Chapter 9 ENERGY ANALYSIS OF CLOSED SYSTEM The


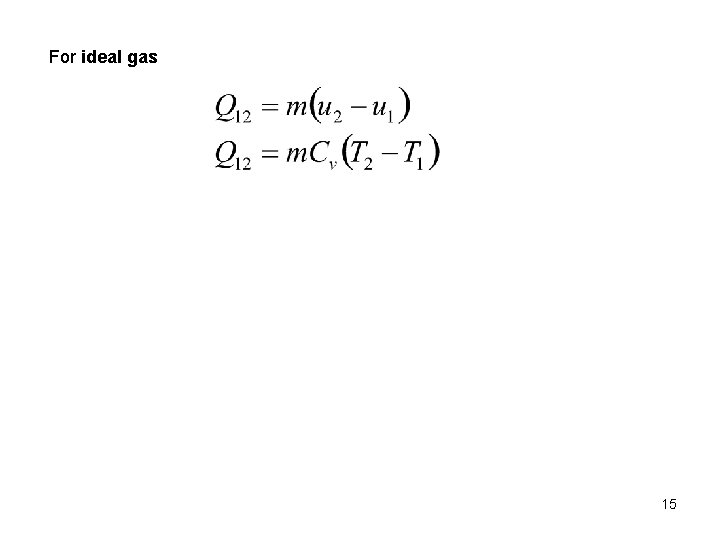
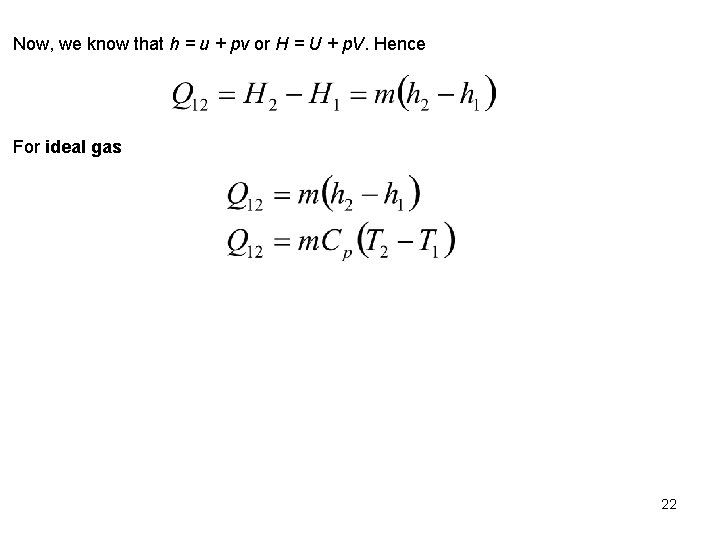


- Slides: 47

Chapter 9 ENERGY ANALYSIS OF CLOSED SYSTEM

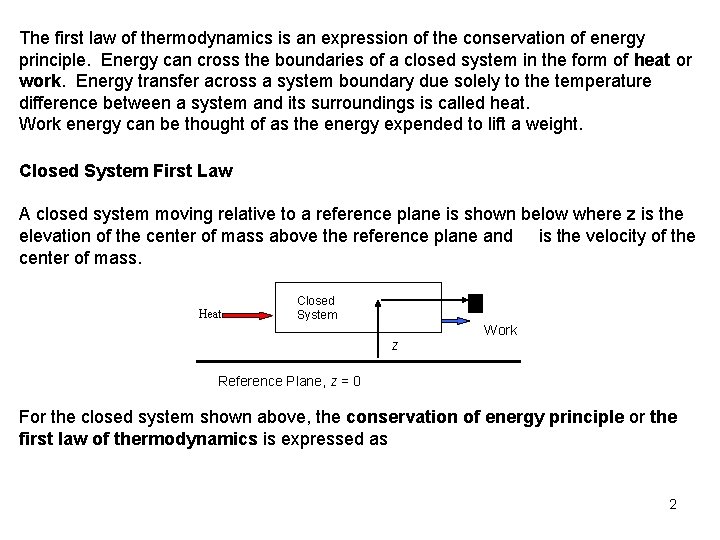
The first law of thermodynamics is an expression of the conservation of energy principle. Energy can cross the boundaries of a closed system in the form of heat or work. Energy transfer across a system boundary due solely to the temperature difference between a system and its surroundings is called heat. Work energy can be thought of as the energy expended to lift a weight. Closed System First Law A closed system moving relative to a reference plane is shown below where z is the elevation of the center of mass above the reference plane and is the velocity of the center of mass. Heat Closed System z Work Reference Plane, z = 0 For the closed system shown above, the conservation of energy principle or the first law of thermodynamics is expressed as 2

or According to classical thermodynamics, we consider the energy added to be net heat transfer to the closed system and the energy leaving the closed system to be net work done by the closed system. So Where Normally the stored energy, or total energy, of a system is expressed as the sum of three separate energies. The total energy of the system, Esystem, is given as 3

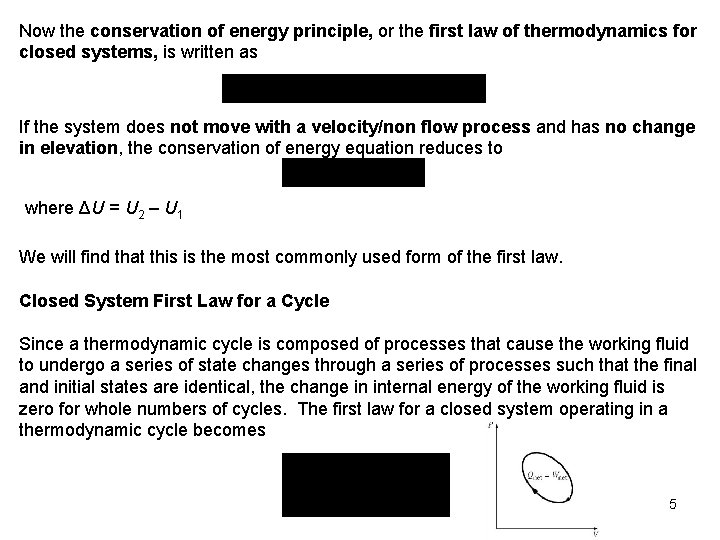
Recall that U is the sum of the energy contained within the molecules of the system other than the kinetic and potential energies of the system as a whole and is called the internal energy. The internal energy U is dependent on the state of the system and the mass of the system. For a system moving relative to a reference plane, the kinetic energy KE and the potential energy PE are given by The change in stored energy for the system is where u 2 and u 1(get from thermodynamic property relations) 4

Now the conservation of energy principle, or the first law of thermodynamics for closed systems, is written as If the system does not move with a velocity/non flow process and has no change in elevation, the conservation of energy equation reduces to where ΔU = U 2 – U 1 We will find that this is the most commonly used form of the first law. Closed System First Law for a Cycle Since a thermodynamic cycle is composed of processes that cause the working fluid to undergo a series of state changes through a series of processes such that the final and initial states are identical, the change in internal energy of the working fluid is zero for whole numbers of cycles. The first law for a closed system operating in a thermodynamic cycle becomes 5

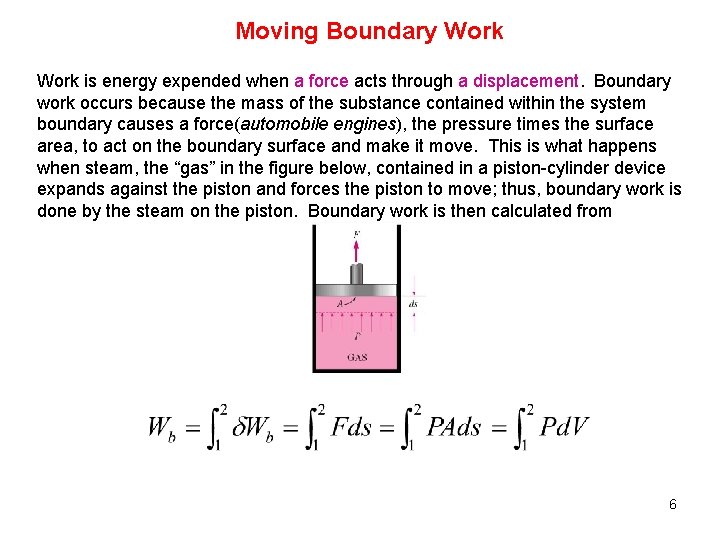
Moving Boundary Work is energy expended when a force acts through a displacement. Boundary work occurs because the mass of the substance contained within the system boundary causes a force(automobile engines), the pressure times the surface area, to act on the boundary surface and make it move. This is what happens when steam, the “gas” in the figure below, contained in a piston-cylinder device expands against the piston and forces the piston to move; thus, boundary work is done by the steam on the piston. Boundary work is then calculated from 6

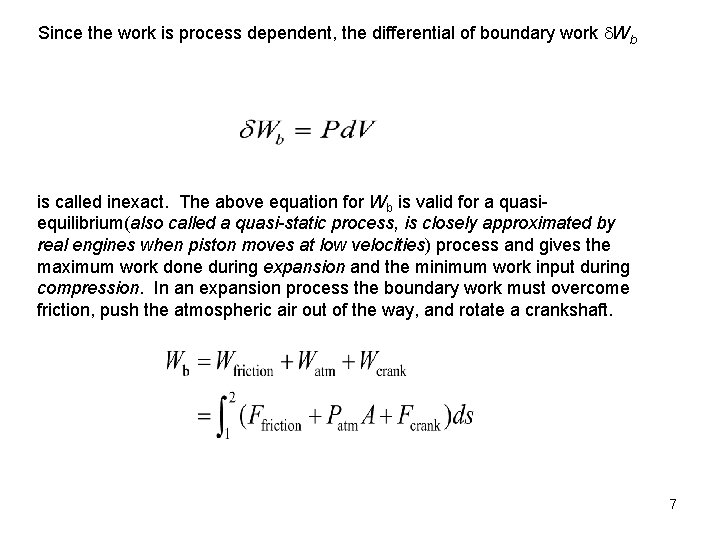
Since the work is process dependent, the differential of boundary work Wb is called inexact. The above equation for Wb is valid for a quasiequilibrium(also called a quasi-static process, is closely approximated by real engines when piston moves at low velocities) process and gives the maximum work done during expansion and the minimum work input during compression. In an expansion process the boundary work must overcome friction, push the atmospheric air out of the way, and rotate a crankshaft. 7

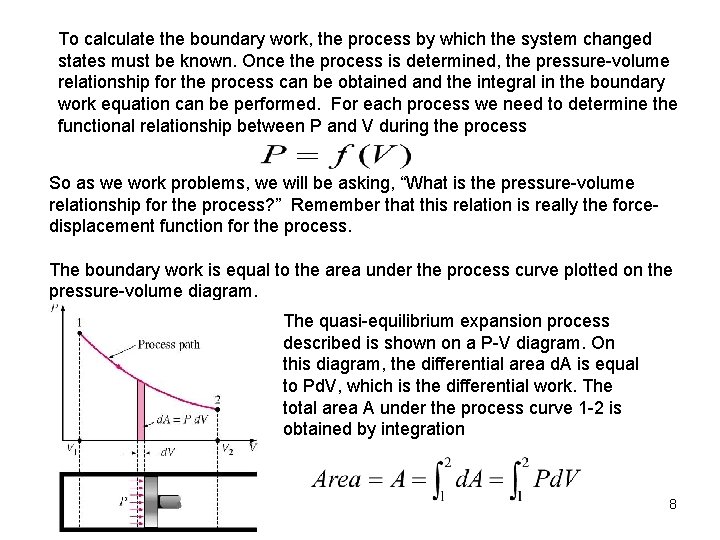
To calculate the boundary work, the process by which the system changed states must be known. Once the process is determined, the pressure-volume relationship for the process can be obtained and the integral in the boundary work equation can be performed. For each process we need to determine the functional relationship between P and V during the process So as we work problems, we will be asking, “What is the pressure-volume relationship for the process? ” Remember that this relation is really the forcedisplacement function for the process. The boundary work is equal to the area under the process curve plotted on the pressure-volume diagram. The quasi-equilibrium expansion process described is shown on a P-V diagram. On this diagram, the differential area d. A is equal to Pd. V, which is the differential work. The total area A under the process curve 1 -2 is obtained by integration 8

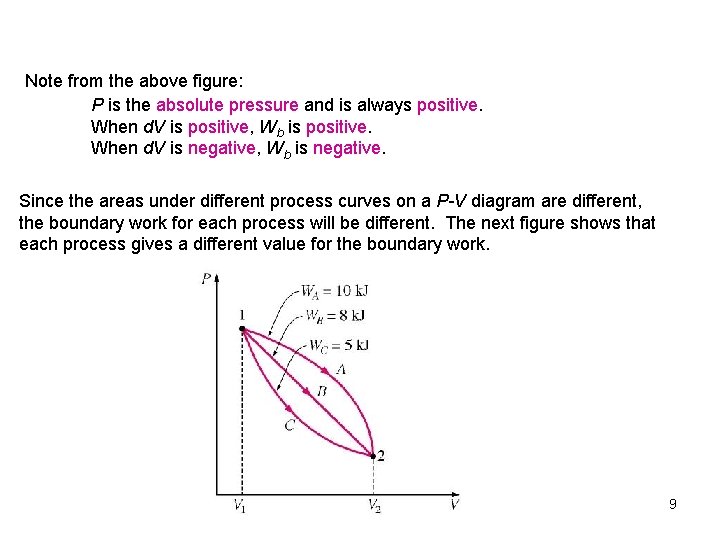
Note from the above figure: P is the absolute pressure and is always positive. When d. V is positive, Wb is positive. When d. V is negative, Wb is negative. Since the areas under different process curves on a P-V diagram are different, the boundary work for each process will be different. The next figure shows that each process gives a different value for the boundary work. 9

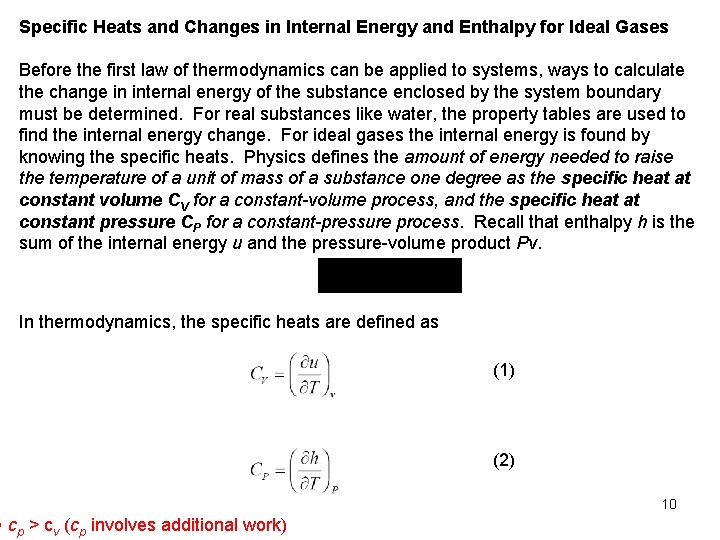
Specific Heats and Changes in Internal Energy and Enthalpy for Ideal Gases Before the first law of thermodynamics can be applied to systems, ways to calculate the change in internal energy of the substance enclosed by the system boundary must be determined. For real substances like water, the property tables are used to find the internal energy change. For ideal gases the internal energy is found by knowing the specific heats. Physics defines the amount of energy needed to raise the temperature of a unit of mass of a substance one degree as the specific heat at constant volume CV for a constant-volume process, and the specific heat at constant pressure CP for a constant-pressure process. Recall that enthalpy h is the sum of the internal energy u and the pressure-volume product Pv. In thermodynamics, the specific heats are defined as cp > cv (cp involves additional work) (1) (2) 10

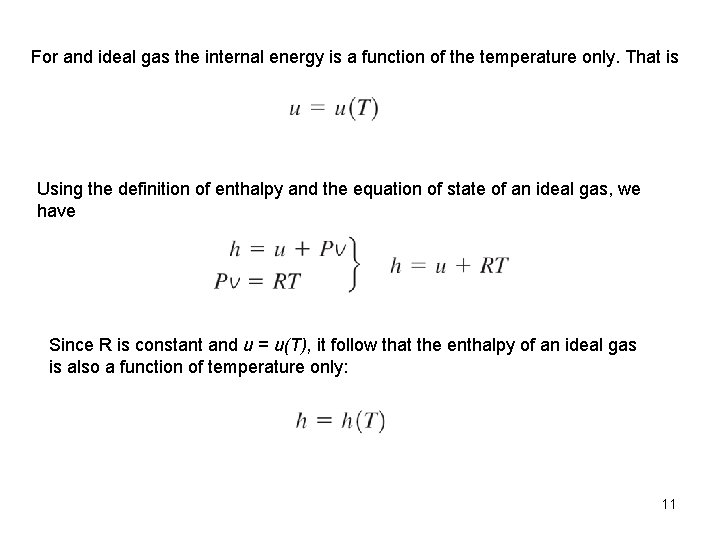
For and ideal gas the internal energy is a function of the temperature only. That is Using the definition of enthalpy and the equation of state of an ideal gas, we have Since R is constant and u = u(T), it follow that the enthalpy of an ideal gas is also a function of temperature only: 11

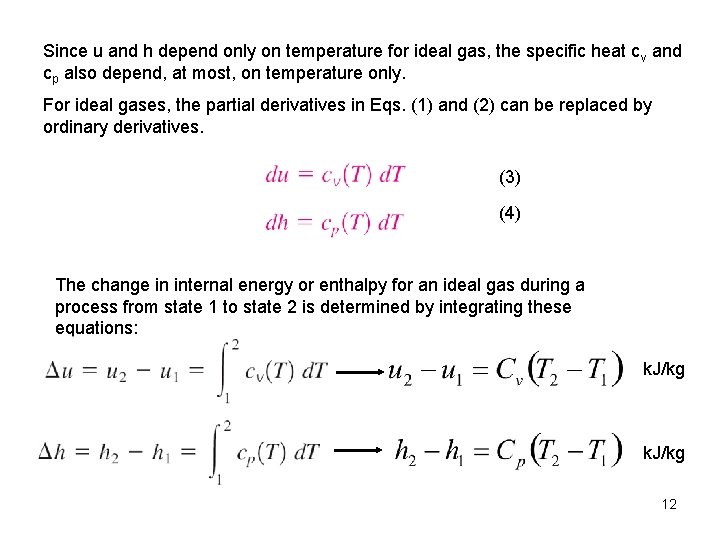
Since u and h depend only on temperature for ideal gas, the specific heat cv and cp also depend, at most, on temperature only. For ideal gases, the partial derivatives in Eqs. (1) and (2) can be replaced by ordinary derivatives. (3) (4) The change in internal energy or enthalpy for an ideal gas during a process from state 1 to state 2 is determined by integrating these equations: k. J/kg 12

Relation between CP and CV for Ideal Gases Using the definition of enthalpy (h = u + Pv) and writing the differential of enthalpy, the relationship between the specific heats for ideal gases is where R is the particular gas constant. The specific heat ratio k (fluids texts often use instead of k) is defined as 13

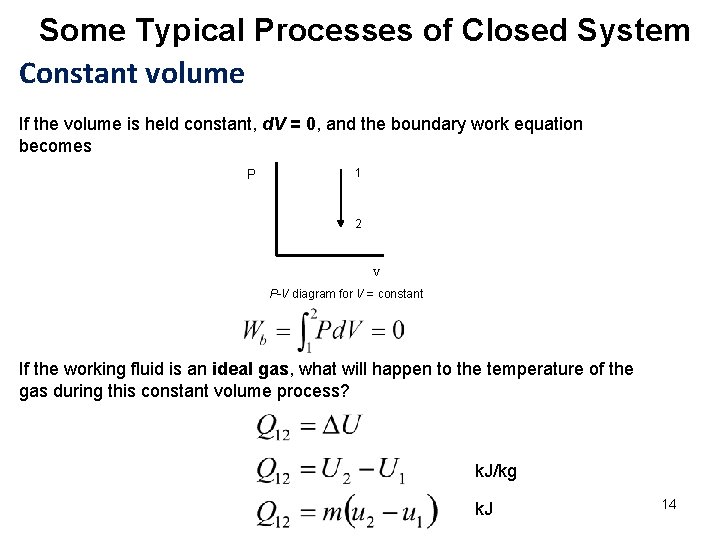
Some Typical Processes of Closed System Constant volume If the volume is held constant, d. V = 0, and the boundary work equation becomes P 1 2 V P-V diagram for V = constant If the working fluid is an ideal gas, what will happen to the temperature of the gas during this constant volume process? k. J/kg k. J 14
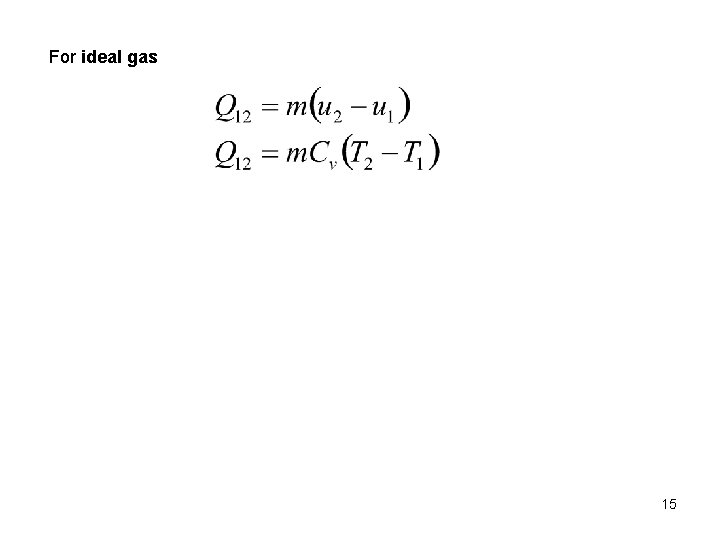
For ideal gas 15

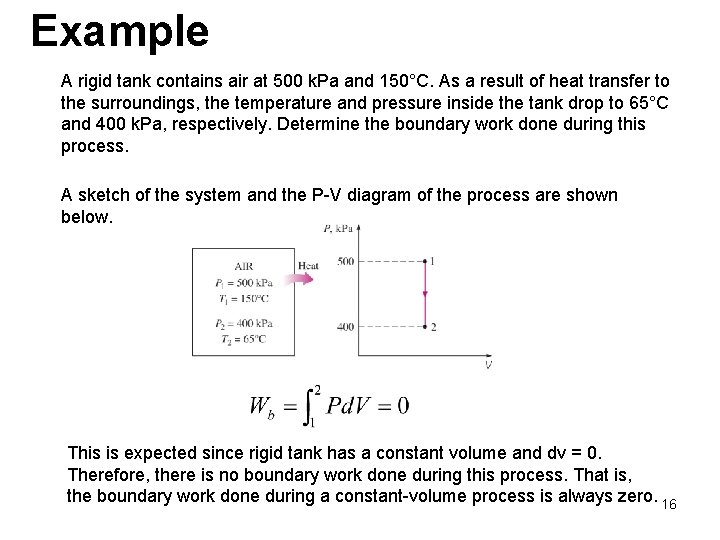
Example A rigid tank contains air at 500 k. Pa and 150°C. As a result of heat transfer to the surroundings, the temperature and pressure inside the tank drop to 65°C and 400 k. Pa, respectively. Determine the boundary work done during this process. A sketch of the system and the P-V diagram of the process are shown below. This is expected since rigid tank has a constant volume and dv = 0. Therefore, there is no boundary work done during this process. That is, the boundary work done during a constant-volume process is always zero. 16

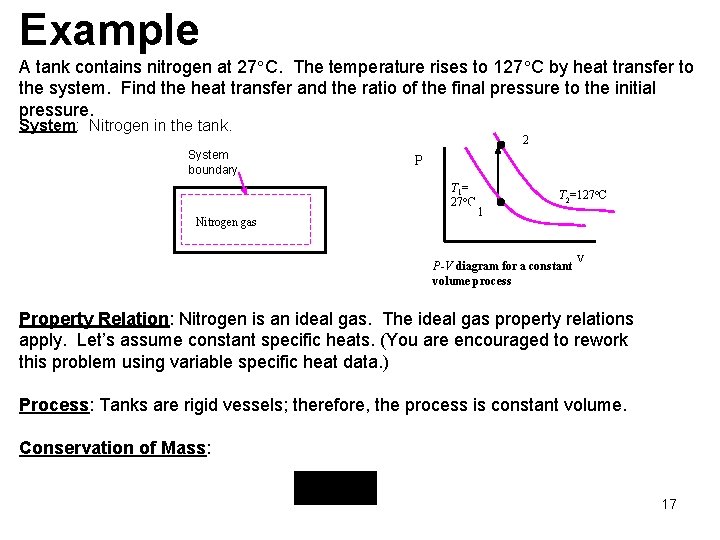
Example A tank contains nitrogen at 27 C. The temperature rises to 127 C by heat transfer to the system. Find the heat transfer and the ratio of the final pressure to the initial pressure. System: Nitrogen in the tank. System boundary 2 P T 1= 27 C Nitrogen gas T 2=127 C 1 P-V diagram for a constant volume process V Property Relation: Nitrogen is an ideal gas. The ideal gas property relations apply. Let’s assume constant specific heats. (You are encouraged to rework this problem using variable specific heat data. ) Process: Tanks are rigid vessels; therefore, the process is constant volume. Conservation of Mass: 17

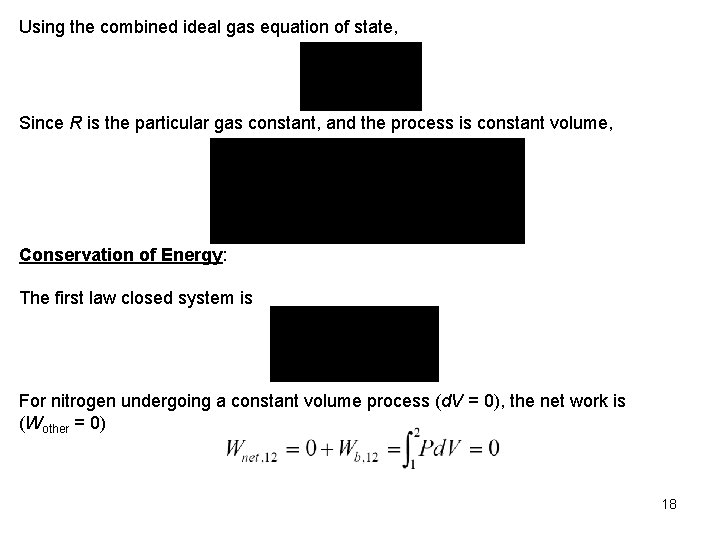
Using the combined ideal gas equation of state, Since R is the particular gas constant, and the process is constant volume, Conservation of Energy: The first law closed system is For nitrogen undergoing a constant volume process (d. V = 0), the net work is (Wother = 0) 18

Using the ideal gas relations with Wnet = 0, the first law becomes (constant specific heats) The heat transfer per unit mass is 19

Example The specific internal energy of a fluid is increased from 120 k. J/kg to 180 k. J/kg during a constant volume process. Determine the amount of heat energy required to bring about this increase for 2 kg of fluid. The non flow energy equation is Qnet – Wnet = U 2 – U 1 For a constant volume process W=0 and the equation becomes Q = U 2 – U 1 Q = 180 – 120 = 60 k. J/kg Therefore for 2 kg of fluid Q = 60 x 2 = 120 k. J i. e. 120 k. J of heat energy would be required. 20

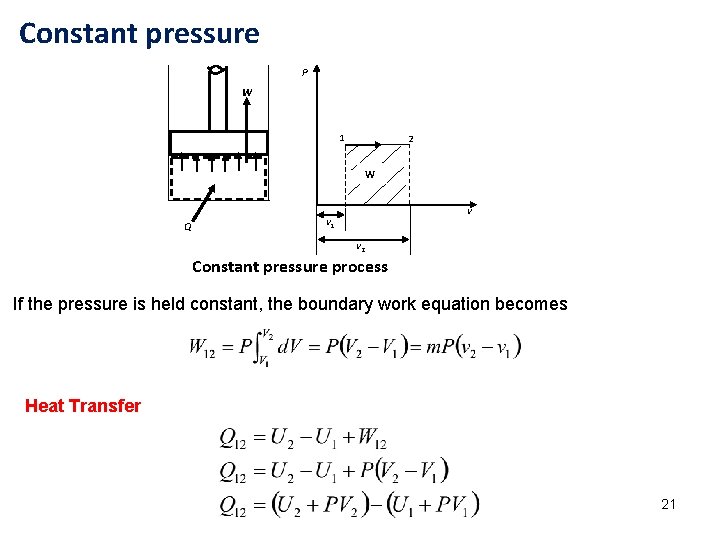
Constant pressure P W 1 2 W Q v v 1 v 2 Constant pressure process If the pressure is held constant, the boundary work equation becomes Heat Transfer 21
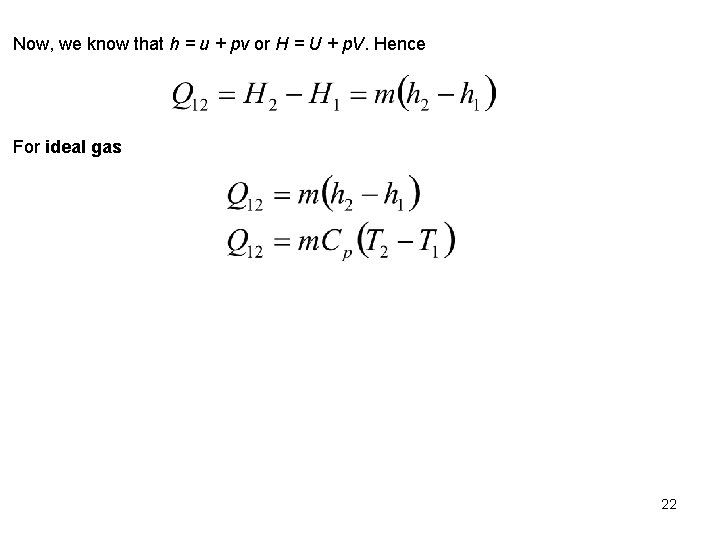
Now, we know that h = u + pv or H = U + p. V. Hence For ideal gas 22

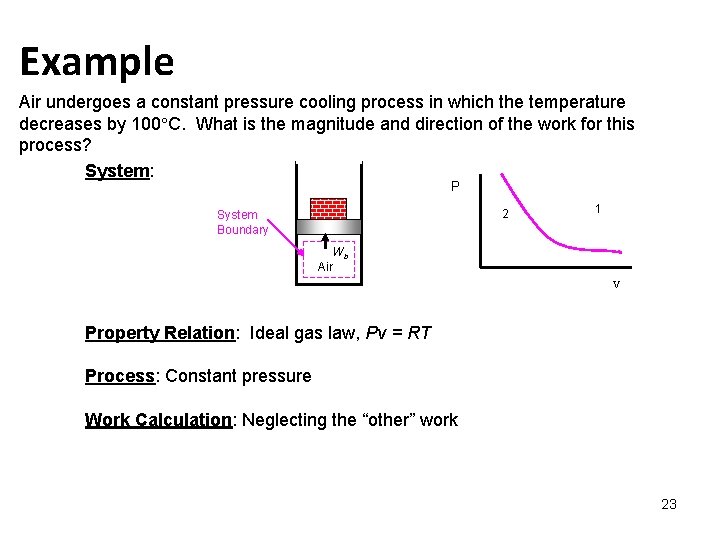
Example Air undergoes a constant pressure cooling process in which the temperature decreases by 100 C. What is the magnitude and direction of the work for this process? System: P 2 System Boundary 1 Wb Air V Property Relation: Ideal gas law, Pv = RT Process: Constant pressure Work Calculation: Neglecting the “other” work 23

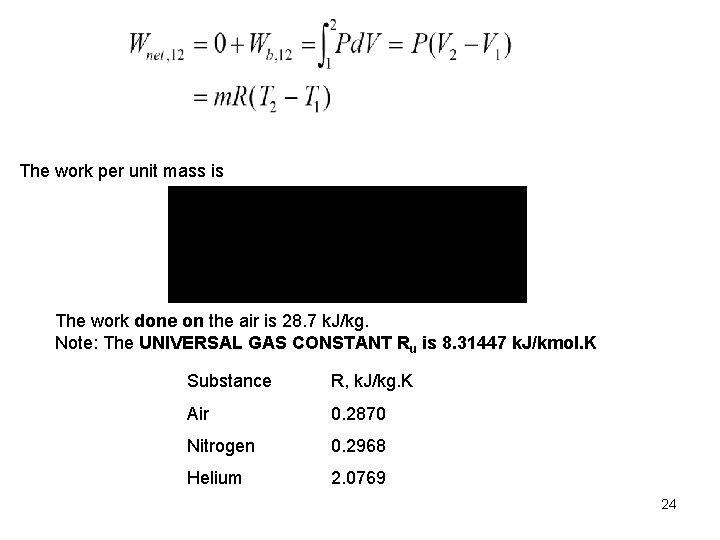
The work per unit mass is The work done on the air is 28. 7 k. J/kg. Note: The UNIVERSAL GAS CONSTANT Ru is 8. 31447 k. J/kmol. K Substance R, k. J/kg. K Air 0. 2870 Nitrogen 0. 2968 Helium 2. 0769 24

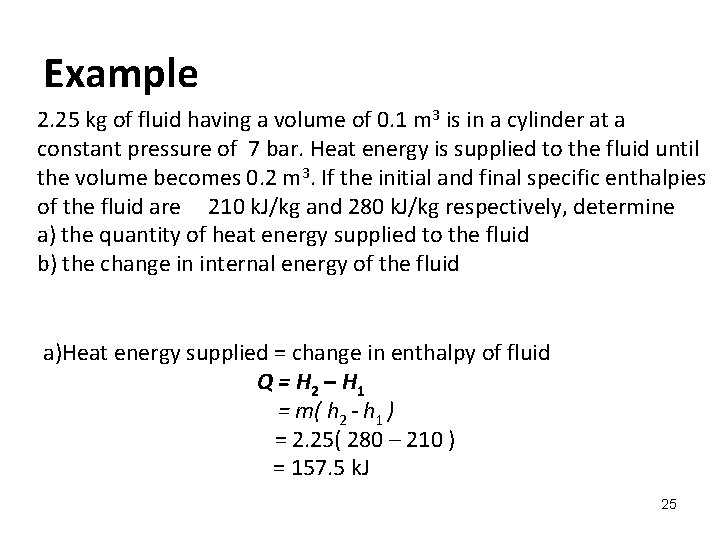
Example 2. 25 kg of fluid having a volume of 0. 1 m 3 is in a cylinder at a constant pressure of 7 bar. Heat energy is supplied to the fluid until the volume becomes 0. 2 m 3. If the initial and final specific enthalpies of the fluid are 210 k. J/kg and 280 k. J/kg respectively, determine a) the quantity of heat energy supplied to the fluid b) the change in internal energy of the fluid a)Heat energy supplied = change in enthalpy of fluid Q = H 2 – H 1 = m( h 2 - h 1 ) = 2. 25( 280 – 210 ) = 157. 5 k. J 25

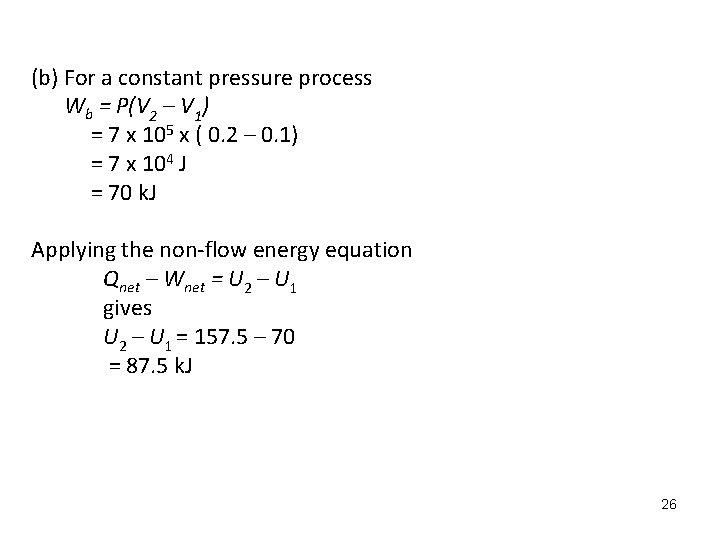
(b) For a constant pressure process Wb = P(V 2 – V 1) = 7 x 105 x ( 0. 2 – 0. 1) = 7 x 104 J = 70 k. J Applying the non-flow energy equation Qnet – Wnet = U 2 – U 1 gives U 2 – U 1 = 157. 5 – 70 = 87. 5 k. J 26

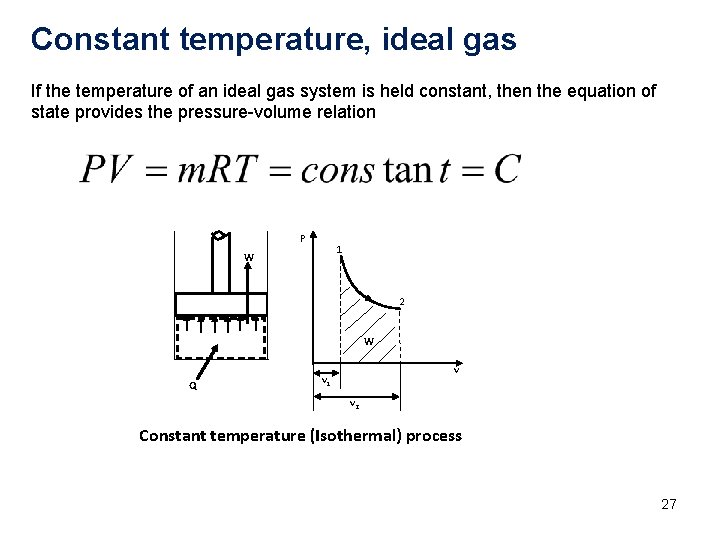
Constant temperature, ideal gas If the temperature of an ideal gas system is held constant, then the equation of state provides the pressure-volume relation P 1 W 2 W Q v v 1 v 2 Constant temperature (Isothermal) process 27

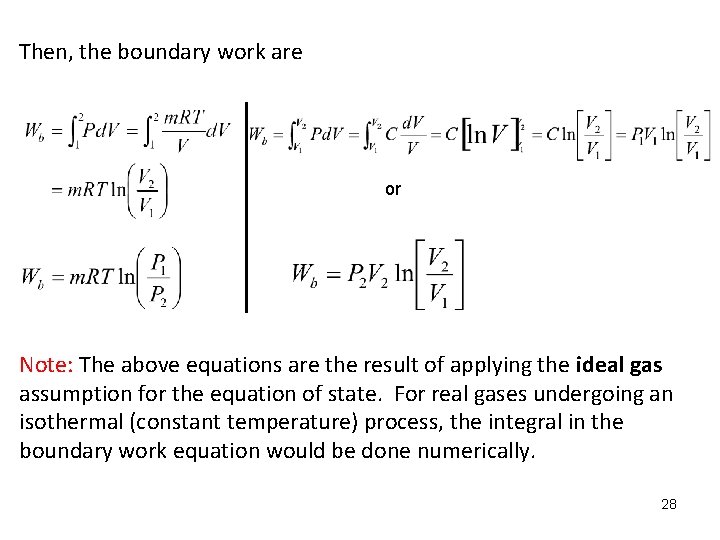
Then, the boundary work are or Note: The above equations are the result of applying the ideal gas assumption for the equation of state. For real gases undergoing an isothermal (constant temperature) process, the integral in the boundary work equation would be done numerically. 28

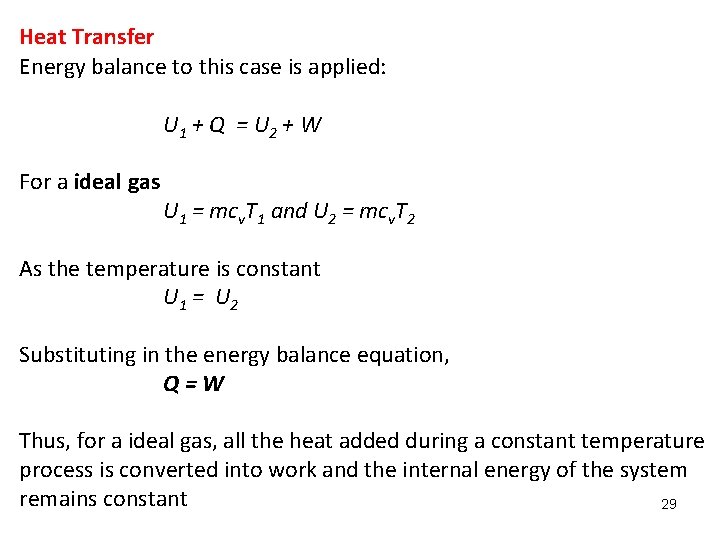
Heat Transfer Energy balance to this case is applied: U 1 + Q = U 2 + W For a ideal gas U 1 = mcv. T 1 and U 2 = mcv. T 2 As the temperature is constant U 1 = U 2 Substituting in the energy balance equation, Q=W Thus, for a ideal gas, all the heat added during a constant temperature process is converted into work and the internal energy of the system remains constant 29

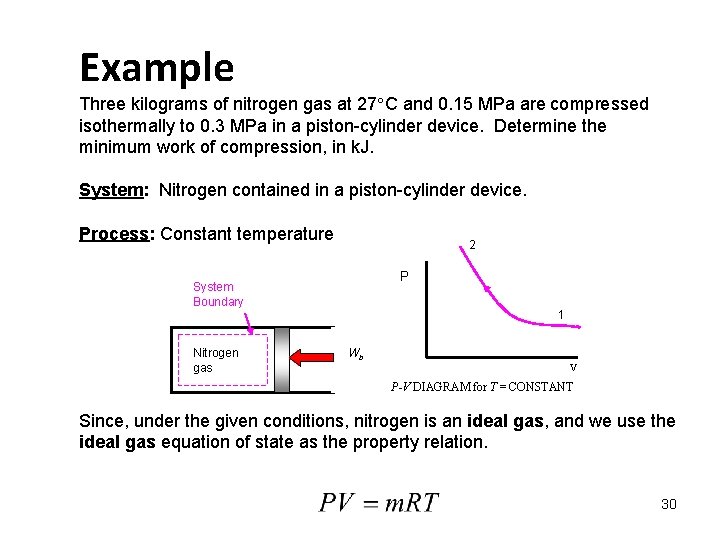
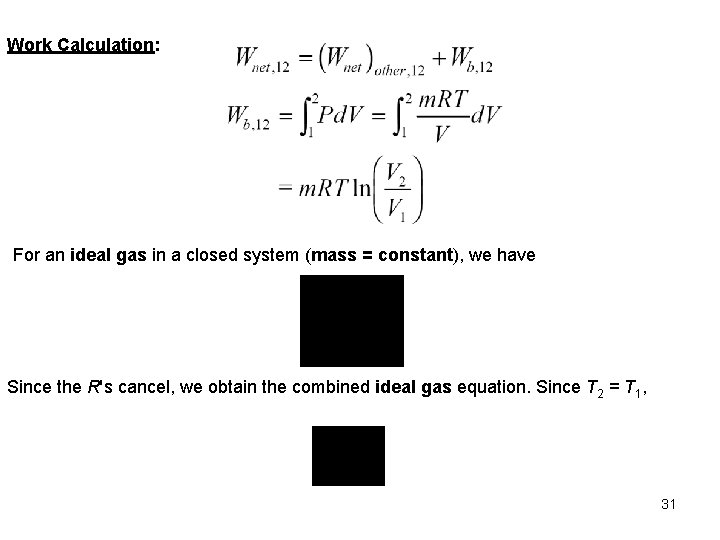
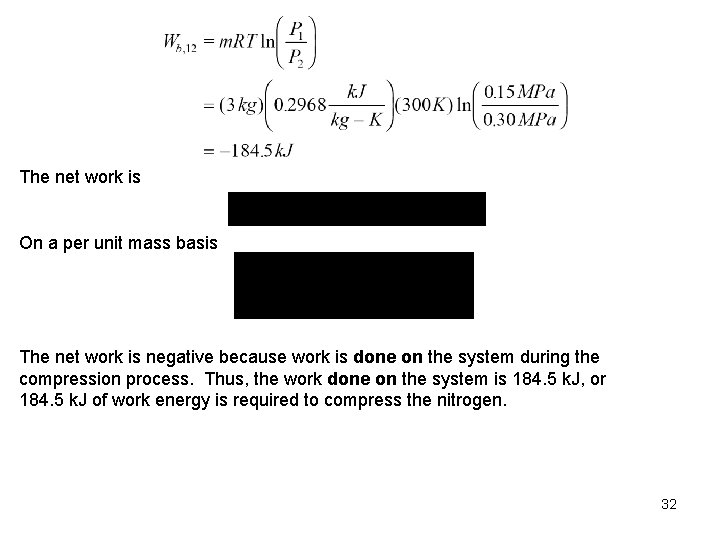
Example Three kilograms of nitrogen gas at 27 C and 0. 15 MPa are compressed isothermally to 0. 3 MPa in a piston-cylinder device. Determine the minimum work of compression, in k. J. System: Nitrogen contained in a piston-cylinder device. Process: Constant temperature 2 P System Boundary Nitrogen gas 1 Wb V P-V DIAGRAM for T = CONSTANT Since, under the given conditions, nitrogen is an ideal gas, and we use the ideal gas equation of state as the property relation. 30

Work Calculation: For an ideal gas in a closed system (mass = constant), we have Since the R's cancel, we obtain the combined ideal gas equation. Since T 2 = T 1, 31

The net work is On a per unit mass basis The net work is negative because work is done on the system during the compression process. Thus, the work done on the system is 184. 5 k. J, or 184. 5 k. J of work energy is required to compress the nitrogen. 32

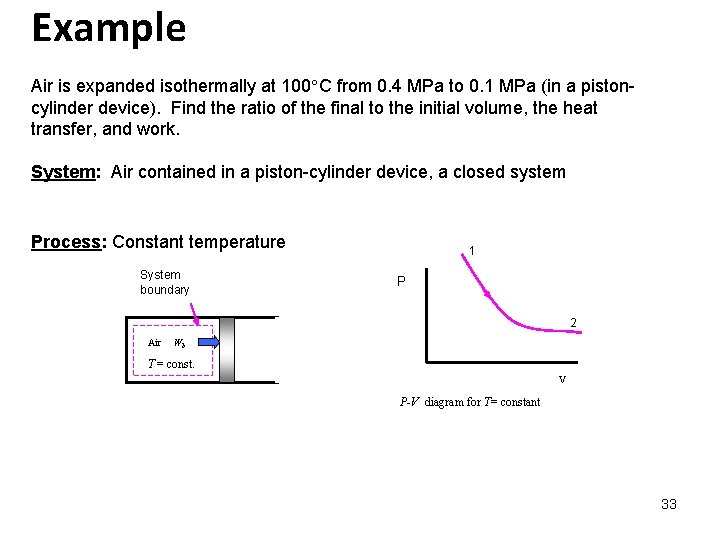
Example Air is expanded isothermally at 100 C from 0. 4 MPa to 0. 1 MPa (in a pistoncylinder device). Find the ratio of the final to the initial volume, the heat transfer, and work. System: Air contained in a piston-cylinder device, a closed system Process: Constant temperature System boundary 1 P 2 Air Wb T = const. V P-V diagram for T= constant 33

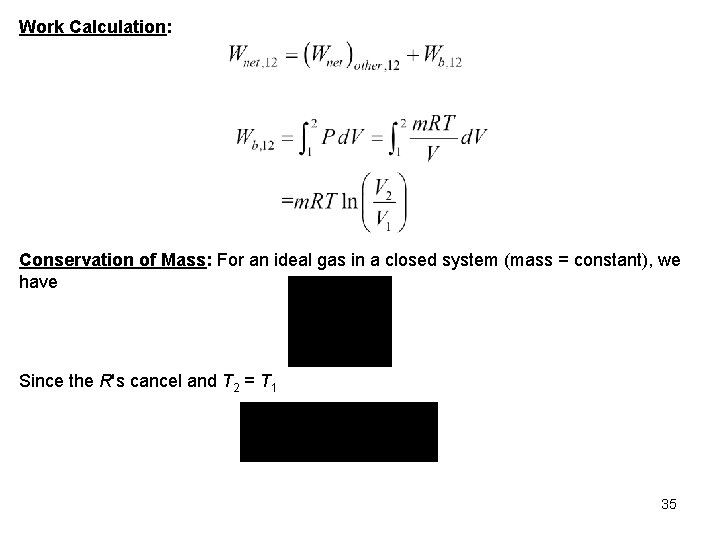
Property Relation: Assume air is an ideal gas and use the ideal gas property relations with constant specific heats. Conservation of Energy: The system mass is constant but is not given and cannot be calculated; therefore, let’s find the work and heat transfer per unit mass. 34

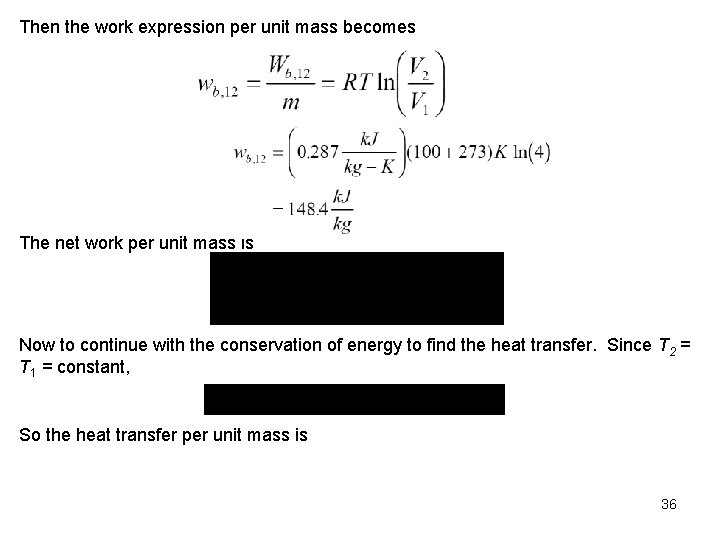
Work Calculation: Conservation of Mass: For an ideal gas in a closed system (mass = constant), we have Since the R's cancel and T 2 = T 1 35

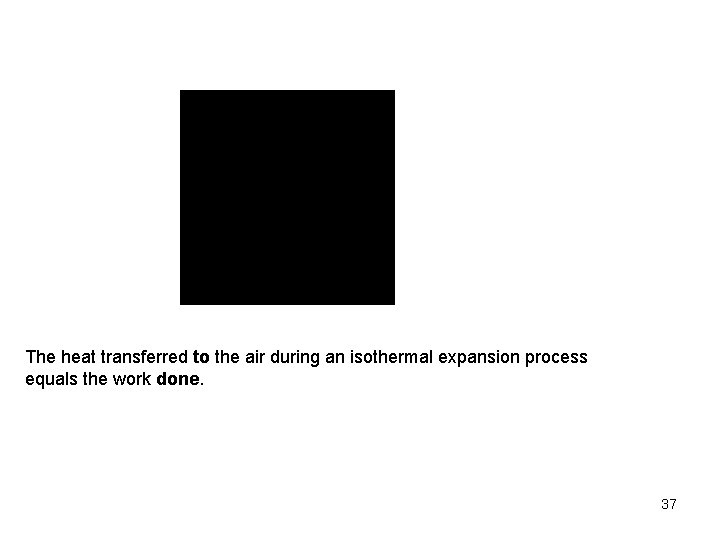
Then the work expression per unit mass becomes The net work per unit mass is Now to continue with the conservation of energy to find the heat transfer. Since T 2 = T 1 = constant, So the heat transfer per unit mass is 36

The heat transferred to the air during an isothermal expansion process equals the work done. 37

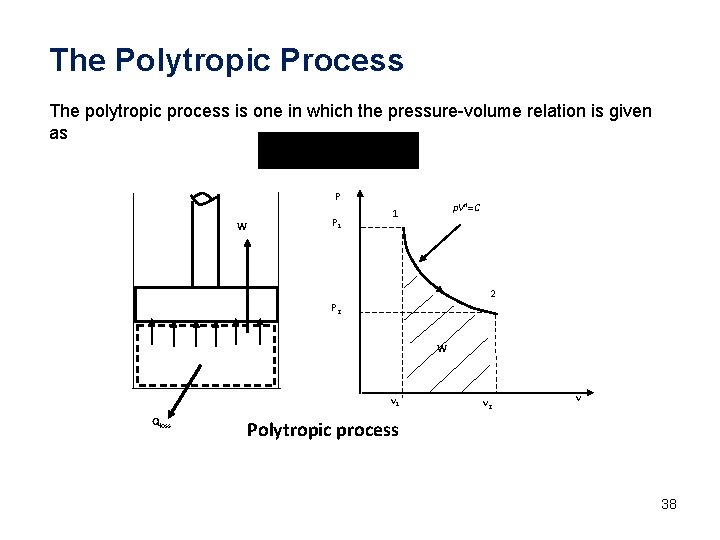
The Polytropic Process The polytropic process is one in which the pressure-volume relation is given as P W P 1 p. Vn=C 1 2 P 2 W v 1 Qloss v 2 v Polytropic process 38

The exponent n may have any value from minus infinity to plus infinity depen-ding on the process. Some of the more common values are given below. Process Constant pressure Constant volume Isothermal & ideal gas Adiabatic & ideal gas Exponent n 0 1 k = CP/CV Here, k is the ratio of the specific heat at constant pressure CP to specific heat at constant volume CV. The specific heats will be discussed later. The boundary work done during the polytropic process is found by substituting the pressure-volume relation into the boundary work equation. The result is 39

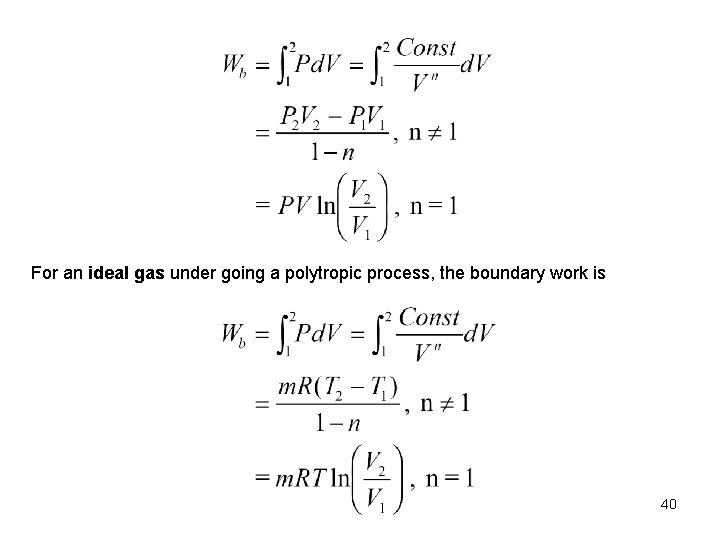
For an ideal gas under going a polytropic process, the boundary work is 40

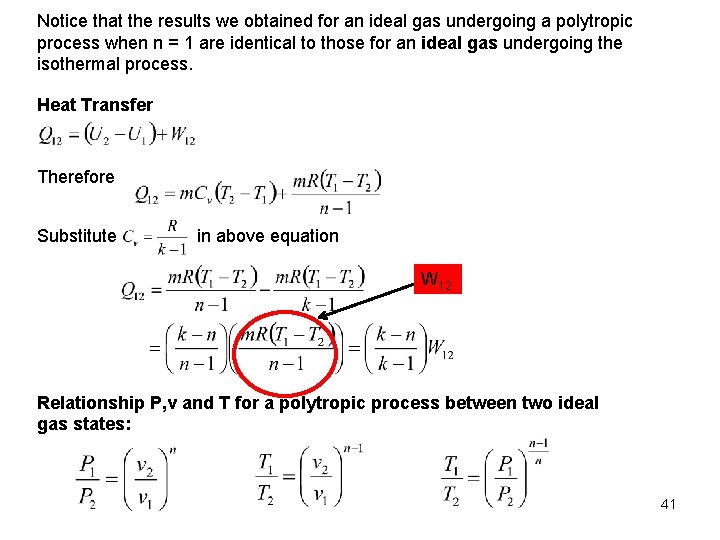
Notice that the results we obtained for an ideal gas undergoing a polytropic process when n = 1 are identical to those for an ideal gas undergoing the isothermal process. Heat Transfer Therefore Substitute in above equation W 12 Relationship P, v and T for a polytropic process between two ideal gas states: 41

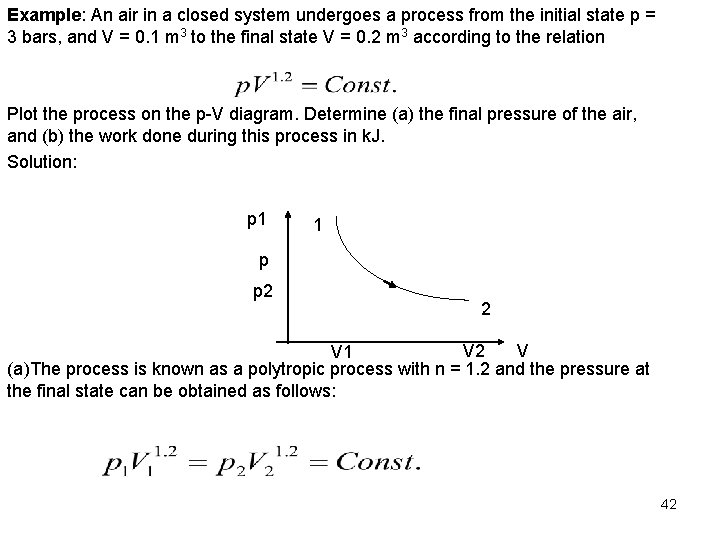
Example: An air in a closed system undergoes a process from the initial state p = 3 bars, and V = 0. 1 m 3 to the final state V = 0. 2 m 3 according to the relation Plot the process on the p-V diagram. Determine (a) the final pressure of the air, and (b) the work done during this process in k. J. Solution: p 1 1 p p 2 2 V V 1 (a)The process is known as a polytropic process with n = 1. 2 and the pressure at the final state can be obtained as follows: 42

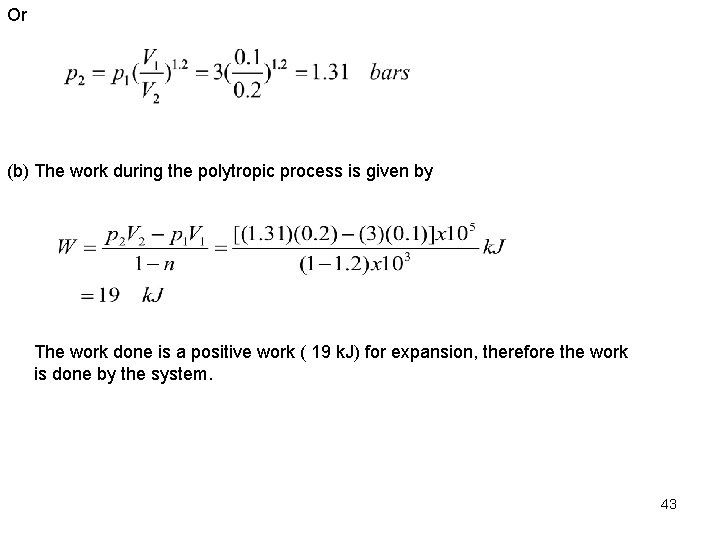
Or (b) The work during the polytropic process is given by The work done is a positive work ( 19 k. J) for expansion, therefore the work is done by the system. 43

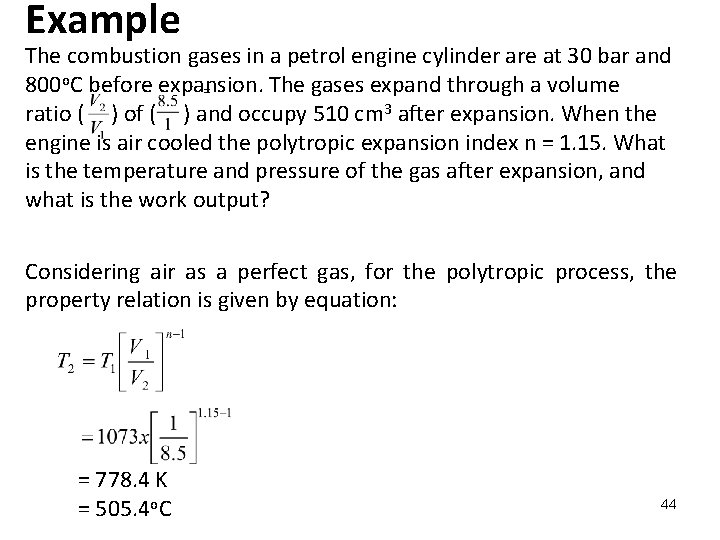
Example The combustion gases in a petrol engine cylinder are at 30 bar and 800 o. C before expansion. The gases expand through a volume = ratio ( ) of ( ) and occupy 510 cm 3 after expansion. When the engine is air cooled the polytropic expansion index n = 1. 15. What is the temperature and pressure of the gas after expansion, and what is the work output? Considering air as a perfect gas, for the polytropic process, the property relation is given by equation: = 778. 4 K = 505. 4 o. C 44

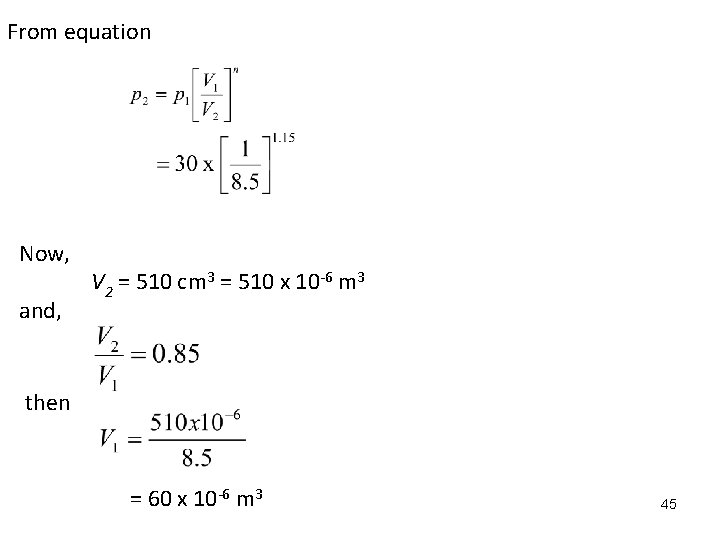
From equation Now, and, V 2 = 510 cm 3 = 510 x 10 -6 m 3 then = 60 x 10 -6 m 3 45

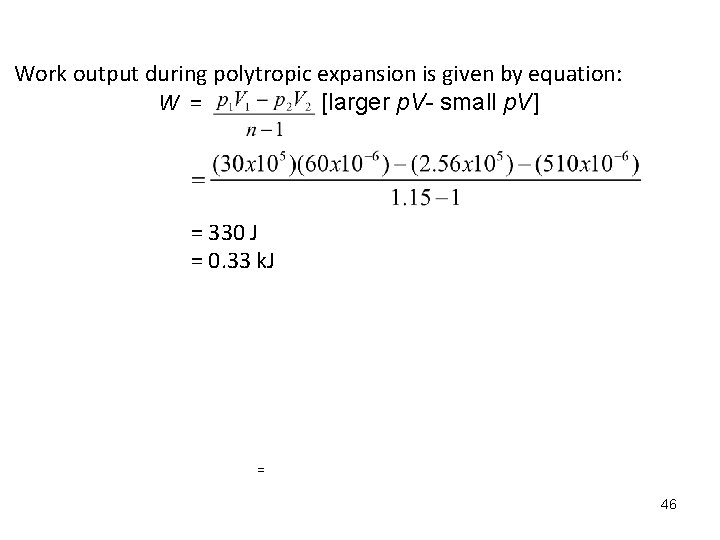
Work output during polytropic expansion is given by equation: W = [larger p. V- small p. V] = 330 J = 0. 33 k. J = 46

The Systematic Thermodynamics Solution Procedure When we apply a methodical solution procedure, thermodynamics problems are relatively easy to solve. Each thermodynamics problem is approached the same way as shown in the following, which is a modification of the procedure given in the text: Thermodynamics Solution Method 1. Sketch the system and show energy interactions across the boundaries. 2. Determine the property relation. Is the working substance an ideal gas or a real substance? Begin to set up and fill in a property table. 3. Determine the process and sketch the process diagram. Continue to fill in the property table. 4. Apply conservation of mass and conservation of energy principles. 5. Bring in other information from the problem statement, called physical constraints, such as the volume doubles or the pressure is halved during the process. 6. Develop enough equations for the unknowns and solve. 47