Naming Organic Compounds 1 Naming Organic Compounds Originally







- Slides: 39

Naming Organic Compounds 1

Naming Organic Compounds • • Originally compounds were named based on their source or use Many organic compounds were given common names which are still in use However many ambiguities resulted With the large number of organic compounds, a method for systematically naming them is very important 2

IUPAC Names • • • The International Union of Pure and Applied Chemists (IUPAC) developed a system for naming organic compounds. This system eliminated many of the ambiguities that plagued earlier naming systems Common names for many substances are still widely used 3

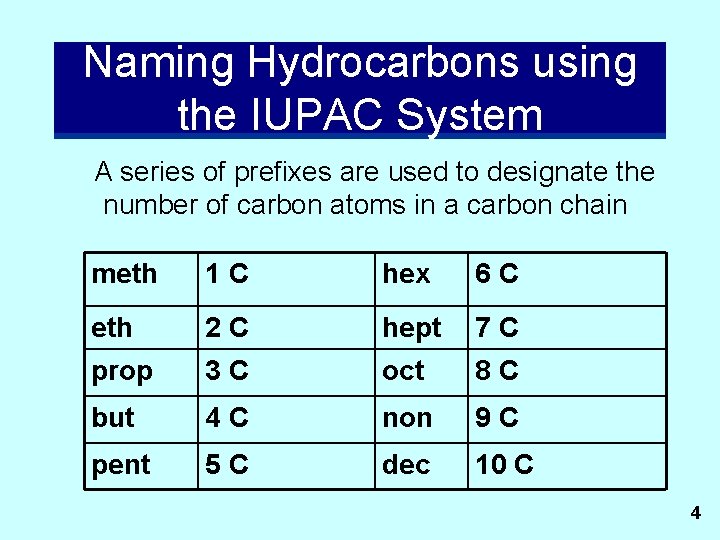
Naming Hydrocarbons using the IUPAC System A series of prefixes are used to designate the number of carbon atoms in a carbon chain meth 1 C hex 6 C eth 2 C hept 7 C prop 3 C oct 8 C but 4 C non 9 C pent 5 C dec 10 C 4

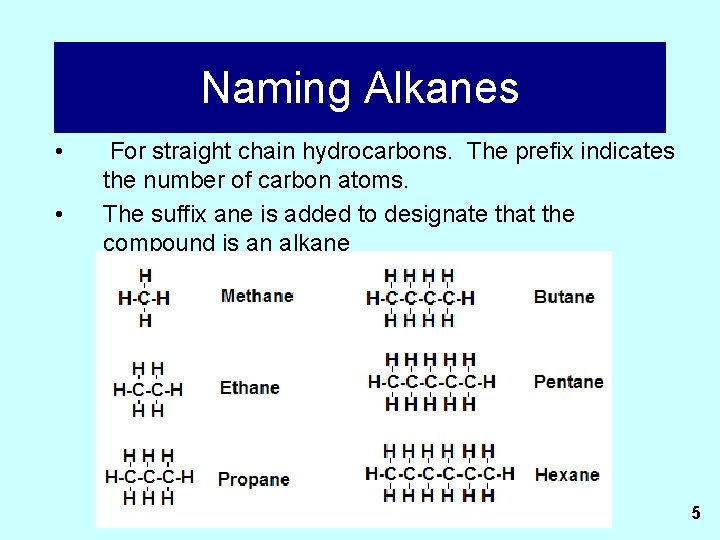
Naming Alkanes • • For straight chain hydrocarbons. The prefix indicates the number of carbon atoms. The suffix ane is added to designate that the compound is an alkane 5

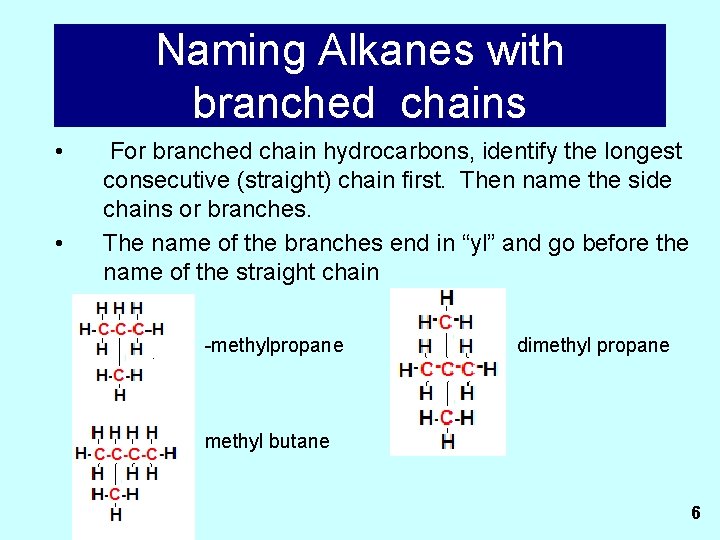
Naming Alkanes with branched chains • • For branched chain hydrocarbons, identify the longest consecutive (straight) chain first. Then name the side chains or branches. The name of the branches end in “yl” and go before the name of the straight chain -methylpropane dimethyl propane methyl butane 6

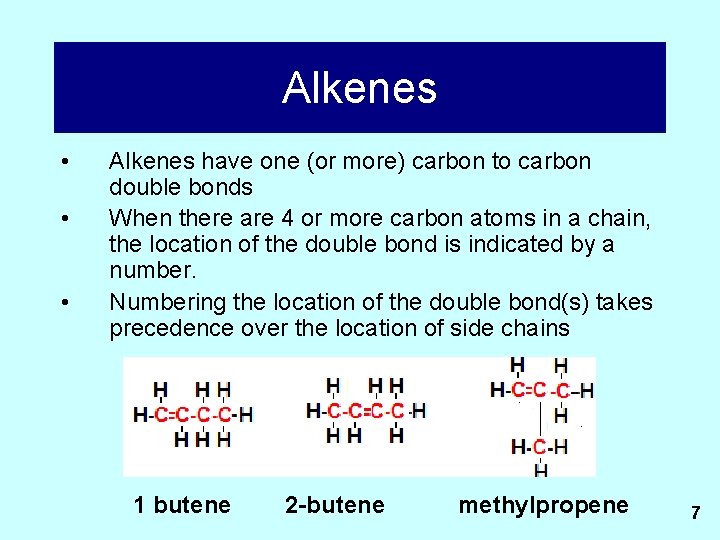
Alkenes • • • Alkenes have one (or more) carbon to carbon double bonds When there are 4 or more carbon atoms in a chain, the location of the double bond is indicated by a number. Numbering the location of the double bond(s) takes precedence over the location of side chains 1 butene 2 -butene methylpropene 7

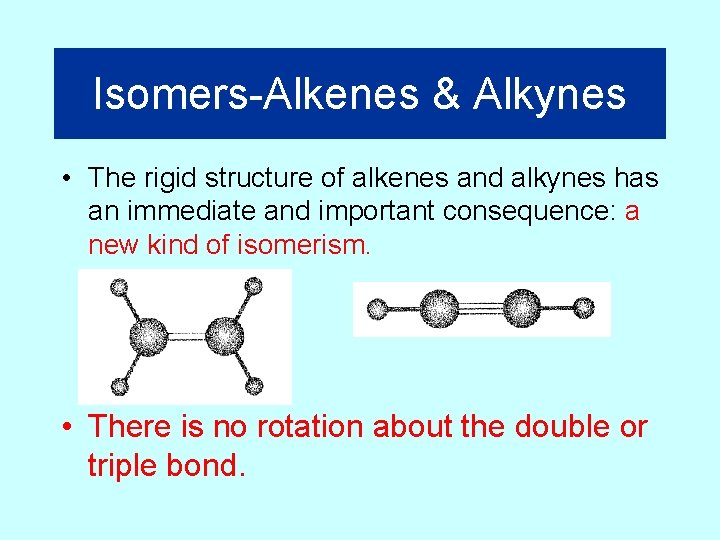
Isomers-Alkenes & Alkynes • The rigid structure of alkenes and alkynes has an immediate and important consequence: a new kind of isomerism. • There is no rotation about the double or triple bond.

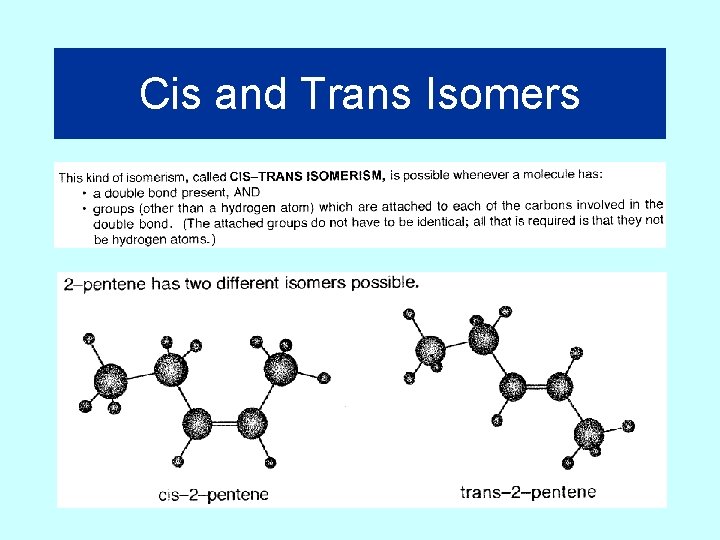
Cis and Trans Isomers

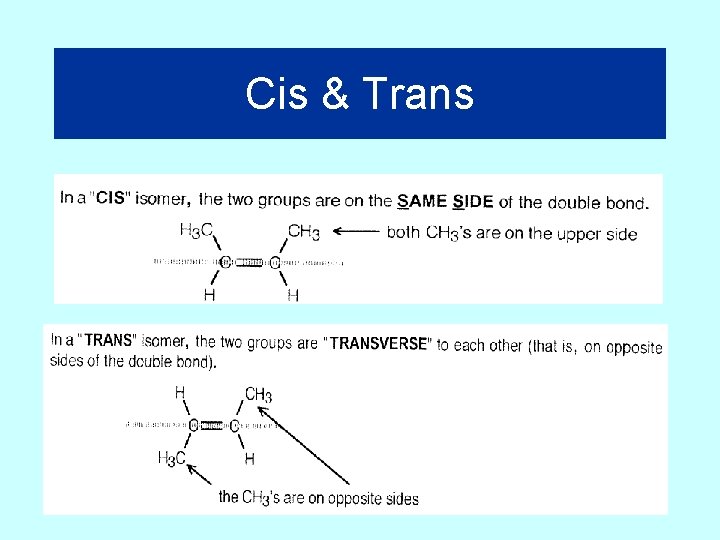
Cis & Trans

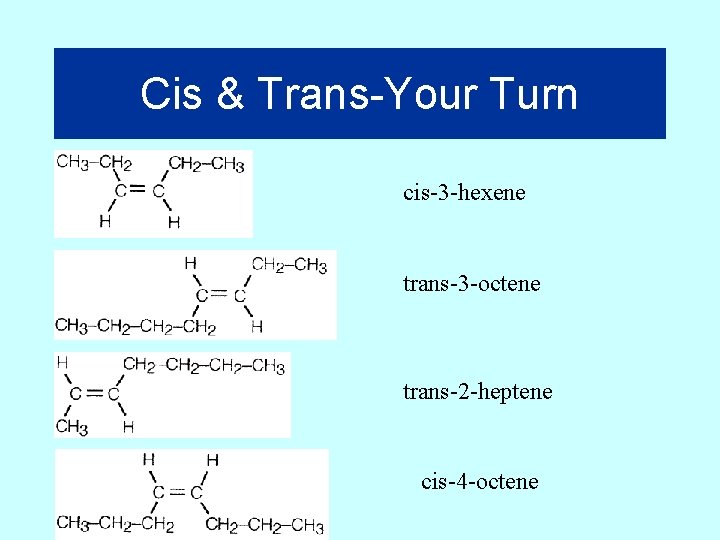
Cis & Trans-Your Turn cis-3 -hexene trans-3 -octene trans-2 -heptene cis-4 -octene

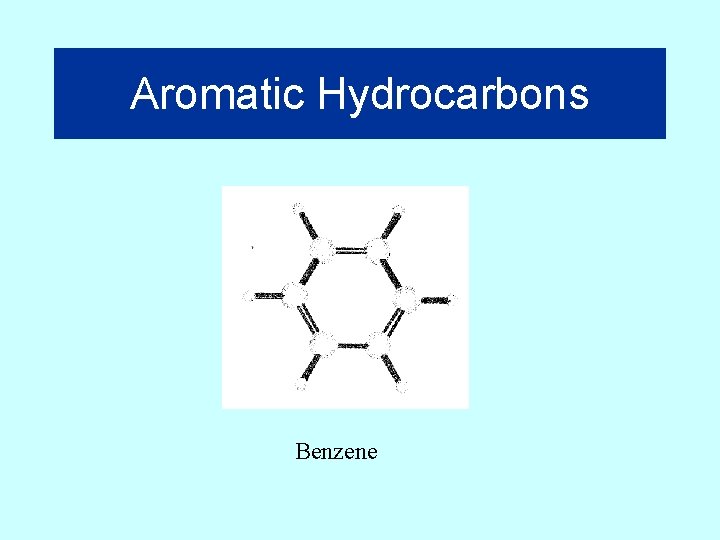
Aromatic Hydrocarbons Benzene

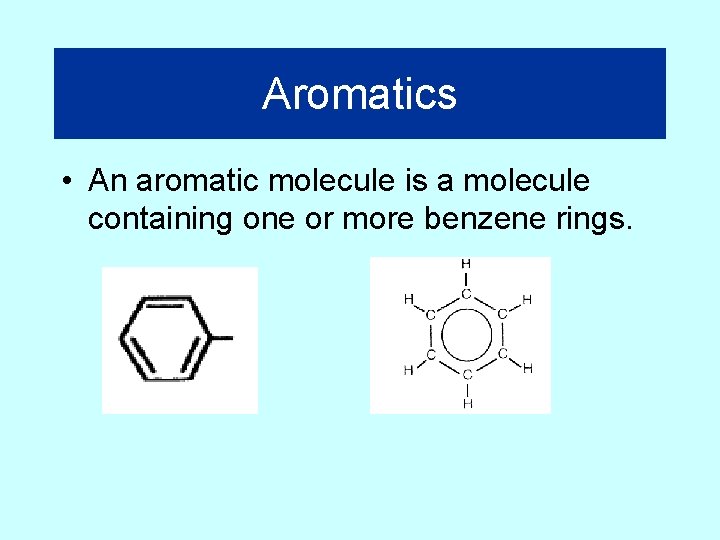
Aromatics • An aromatic molecule is a molecule containing one or more benzene rings.

Naming Aromatics • The naming of simple aromatic compounds formed by adding groups to a benzene ring is almost identical to the naming procedure for other cyclic hydrocarbons.

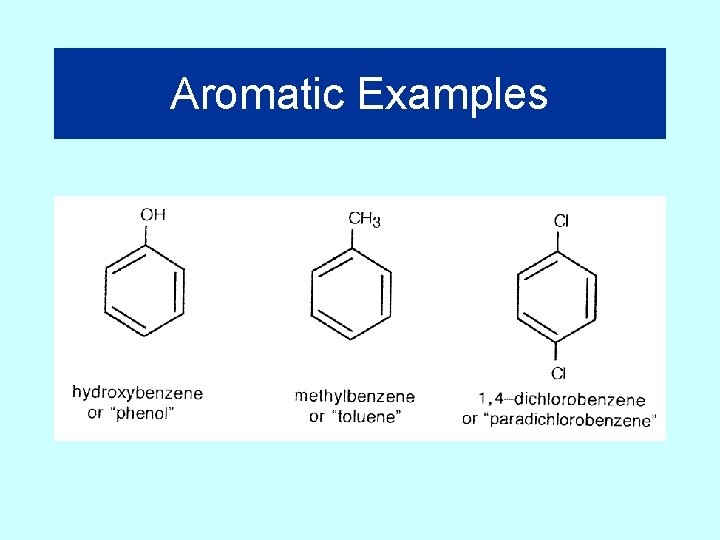
Aromatic Examples

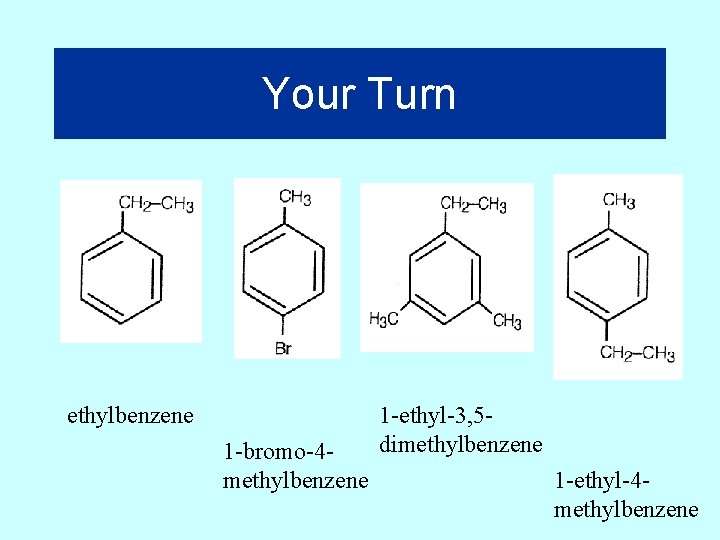
Your Turn ethylbenzene 1 -bromo-4 methylbenzene 1 -ethyl-3, 5 dimethylbenzene 1 -ethyl-4 methylbenzene

Naming Compounds With Functional Groups Various functional groups have unique suffixes that designate the functional group. The functional group takes precedence in numbering the carbon chain. Branches to the carbon chain are named in the usual manner. alcohols “ol” Amides “amide” Aldehydes “al” Amines “amine” or amino as a prefix Ketones “one” Ethers ethoxy as prefix Esters “oate” Carboxylic Acids “oic” 17

Alcohols

Alcohols 1 - Propanol Suffix = “ol” 2 - Propanol 2 -methyl-2 -propanol 19

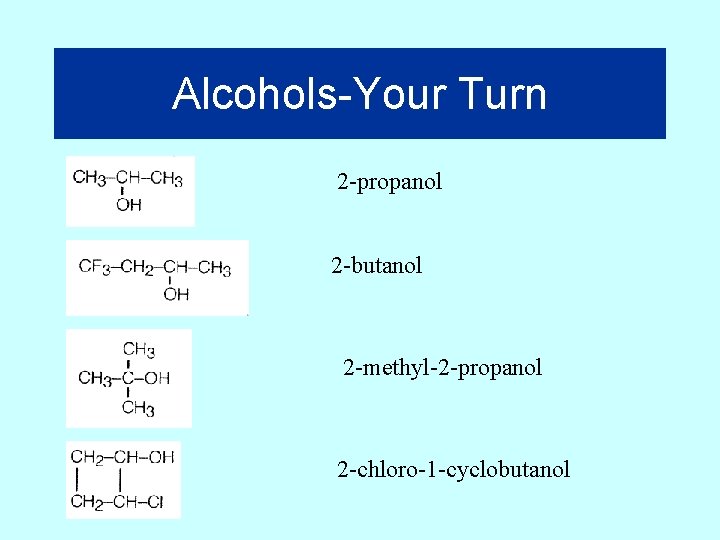
Alcohols-Your Turn 2 -propanol 2 -butanol 2 -methyl-2 -propanol 2 -chloro-1 -cyclobutanol

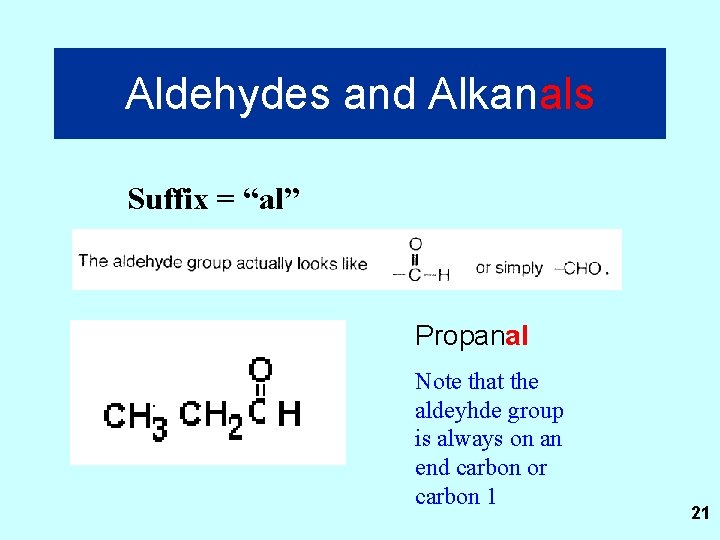
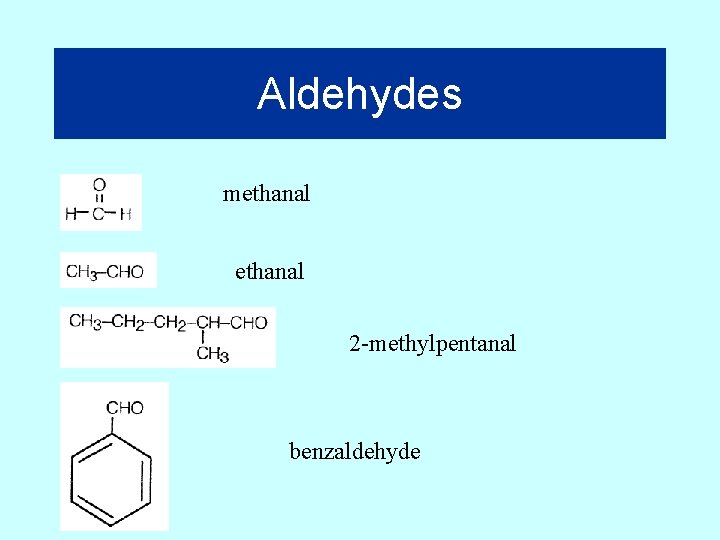
Aldehydes and Alkanals Suffix = “al” Propanal Note that the aldeyhde group is always on an end carbon or carbon 1 21

Aldehydes methanal 2 -methylpentanal benzaldehyde

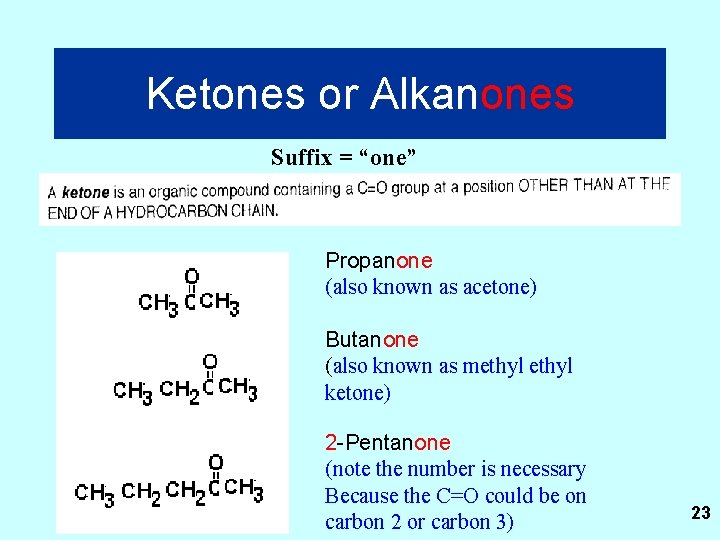
Ketones or Alkanones Suffix = “one” Propanone (also known as acetone) Butanone (also known as methyl ketone) 2 -Pentanone (note the number is necessary Because the C=O could be on carbon 2 or carbon 3) 23

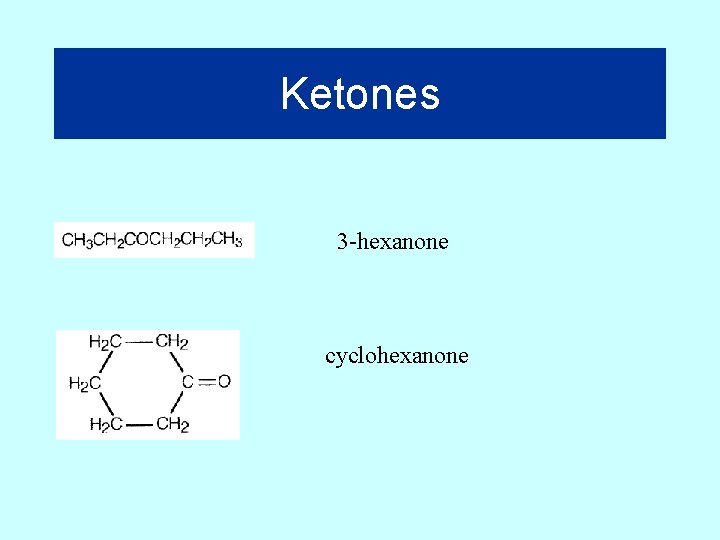
Ketones 3 -hexanone cyclohexanone

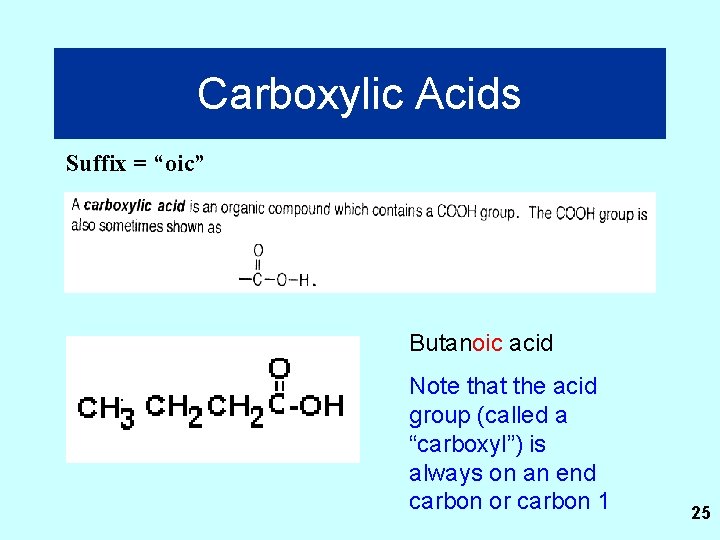
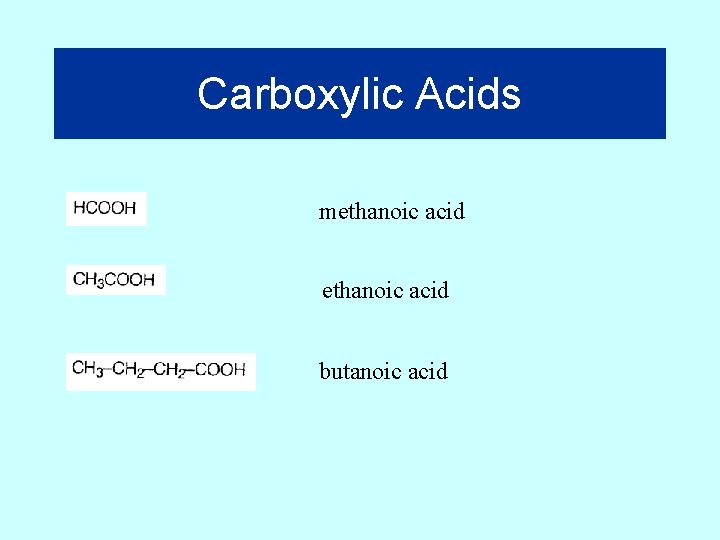
Carboxylic Acids Suffix = “oic” Butanoic acid Note that the acid group (called a “carboxyl”) is always on an end carbon or carbon 1 25

Carboxylic Acids methanoic acid butanoic acid

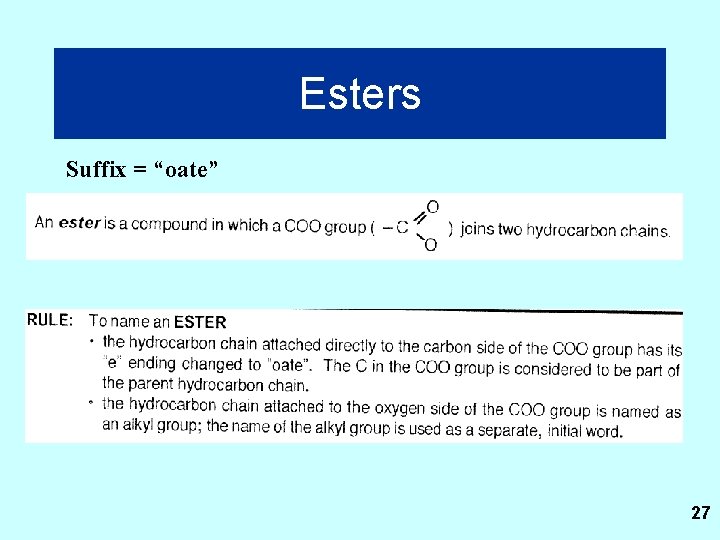
Esters Suffix = “oate” 27

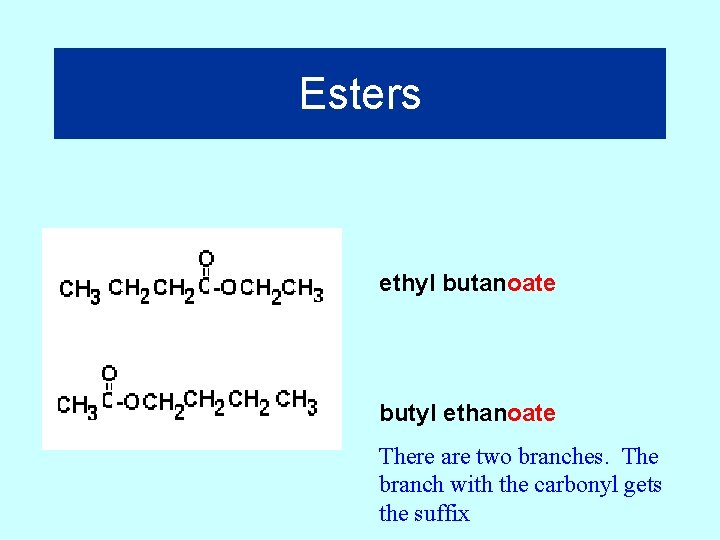
Esters ethyl butanoate butyl ethanoate There are two branches. The branch with the carbonyl gets the suffix

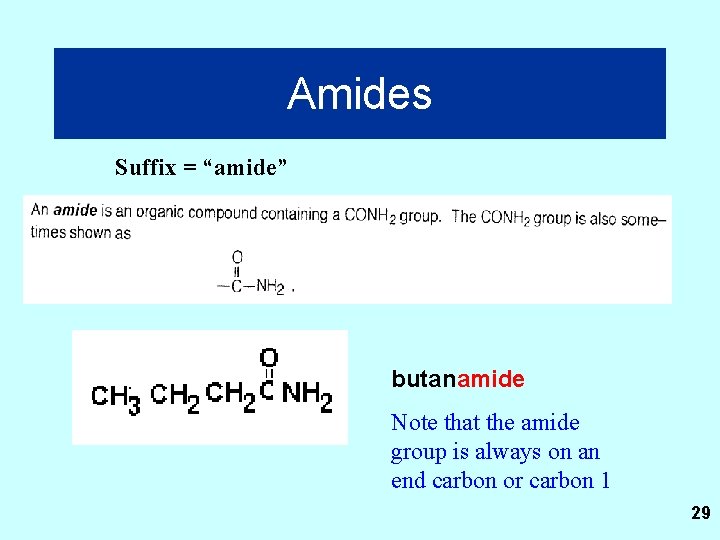
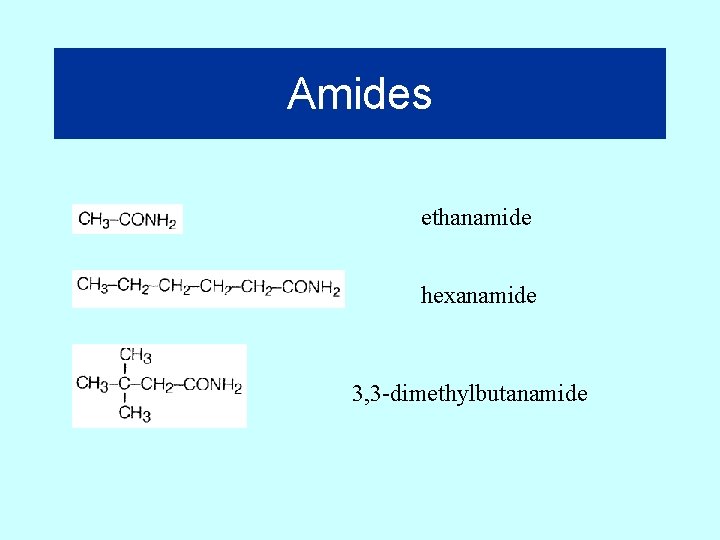
Amides Suffix = “amide” butanamide Note that the amide group is always on an end carbon or carbon 1 29

Amides ethanamide hexanamide 3, 3 -dimethylbutanamide

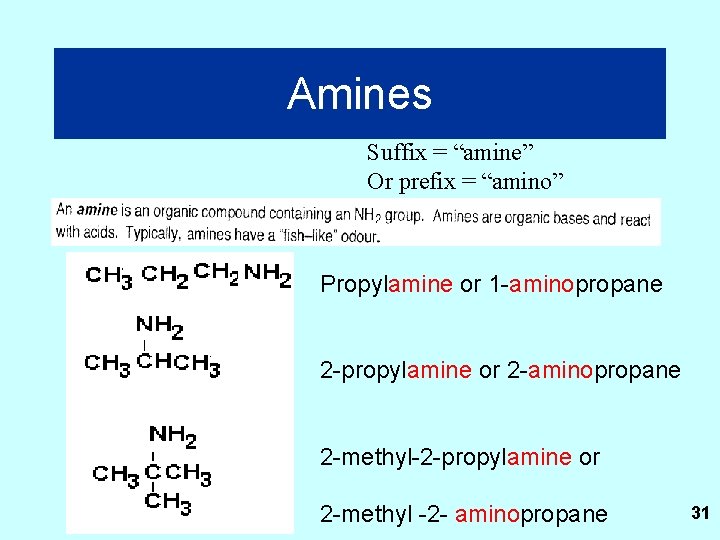
Amines Suffix = “amine” Or prefix = “amino” Propylamine or 1 -aminopropane 2 -propylamine or 2 -aminopropane 2 -methyl-2 -propylamine or 2 -methyl -2 - aminopropane 31

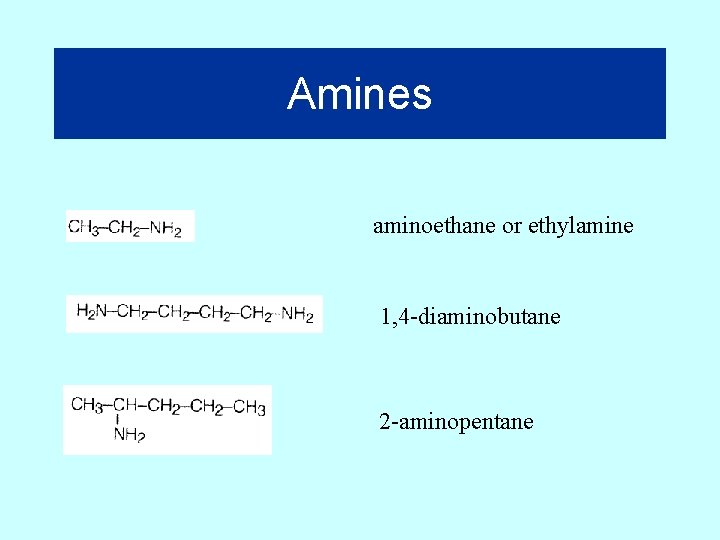
Amines aminoethane or ethylamine 1, 4 -diaminobutane 2 -aminopentane

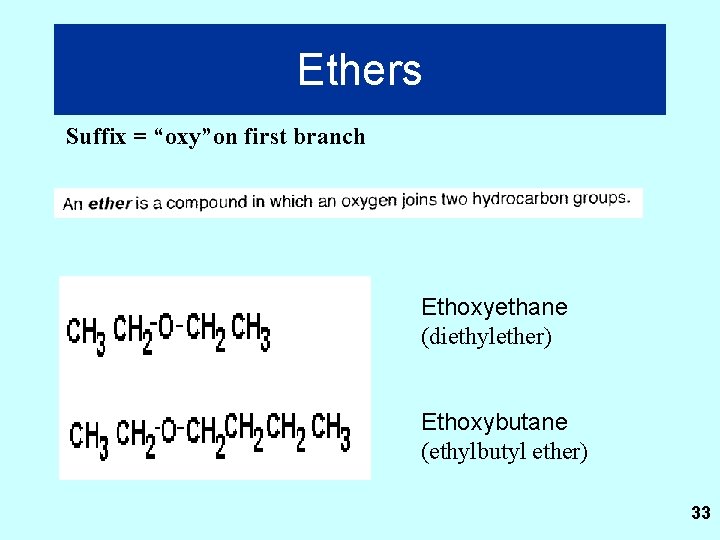
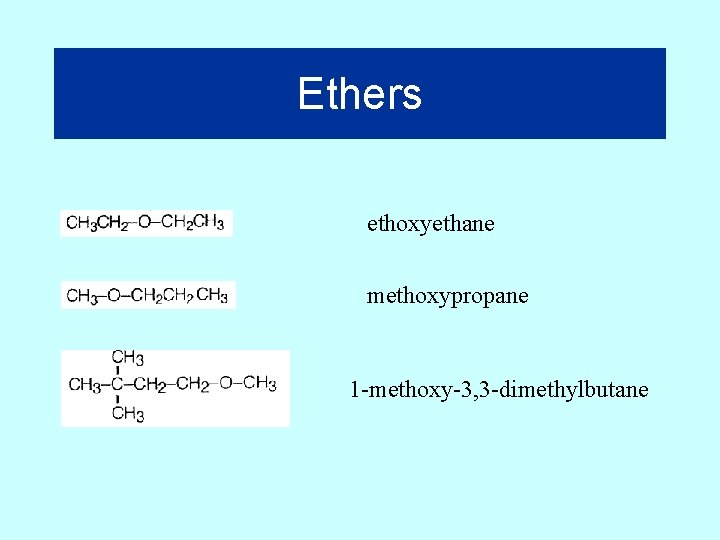
Ethers Suffix = “oxy”on first branch Ethoxyethane (diethylether) Ethoxybutane (ethylbutyl ether) 33

Ethers ethoxyethane methoxypropane 1 -methoxy-3, 3 -dimethylbutane

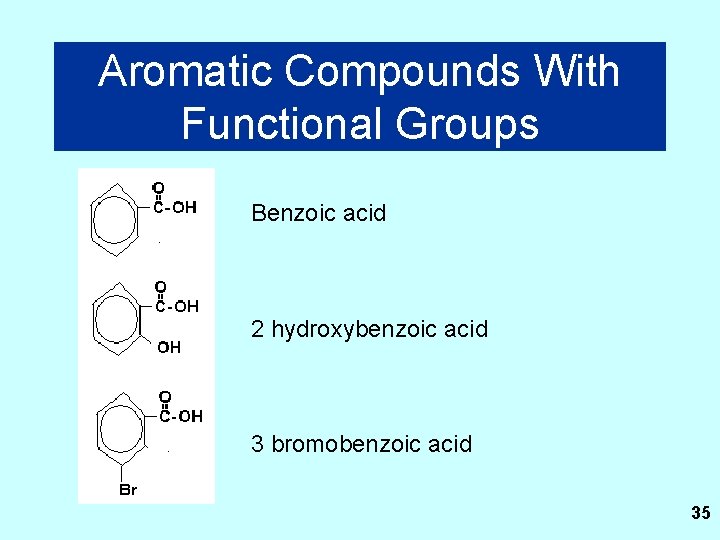
Aromatic Compounds With Functional Groups Benzoic acid 2 hydroxybenzoic acid 3 bromobenzoic acid 35

Nitriles have a cyanide group. The name is based on the longest carbon chain that includes the carbon atom in the nitrile group. 36

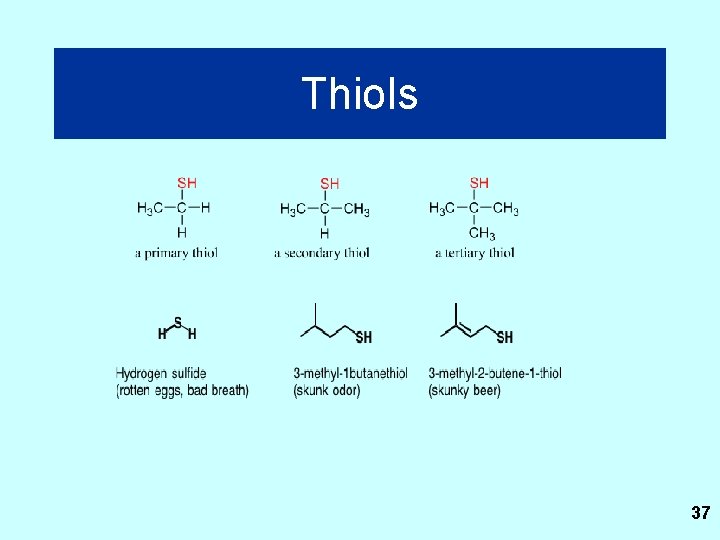
Thiols 37

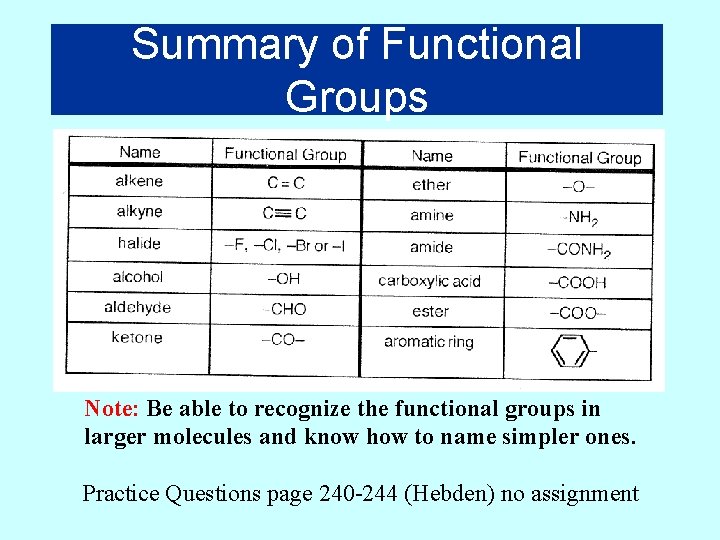
Summary of Functional Groups Note: Be able to recognize the functional groups in larger molecules and know how to name simpler ones. Practice Questions page 240 -244 (Hebden) no assignment

Finis End of Chemistry 11 Thank You for being a Great Class! Congratulations!!