ATOMS ELEMENTS AND MATTER Matter Anything that has













































- Slides: 54

ATOMS, ELEMENTS, AND MATTER

Matter Anything that has mass and volume.


Mass The amount of matter in an object

Volume The amount of space an object takes up.

Matter is found in four forms solids, liquids, gasses, and plasma

Solids Have a definite shape and volume. It holds its shape

Liquid Has definite volume Shape is defined by its container Particles move more than in a solid


Gas No definite shape No definite volume Particles move freely in all directions.

Plasmas A collection of free moving electrons and ions – usually a form of ionized gas Most common form of matter Uncommon on Earth The auroras, stars, and lightning are all plasma Fluorescent lights are a form of plasma


Law of Conservation of Matter can change forms, but the total amount of it does not change, ever. Not even with a physical or chemical change

Example 100 pounds of timber would equal 100 pounds of ash and smoke after burning

Physical Change A change that alters the form or appearance of matter but doesn't change the material into a new substance.

Examples of Physical Changes

Chemical Change A change in matter that forms one or more new substances

Examples of a chemical change

Signs of a Chemical Change 1) Color Change 2) Odor is produced 3) A gas (bubbles) or a solid is produced 4) Temperature change 5)Energy is released or absorbed

Atoms The basic units of matter. All atoms are made up of protons (+), electrons (), and neutrons.

Elements Consist of atoms of only one type Can not be broken down into simpler substances by ordinary chemical changes. The identy of an atom is determined by the number of protons in its nucleus which is its atomic number.

Helium element

Elements Each of the 92 naturally occuring elements can be identified by a symbol

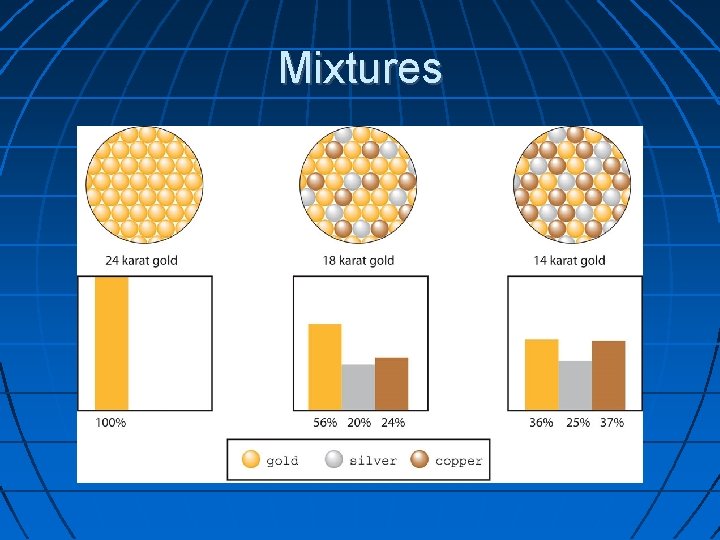
Mixture A combination of two or more substances that do not combine chemically but remain the same individual substances Mixtures can be separated by physical means. Examples of a mixture are kool-aid, saltwater, and juice

Mixtures

Candy mixture

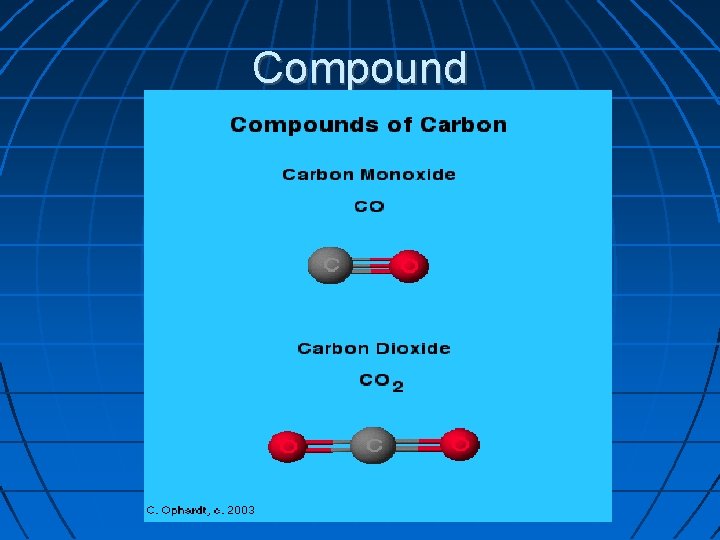
Compound A substance made up of two or more different types of elements bonded together. A compund has different properties than the elements that make it up; therefore, it is a chemical change.

Compound

coumpound

Question Oxygen gas has the chemical formula of O 2 Why would this not be considered a compound?

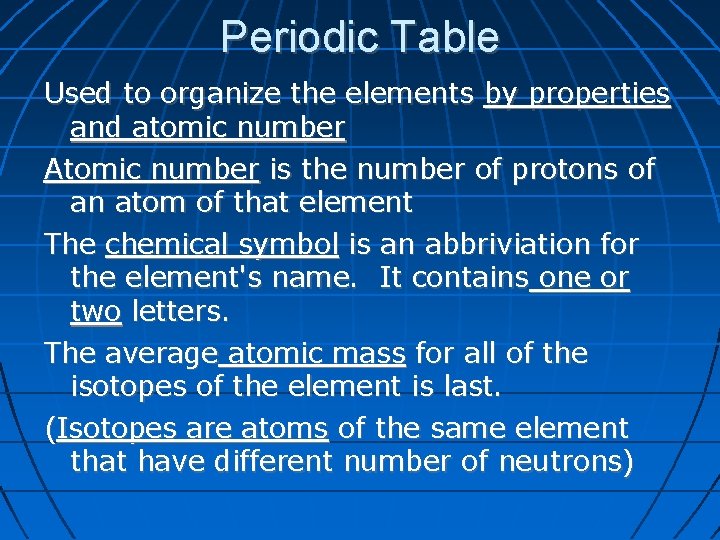
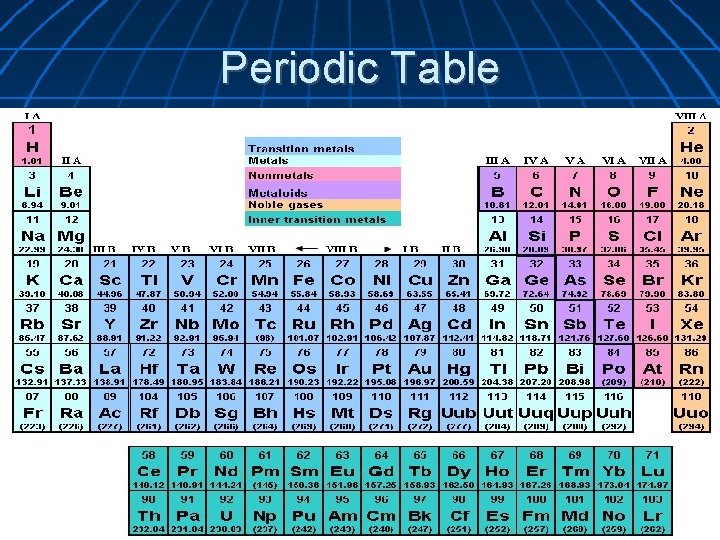
Periodic Table Used to organize the elements by properties and atomic number Atomic number is the number of protons of an atom of that element The chemical symbol is an abbriviation for the element's name. It contains one or two letters. The average atomic mass for all of the isotopes of the element is last. (Isotopes are atoms of the same element that have different number of neutrons)

Periodic Table


Properties of the Periodic Table Groups – All of the elements in a column. They have similar chemical and physical properties. They are numbered on top.

Groups

Properties of the Periodic Table Period– Each horizontal row in the periodic table.

Periods

Metals Are located to the left of the table (except Hydrogen) and zigzag through group 13, 14, and 15. Most can be shaped easily Most are solid at room temperature Most conduct electricity easily. Most have a high melting point. Examples are gold, silver, sodium, copper, aluminum tungsten, and zinc

metals

Nonmetals Found on the right hand side of the periodic table. Many are gases at room temperature Solid nonmetals are often dull and cannnot be shaped by hammering. They are generally poor conductors of heat and electricity.

Nonmetals

Halogens The elements in group 17 Halogen comes from the Greek word meaning “forming salts” They are very reactive nonmetals that combine easily with metals to form salts Also used to kill harmful microorganisms (Chlorine & Iodine)


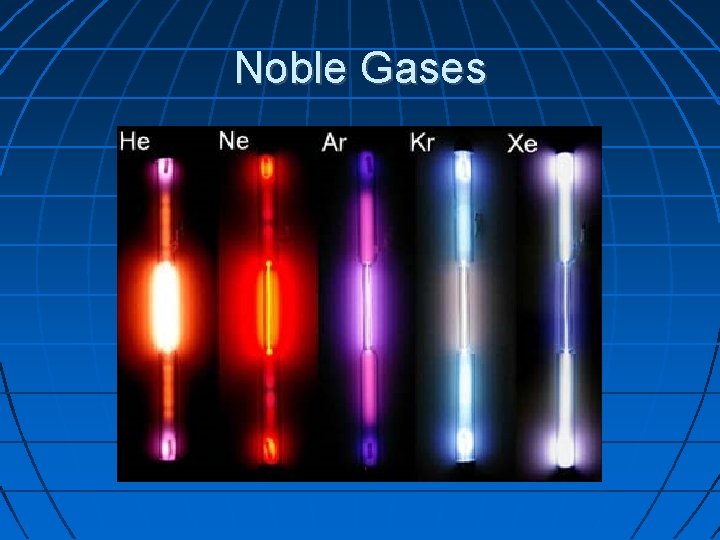
Noble Gases Found in Group 18 They almost never react with other elements Examples are argon, neon, krypton, and xenon

Noble Gases

Acids Is a substance that can donate a hydrogen ion (a proton) to another substance. They react with most metals Taste sour (vinegar) Burns the skin Turns litmus paper red

Acids

Bases A substance that can accept a hydrogen ion from another substance. Taste bitter Feel slippery Turns litmus paper blue

Bases

PH The concentration of hydrogen ions in a solutions A measurement of acidity Numbers range from 0 to 14 with 7 being neutral Numbers below 7 are acids Numbers above 7 are bases


Acids and Bases Neutralize Each Other Acids will react with bases to form a neutral substance Common example is swallowing an antacid tablet to releive an upset stomach.

