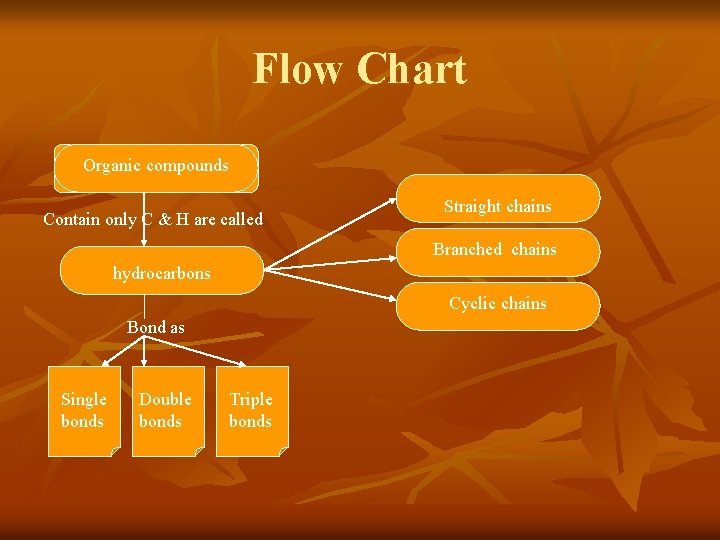
Flow Chart Organic compounds Contain only C & H are called Straight chains Branched chains hydrocarbons Cyclic chains Bond as Single bonds Double bonds Triple bonds

Section 2 Other Organic Compounds n n What You’ll Learn: What aromatic compounds are What alcohols and acids are Some organic compounds you use everyday

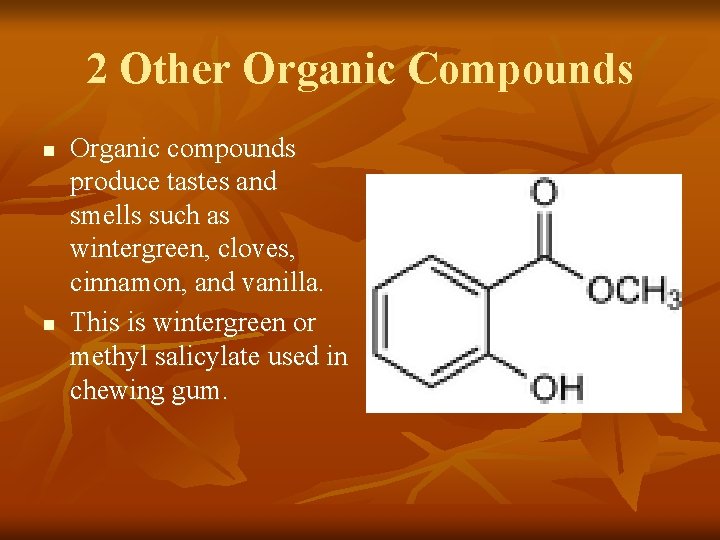
2 Other Organic Compounds n n Organic compounds produce tastes and smells such as wintergreen, cloves, cinnamon, and vanilla. This is wintergreen or methyl salicylate used in chewing gum.

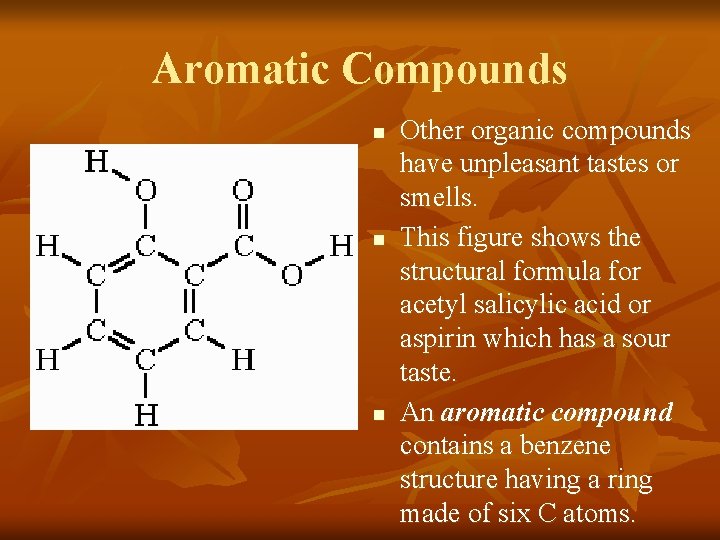
Aromatic Compounds n n n Other organic compounds have unpleasant tastes or smells. This figure shows the structural formula for acetyl salicylic acid or aspirin which has a sour taste. An aromatic compound contains a benzene structure having a ring made of six C atoms.

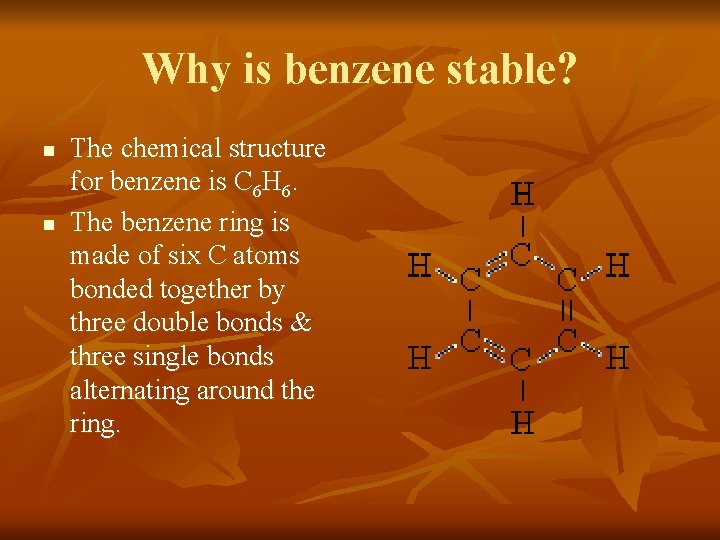
Why is benzene stable? n n The chemical structure for benzene is C 6 H 6. The benzene ring is made of six C atoms bonded together by three double bonds & three single bonds alternating around the ring.

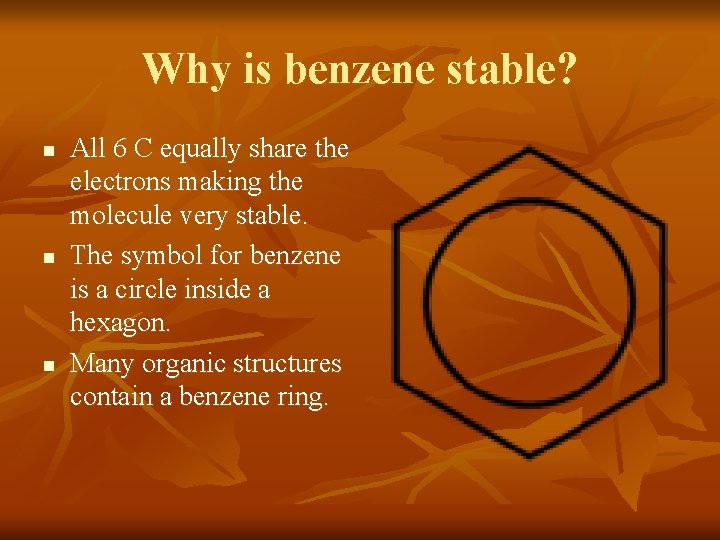
Why is benzene stable? n n n All 6 C equally share the electrons making the molecule very stable. The symbol for benzene is a circle inside a hexagon. Many organic structures contain a benzene ring.

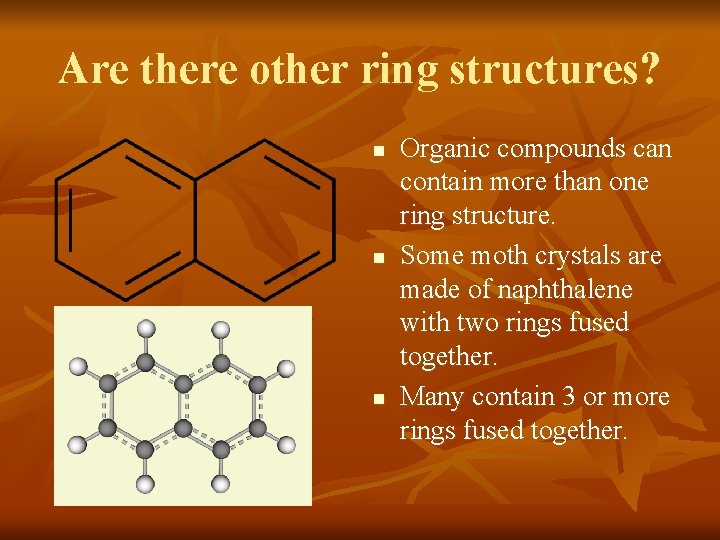
Are there other ring structures? n n n Organic compounds can contain more than one ring structure. Some moth crystals are made of naphthalene with two rings fused together. Many contain 3 or more rings fused together.

Substituted Hydrocarbons n Chemists change hydrocarbons into other compounds with different physical & chemical properties. n n May add a double or triple bond May substitute different atoms or groups of atoms

Substituted Hydrocarbons n n A substituted hydrocarbon is a hydrocarbon that has one or more of its hydrogen atoms replaced by atoms or groups of atoms of other elements. Chemists decide what kinds of properties they want in a new compound and then they choose atoms or groups of atoms or types of bonds that will give those properties.

What are some substituted hydrocarbons? n n An alcohol forms when a hydroxyl group, –OH, replaces one or more hydrogen atoms in a hydrocarbon. This is ethanol. When the sugars in grains or fruits ferments, it produces ethanol

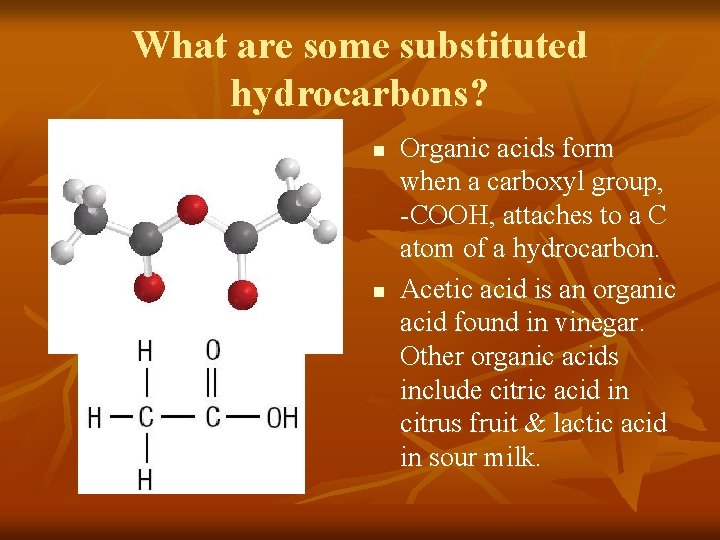
What are some substituted hydrocarbons? n n Organic acids form when a carboxyl group, -COOH, attaches to a C atom of a hydrocarbon. Acetic acid is an organic acid found in vinegar. Other organic acids include citric acid in citrus fruit & lactic acid in sour milk.

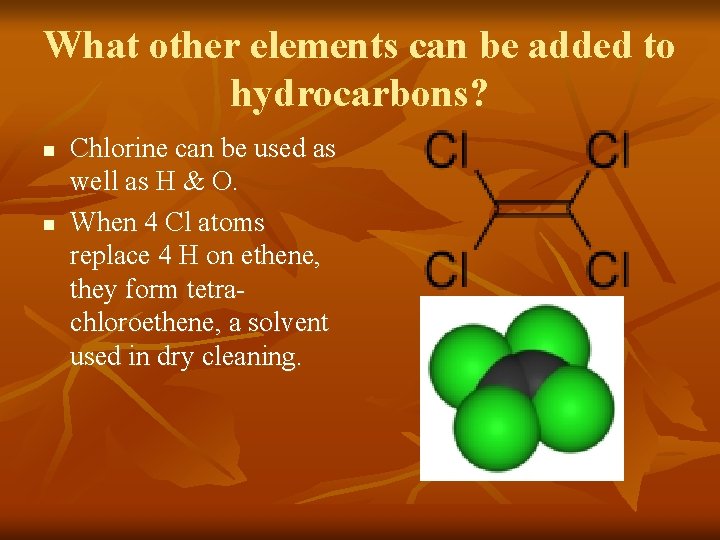
What other elements can be added to hydrocarbons? n n Chlorine can be used as well as H & O. When 4 Cl atoms replace 4 H on ethene, they form tetrachloroethene, a solvent used in dry cleaning.

What other elements can be added to hydrocarbons? n n When 4 Fl atoms replace 4 H atoms, they form a compound that can be made into a black, shiny material used for nonstick cookware. N, Br and S are also used in substituted hydrocarbons. Compounds called thiols are formed when S replaces the O in the –OH group of an alcohol. Thiols are also called mercaptans which smell very bad such as skunk spray.

Section 3 Petroleum- A Source of Carbon Compounds n n What You’ll Learn: How carbon compounds are obtained from petroleum How carbon compounds form long chains of molecules What polymers are

What is Petroleum? n n n Plastic comes from petroleum, a dark, flammable liquid often called crude oil. It exists deep within Earth. Coal, natural gas, & petroleum are all called fossil fuels because they come from fossilized material. Oil wells pump crude oil to Earth’s surface. Engineers separate the mixture by fractional distillation at refineries in metal towers called fractionating towers.

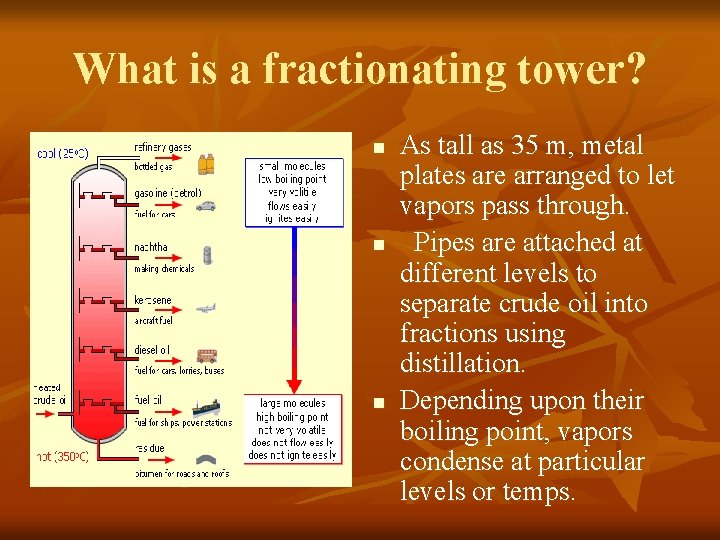
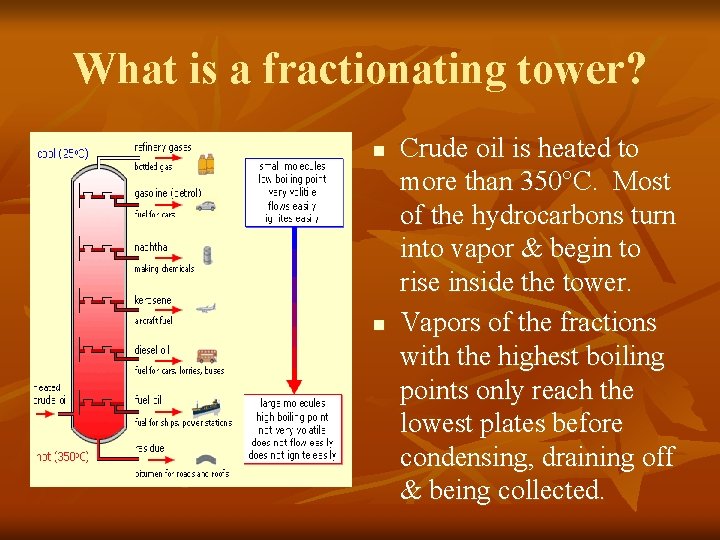
What is a fractionating tower? n n n As tall as 35 m, metal plates are arranged to let vapors pass through. Pipes are attached at different levels to separate crude oil into fractions using distillation. Depending upon their boiling point, vapors condense at particular levels or temps.

What is a fractionating tower? n n Crude oil is heated to more than 350°C. Most of the hydrocarbons turn into vapor & begin to rise inside the tower. Vapors of the fractions with the highest boiling points only reach the lowest plates before condensing, draining off & being collected.

Uses for Petroleum Compounds n n Some fractions are used for fuels. Butane & propane are some of the lightest fractions taken from the top of the tower. Molecules of propane have 3 C atoms; butane has 4 C atoms. Molecules with 5 -10 C atoms/molecule condense on the upper plate & are used for gasoline & solvents. Those that condense on lower plates have 12 -18 C atoms like kerosene & jet fuel. Bottom fractions are lubricating oil with leftovers used to make asphalt to pave roads.

Polymers n n n A polymer is a very large molecule made from small molecules linked together like a chain. A monomer is the small molecule that forms a link in the polymer chain. A polymer chain can contain as many as 10, 000 monomers.

What are some common polymers? n n n Plastic is a common polymer made from the monomer ethene or ethylene combined repeatedly to make polyethylene for shopping bags and plastic bottles. Polypropylene is used to make glues and carpets. Copolymers consist of two or more different monomers combined to make one polymer molecule.

What are some common polymers? n n Characteristics include being light & flexible. So strong used to make plastic pipes, boats, car bodies; Used in place of wood & metal in buildings. Some people call this the age of plastics.

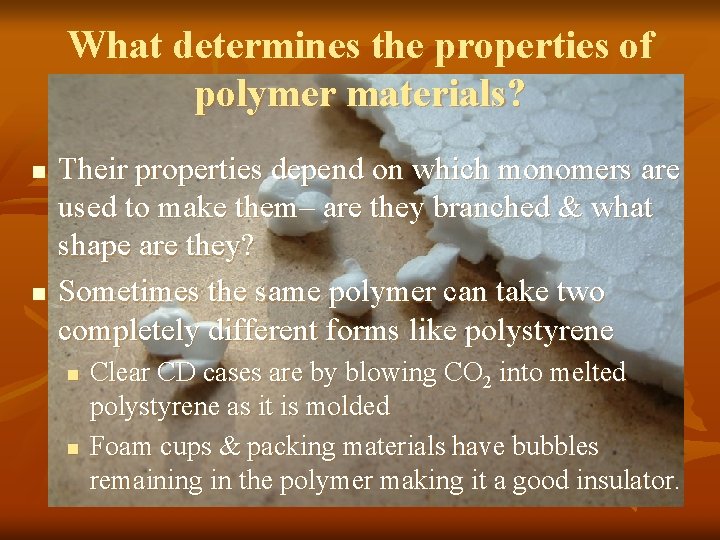
What determines the properties of polymer materials? n n Their properties depend on which monomers are used to make them– are they branched & what shape are they? Sometimes the same polymer can take two completely different forms like polystyrene n n Clear CD cases are by blowing CO 2 into melted polystyrene as it is molded Foam cups & packing materials have bubbles remaining in the polymer making it a good insulator.

What determines the properties of polymer materials? n n Polymers can be spun into thread & made into tough fabrics for suitcases & backpacks or for bulletproof vests. Polymer fibers can stretch and return to their original shape for exercise clothing.

What are some other petroleum products? n Other products are made by separating individual compounds from the petroleum fractions & then changed into substituted hydrocarbons to make: n n n Aspirin Insecticides Printing ink Flavorings Dyes

Are there some problems with polymers? n n n Disposing of things made with polymers is a problem because they do not decompose. Recycling reuses clean plastics to make new products. Depolymerization uses heat or chemicals to break long polymer chains into monomer fragments which then can be used to make other polymers. Too expensive to be practical now due to different process for each polymer.

Section 4 Biological Compounds n n What You’ll Learn: About proteins, nucleic acids, carbohydrates, and lipids Polymers in food Biological polymers

Biological Polymers n Many important biological compounds in your body are biological polymers: n n n Huge molecules made of monomers Larger than monomers of other polymers Examples include proteins, nucleic acids, carbohydrates, & lipids.

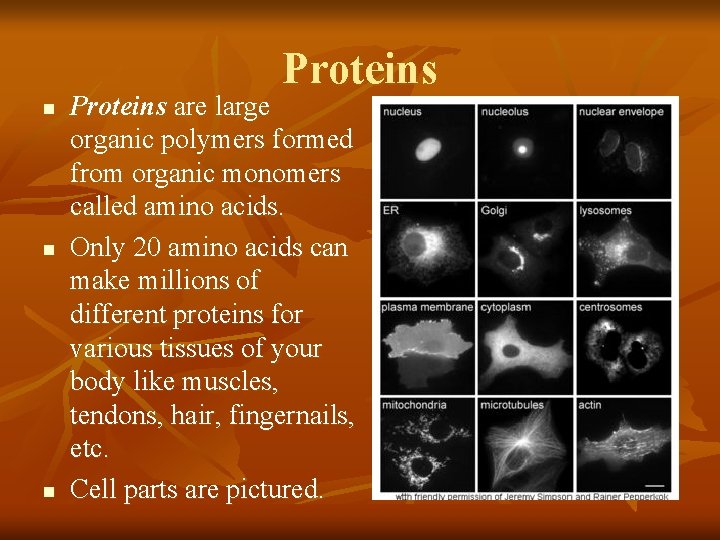
Proteins n n n Proteins are large organic polymers formed from organic monomers called amino acids. Only 20 amino acids can make millions of different proteins for various tissues of your body like muscles, tendons, hair, fingernails, etc. Cell parts are pictured.

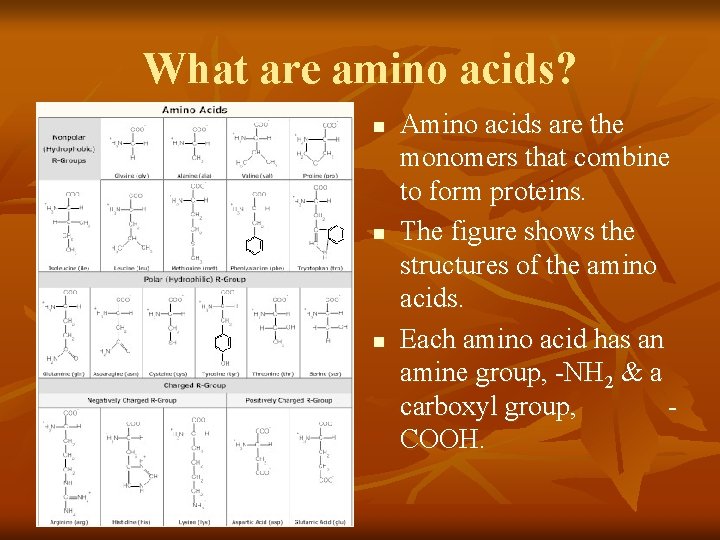
What are amino acids? n n n Amino acids are the monomers that combine to form proteins. The figure shows the structures of the amino acids. Each amino acid has an amine group, -NH 2 & a carboxyl group, COOH.

What are amino acids? n n n The amine group of one amino acid can combine with the carboxyl group of another amino acid. This compound is a peptide with a peptide bond joining them. A molecule containing 50 or more amino acids is called a protein.

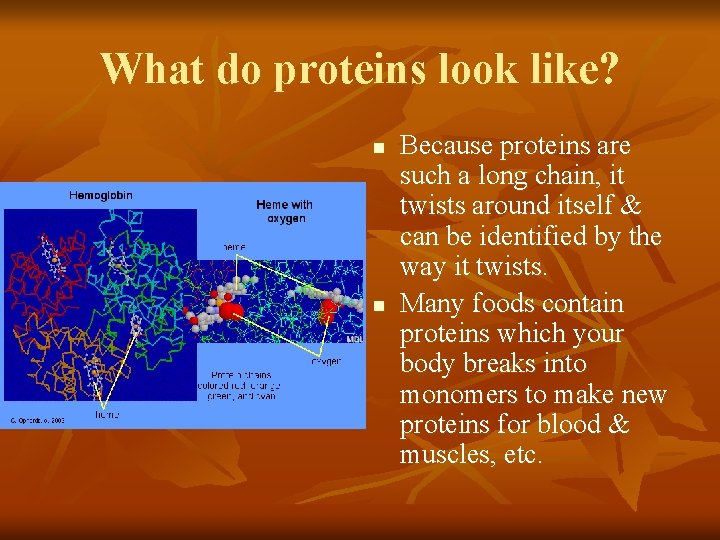
What do proteins look like? n n Because proteins are such a long chain, it twists around itself & can be identified by the way it twists. Many foods contain proteins which your body breaks into monomers to make new proteins for blood & muscles, etc.

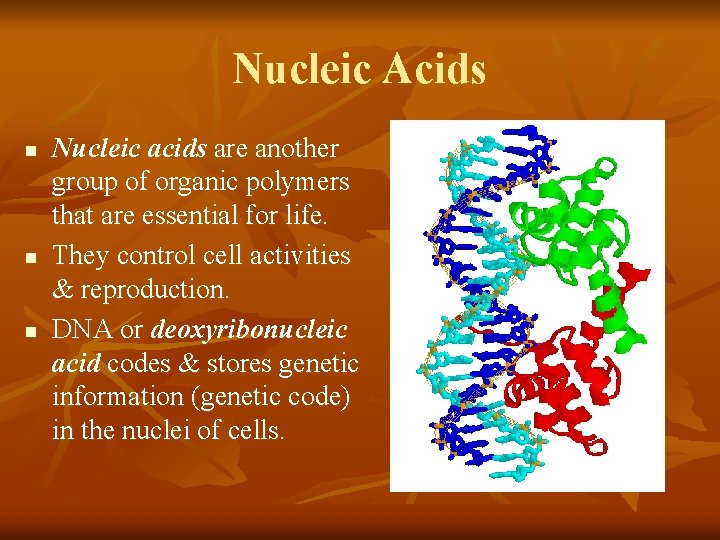
Nucleic Acids n n n Nucleic acids are another group of organic polymers that are essential for life. They control cell activities & reproduction. DNA or deoxyribonucleic acid codes & stores genetic information (genetic code) in the nuclei of cells.

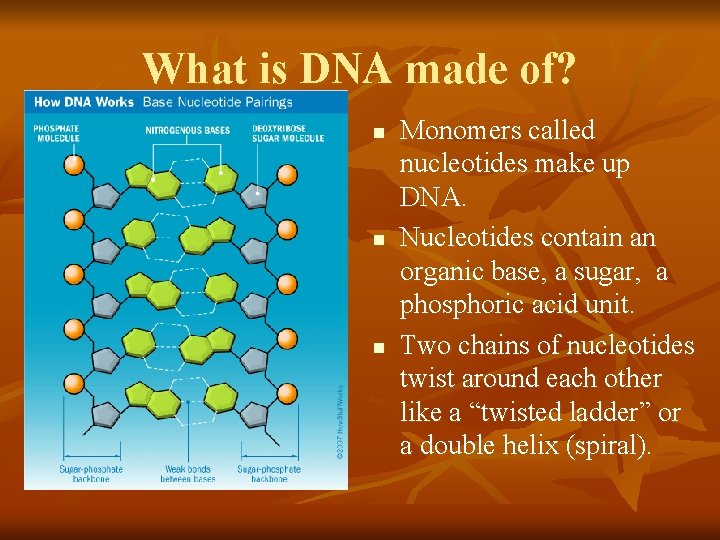
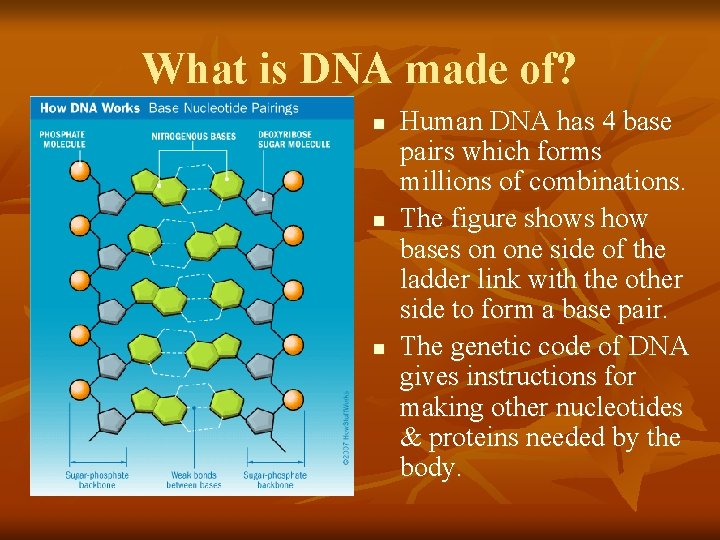
What is DNA made of? n n n Monomers called nucleotides make up DNA. Nucleotides contain an organic base, a sugar, a phosphoric acid unit. Two chains of nucleotides twist around each other like a “twisted ladder” or a double helix (spiral).

What is DNA made of? n n n Human DNA has 4 base pairs which forms millions of combinations. The figure shows how bases on one side of the ladder link with the other side to form a base pair. The genetic code of DNA gives instructions for making other nucleotides & proteins needed by the body.

What is DNA fingerprinting? n n Each molecule of DNA in your body has more than 5 million base pairs. Your DNA is unique unless you have an identical twin. DNA can be used to solve crimes by removing the DNA from hair, blood, or saliva left at a crime scene. By breaking the polymer into monomers & comparing the pattern to a suspect’s DNA, they can link the suspect to the crime scene.

Carbohydrates n n n Carbohydrates are organic compounds made of C, H & O with twice as many H atoms as O atoms. Carbohydrates include sugars & starches. Foods like bread & pasta contain carbohydrates.

What are sugars? n Sucrose C 12 H 22 O 11 n n n Sucrose is table sugar which the body breaks down into fructose & glucose or more simple sugars. Fruit contains fructose. Glucose is found in your blood & in fruit & honey. Eating sugar-rich food gives you a quick boost of energy. Glucose C 6 H 12 O 6

What is a starch? n n n Starch is a polymer carbohydrate made of monomers of glucose. Your body breaks it into sugars which release energy into your cells. Athletes use starches for long lasting energy stored in the liver & muscle cells as glycogen for a fresh burst of power.

Lipids n n n Lipids are organic compounds like fats and oils such as butter and corn oil. Lipids are made of C, H & O but with fewer O atoms than carbohydrates. Another difference is that lipids contain carboxyl groups, -COOH while carbohydrates do not.

What are some lipids in your diet? n n Fats & oils are similar in structure to hydrocarbons. If they only have single bonds between C atoms, they are saturated fats. Unsaturated fat that has only one double bond is monounsaturated. An unsaturated fat that has two or more double bonds is polyunsaturated

What are some lipids in your diet? n n Fats are lipids that come from animals. Usually saturated & solid at room temperature. Oils are unsaturated & usually liquid at room temperature. Sometimes H is added to vegetable oil to saturate the C & make it solid.

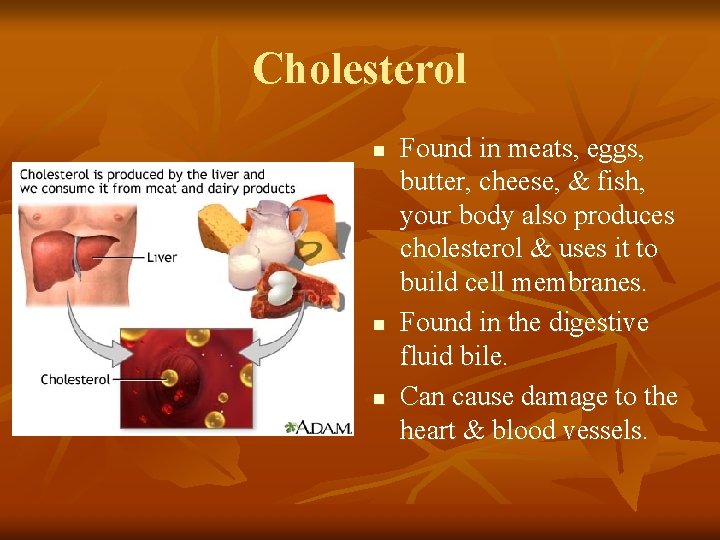
Cholesterol n n n Found in meats, eggs, butter, cheese, & fish, your body also produces cholesterol & uses it to build cell membranes. Found in the digestive fluid bile. Can cause damage to the heart & blood vessels.

Cholesterol n n n Eating too many foods with high amounts of saturated fats & cholesterol may cause heart disease. Some unsaturated fats may protect the heart from disease. A balanced diet contains some fats as well as proteins & carbohydrates.