Elements Atoms Atoms Matter is anything that takes





- Slides: 12

Elements & Atoms

Atoms • Matter is anything that takes up space and has mass. • All matter is made of atoms • Atoms are the building blocks of matter, sort of how bricks are the building blocks of houses.

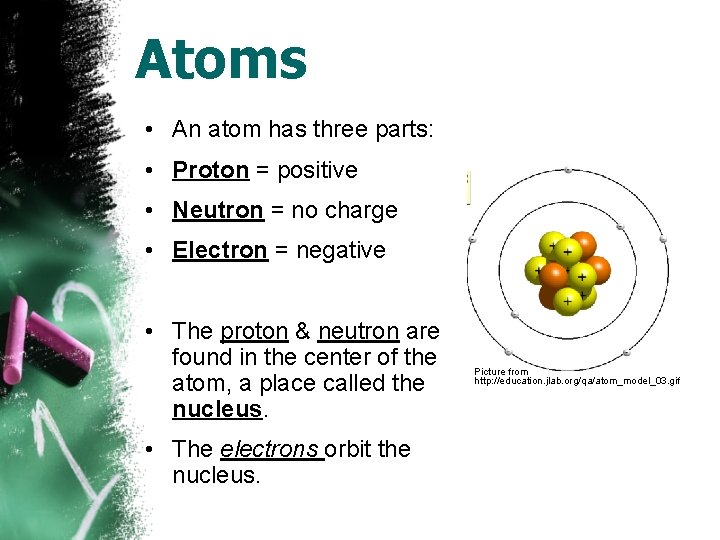
Atoms • An atom has three parts: • Proton = positive • Neutron = no charge • Electron = negative • The proton & neutron are found in the center of the atom, a place called the nucleus. • The electrons orbit the nucleus. Picture from http: //education. jlab. org/qa/atom_model_03. gif

What are elements? • Elements are pure substances because they are made up only one kind of atom.

Graphic from http: //education. jlab. org/atomtour/fact 2. html

More about Elements… • Elements are the building blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things…

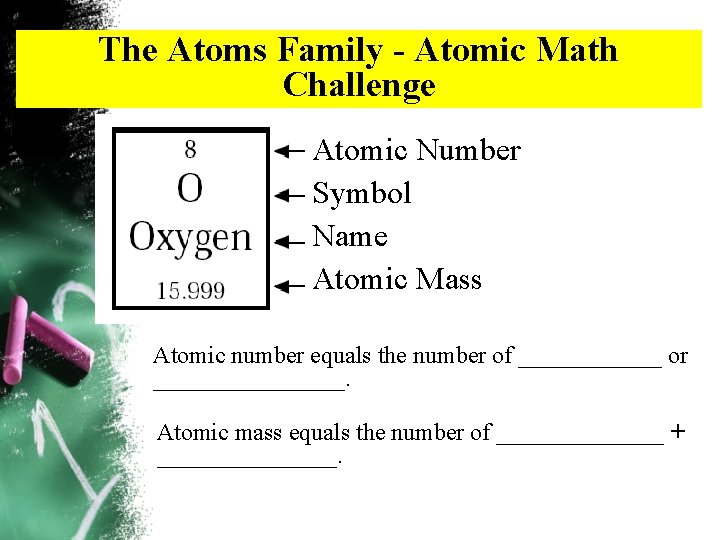
Periodic Table Atomic Number: Number of protons and it is also the number of electrons in an atom of an element. Element’s Symbol: An abbreviation for the element. 8 O Oxygen Elements Name 16 Atomic Mass/Weight: Number of protons + neutrons.

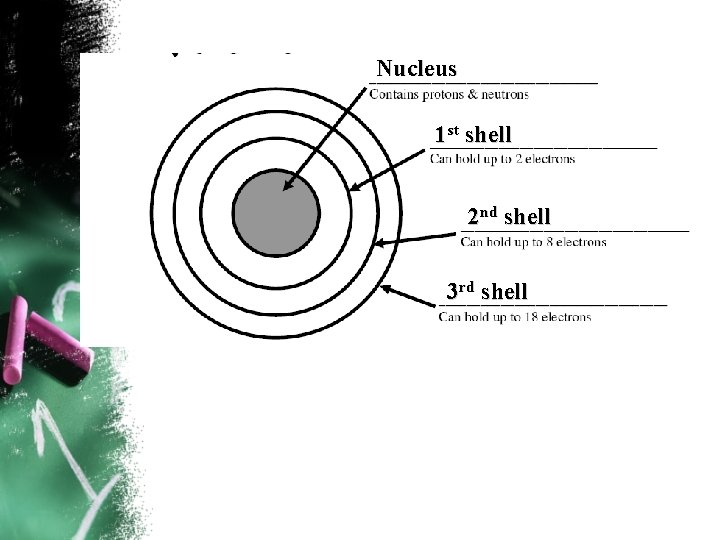
Electrons have special rules…. • You can’t just shove all of the electrons into the first orbit of an element. • Electrons live in something called shells or energy levels. • Only so many can be in any certain shell. • The electrons in the outer most shell of any element are called valance electrons.

Nucleus 1 st shell 2 nd shell 3 rd shell

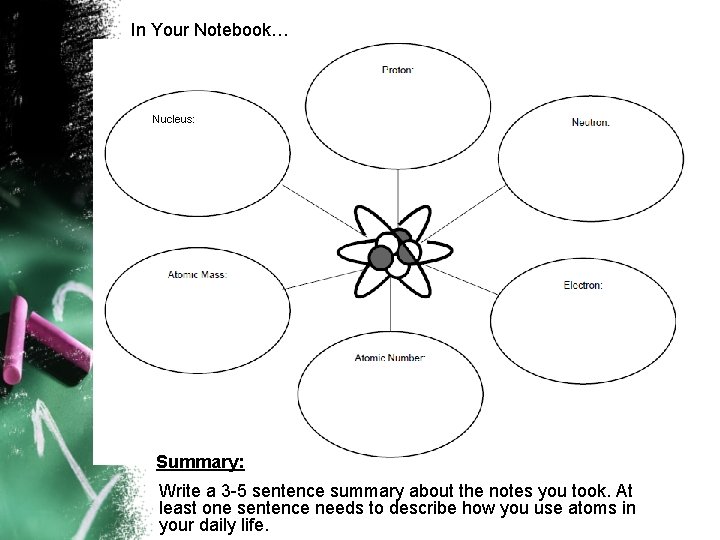
In Your Notebook… Nucleus: Summary: Write a 3 -5 sentence summary about the notes you took. At least one sentence needs to describe how you use atoms in your daily life.

The Atoms Family - Atomic Math Challenge Atomic Number Symbol Name Atomic Mass Atomic number equals the number of ______ or ________. Atomic mass equals the number of _______ + ________.

 Anything that takes up space and has mass is
Anything that takes up space and has mass is Something that takes up space
Something that takes up space Matter is anything that occupies space and has mass
Matter is anything that occupies space and has mass Defintion of malleability
Defintion of malleability Matter is anything that has mass and
Matter is anything that has mass and Anything that has mass and takes up space
Anything that has mass and takes up space Anything that takes up space and has mass
Anything that takes up space and has mass Matter is anything that has both
Matter is anything that has both What is anything that has mass and volume
What is anything that has mass and volume What is mass of matter
What is mass of matter Anything that takes up space and has mass
Anything that takes up space and has mass Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block av độ 1
Block av độ 1