Chapter 3 The Aquatic Environment Aquatic ecosystem 75































- Slides: 49

Chapter 3 The Aquatic Environment

• Aquatic ecosystem: 75% of planet surface • The major feature influencing the adaption of organism: salinity saltwater (marine) freshwater

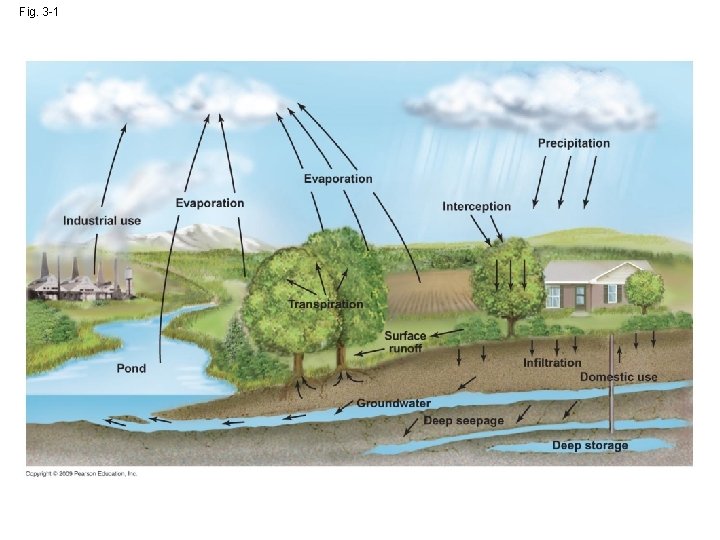
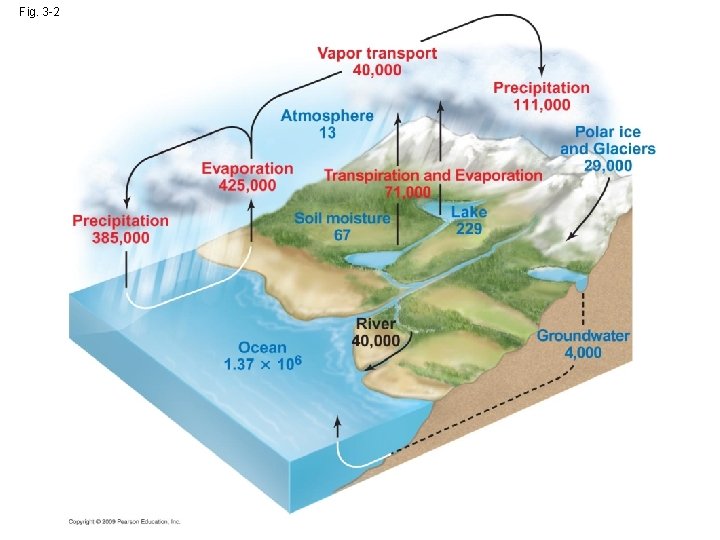
3. 1 Water cycles between earth and the atmosphere • Water cycle:hydrologic cycle • water→ water vapor→ precipitation ↑ solar energy (evaporation) ↓ driving force

Fig. 3 -1

• Interception(攔截): water is intercepted by vegetation, dead organic matter or urban structure and streets → water never infiltrate the ground, but evaporate directly back to the atmosphere. Infiltration(滲透): water reach the soil move into the ground →Infiltration rate: type of soil, slope, vegetation and intensity of the precipitation.

• Surface runoff: overland flow groundwater • Transpiration: the evaporation of water from internal surface of leaves, stem, and other living part • Evapotranspiration:the total flux of evaporating water → evaporation+ transpiration

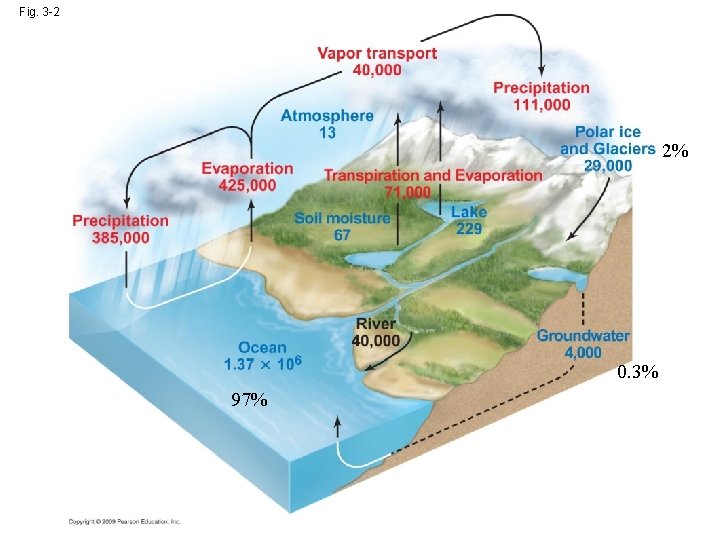
Fig. 3 -2 2% 0. 3% 97%

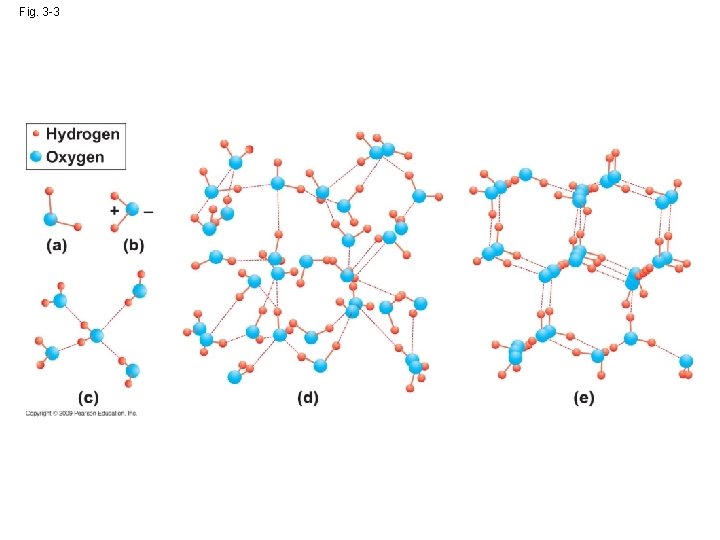
3. 2 Water has important physical properties

Fig. 3 -3

The structure of water is based on hydrogen bonds • Covalent bond(共價鍵)→ hydrogen bond: easily broken and reformed • Polar covalent bond

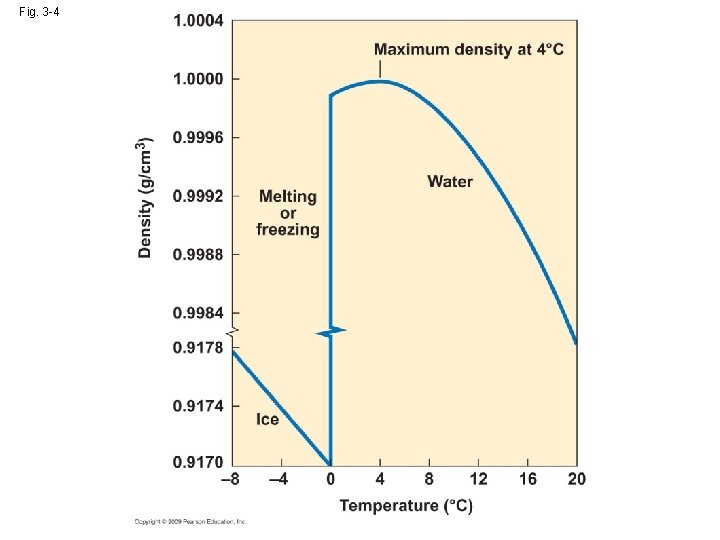
• High Specific heat: the number of calories necessary raise 1 g of water 1℃ →warm up slowly in spring and cool off just as slowly in the fall: prevent the wide seasonal fluctuation in the temperature of the aquatic habitats → thermal regulation of organism: temperature variation is moderated relative to change in ambient temperature

• High Specific heat: the number of calories necessary raise 1 g of water 1℃ Gas→ liquid→ solid ↓ ↓ evaporation ↓ ↓ latent heat: 536 cal 80 cal

Fig. 3 -4

• Surface tension: cohesion(內聚力): hydrogen bond • Viscosity: the resistance of a liquid to flow → the frictional resistance of water is 100 times greater than air: due to high density (860 times) →reduce this frictional resistance→ streamline body • High density: Buoyancy: unnecessary structural material to erect against the force of gravity and movement • water pressure: increase 1 atm (1 kg/cm 2) per 10 m

Water strider

Sperm whale抹香鯨

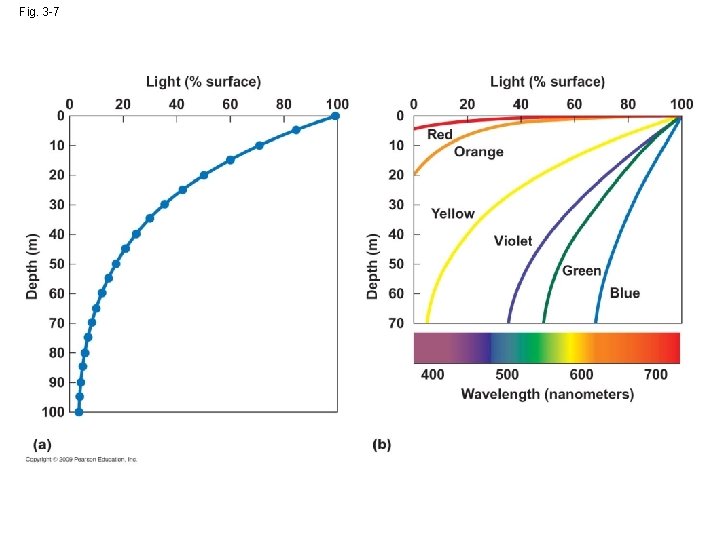
3. 3 Light varies with depth in aquatic environments • The amount of light in the water reduced by two process (1) suspended particles (2) water itself absorbs light

Fig. 3 -7

3. 3 Light varies with depth in aquatic environments • The amount of light in the water reduced by two process (1) suspended particles (2) water itself absorbs light • (1) red and infrared radiation (2) yellow→ green→ violet →leaving only blue: 100 m→ 10% of blue light

• Adaptation: (1) silver gray and deep black (2) lack pigment (3) large eye: maximum light-gathering ability (4) organ: produce light (bioluminescence)

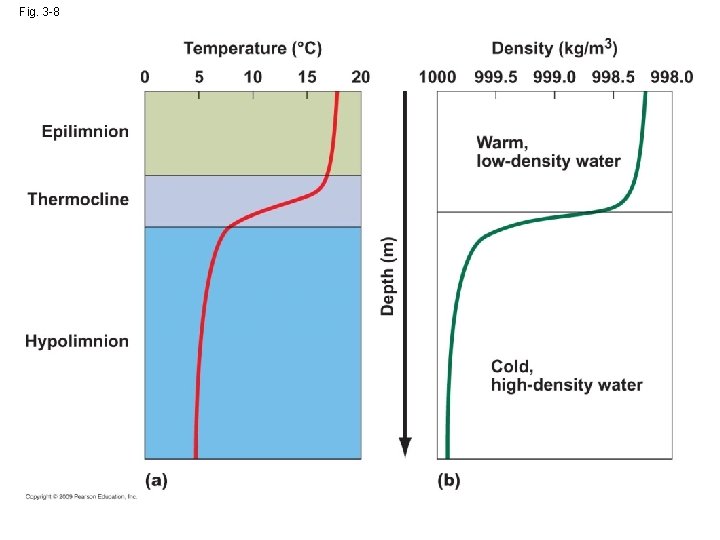
3. 4 Temperature varies with water depth

Fig. 3 -8

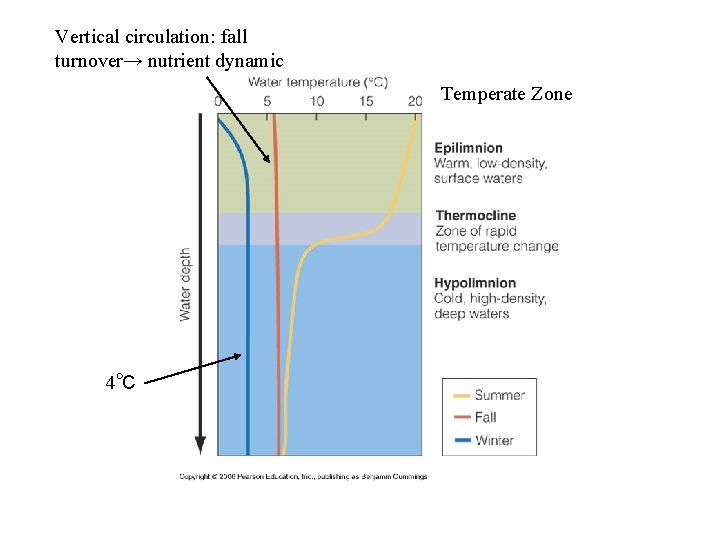
Vertical circulation: fall turnover→ nutrient dynamic Temperate Zone 4℃

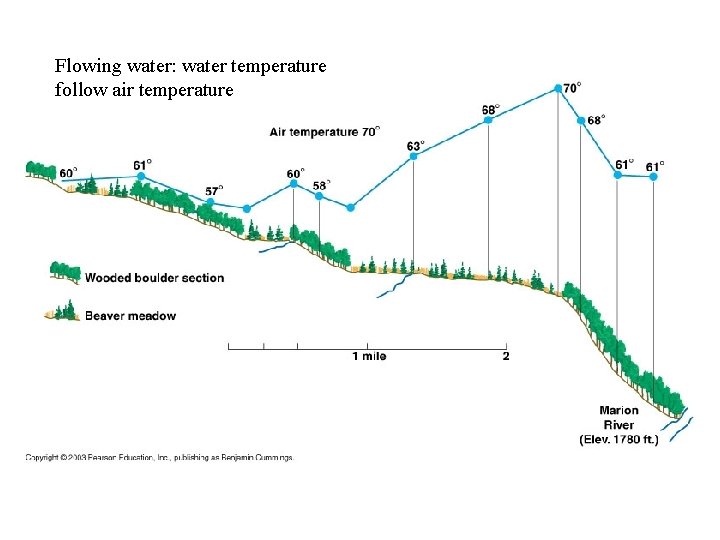
Flowing water: water temperature follow air temperature

3. 5 Water functions as a solvent • Solution: 溶液 solvent: 溶劑 solute: 溶質 → aqueous solution • Water is excellent solvent: biological crucial substance (1) nutrient and waster can be dissolved and transported (2) regulate temperature (3) preserves chemical equilibrium

• The attraction between water and salt > ionic bond→ dissolve • ocean: still(蒸餾器) → the concentration of solute is limited by the maximum solubility of the compound→ deposited • psu: Practical Salinity Units

Fig. 3 -2


3. 6 Oxygen diffuse from the atmosphere to the surface water • Diffusion: molecule to move from high concentration to one of lower concentration • The diffusion process result in a net transfer of oxygen and carbon dioxide from the atmosphere into surface water and from surface to deep water.

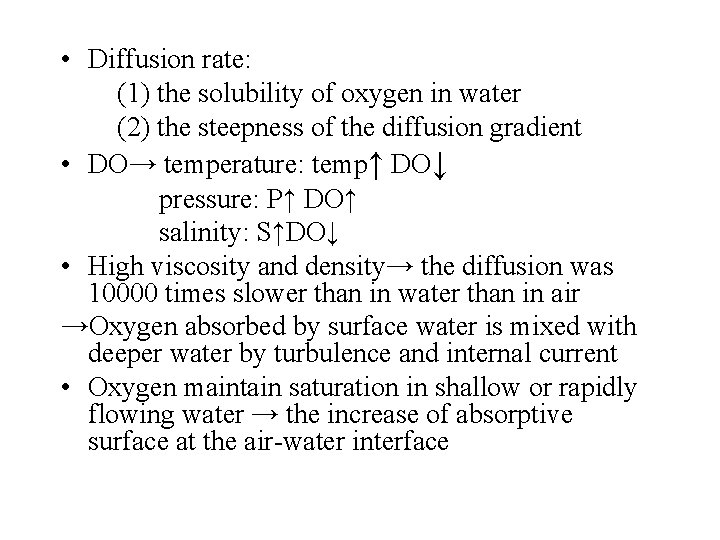
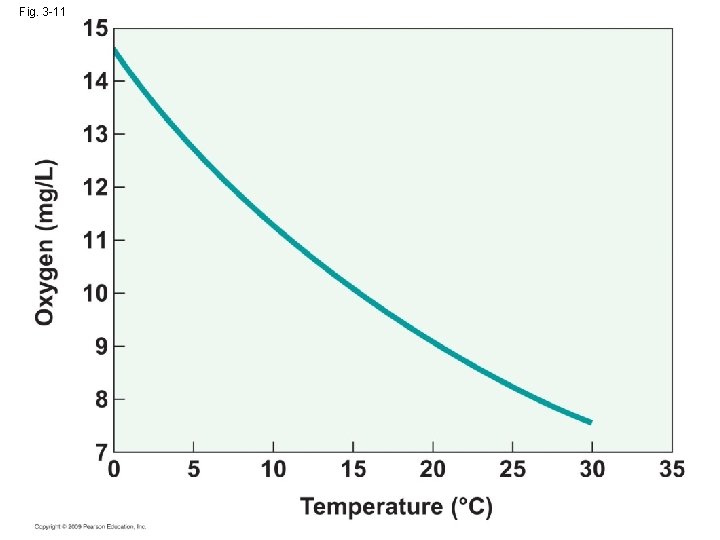
• Diffusion rate: (1) the solubility of oxygen in water (2) the steepness of the diffusion gradient • DO→ temperature: temp↑ DO↓ pressure: P↑ DO↑ salinity: S↑DO↓ • High viscosity and density→ the diffusion was 10000 times slower than in water than in air →Oxygen absorbed by surface water is mixed with deeper water by turbulence and internal current • Oxygen maintain saturation in shallow or rapidly flowing water → the increase of absorptive surface at the air-water interface

Fig. 3 -11

Diffusion, wind and photosynthesis Temp↓ Do↑ Oxygen was replenished

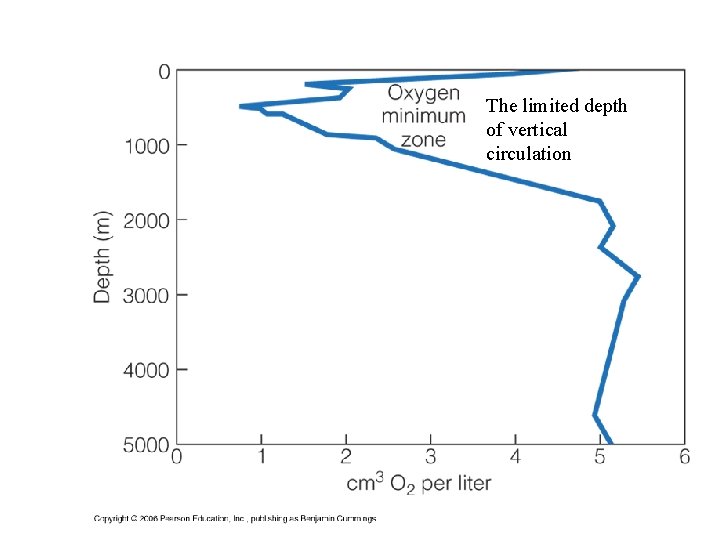
The limited depth of vertical circulation

• The solubility of gases is not great atmosphere: 21% fresh water at 0℃: 1%

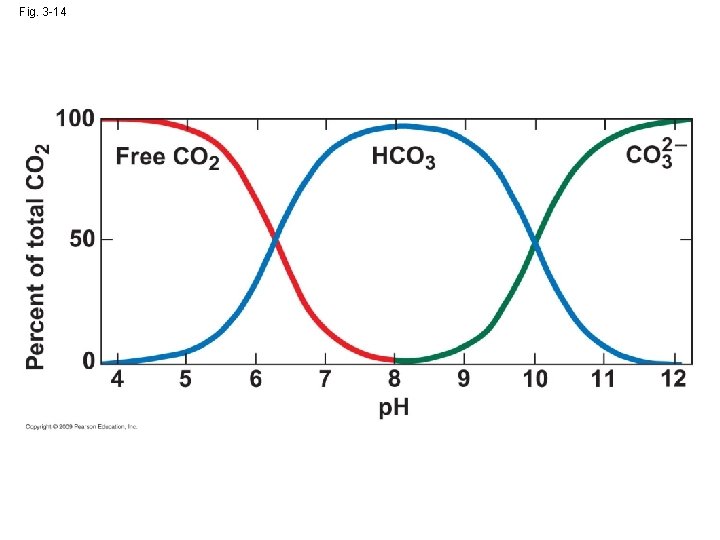
3. 7 Acidity has a widespread influence on aquatic environment • CO 2+H 2 O↔H 2 CO 3↔HCO 3 -1↔H++CO 3 -2 ↓ ↓ carbonic acid bicarbonate ion →a buffer to keep the p. H within a narrow range • Acidity: � H� Alkaline: � OH� • p. H=- log � H� p. H=6 → � H� =10 -6 , � OH� =10 -8

Fig. 3 -14

• Natural water: p. H: 2 -12 • Sea water: 7. 5 -8. 4 • acidity→ influence on the distribution and abundance of organism high acidity→ physiological (direct) or the concentration of toxic heavy metal (indirect) → tolerance limit: 4. 5: low p. H→high Al (highly toxic)

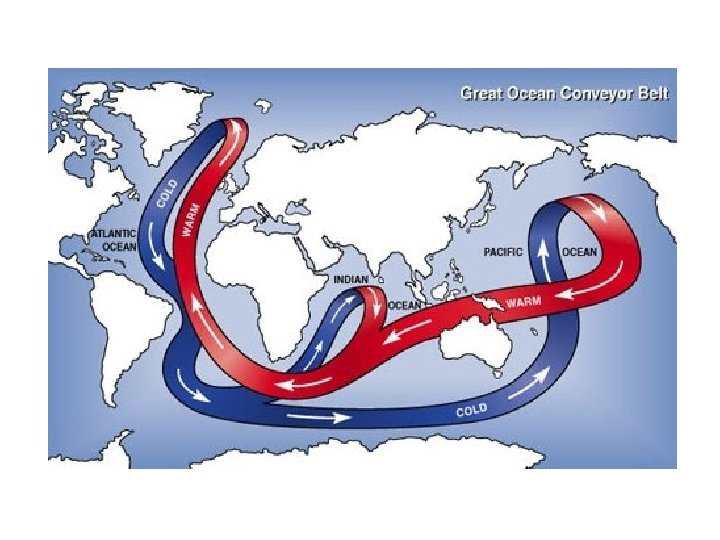
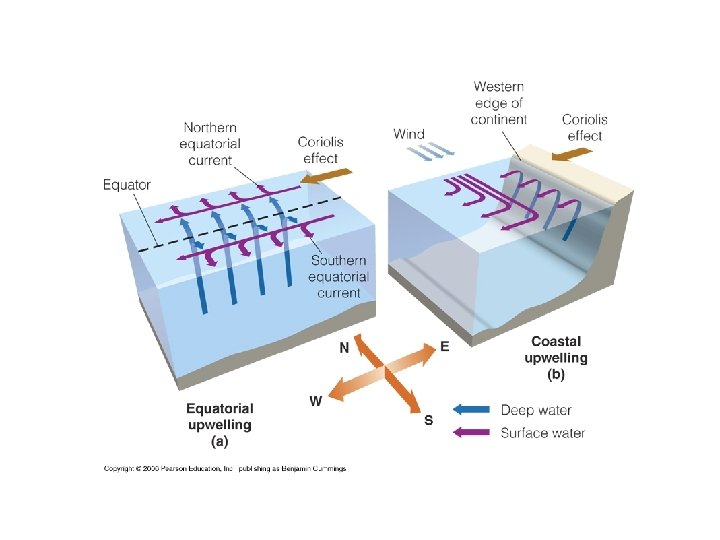
3. 8 Water movements shape freshwater and marine environments • Water movement: current in stream and wave in open body of water • Fast stream: velocity > 50 cm/sec → remove all particle less than 5 mm • Wave: wind→ ripple→ wave →more energy (wind continue blow) → wave continue to grow→ wind energy= energy lost by breaking wave (white cape) →wave approach land: the height of each wave rises until wave front grow to steeps and topples over • Thermohaline circulation • Upwelling

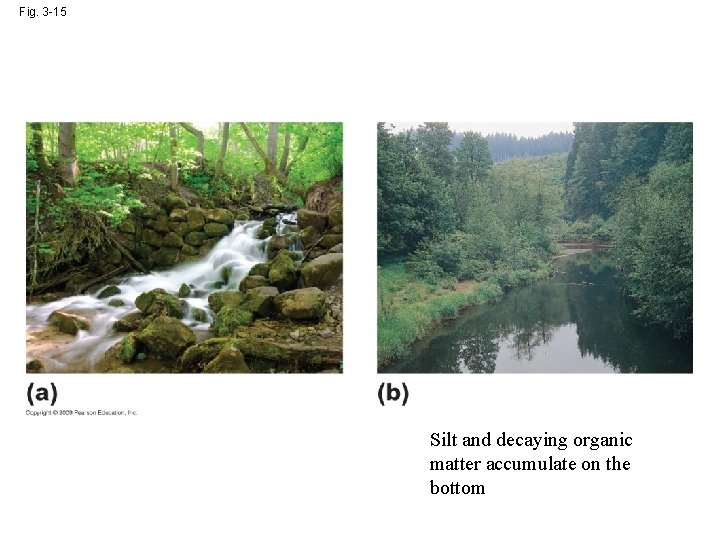
Fig. 3 -15 Silt and decaying organic matter accumulate on the bottom

Fig. 3 -16



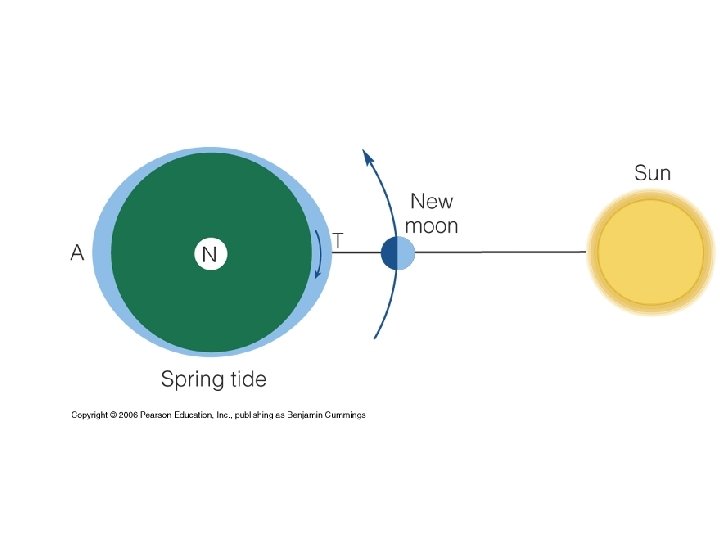
3. 9 Tides dominate the marine environment • tide:the gravitational pull of the sun and moon • moon:two lunar tidal bulges (high tide) every day (12 hour 25 min) ,two of the lows or low tides • Sun: new and full→ brimming fullness → neap tides



• Tide advance: westward • Intertidal zone: dramatic shift in environmental condition

3. 10 The transition zone between freshwater and saltwater environments present unique constraints • Estuary: freshwater join and mix with the salt water (1) homogenous→ current strong enough to mix the water from top to bottom. (2) stratification→ surface: fresh bottom: salty water (2) low tide: homogenous high tide: surface wedge of seawater move upstream more rapidly than the bottom seawater → density is inverted: tidal overmixing


• Horizon • Salinity: left>right