Elements Atoms Refresher Matter is anything that takes








- Slides: 18

Elements & Atoms

Refresher • Matter is anything that takes up space and has mass. • All matter is made of atoms • Atoms are the building blocks of matter, sort of how bricks are the building blocks of houses.

Definitions / Vocabulary • Atom – smallest particle of an element that retains the properties of that element. • Proton – positively charges subatomic particle fount in the nucleus of an atom. Has mass. • Neutron – subatomic particle with no charge found in the nucleus of an atom. Has mass. • Nucleus – dense central portion of an atom, composed of protons and neutrons. • Electron – negatively charges subatomic particle found in an atom, but not in the nucleus. VERY LITTLE MASS. • Isotope – atoms of the same element that have the same atomic number but different atomic masses due to a different number of neutrons

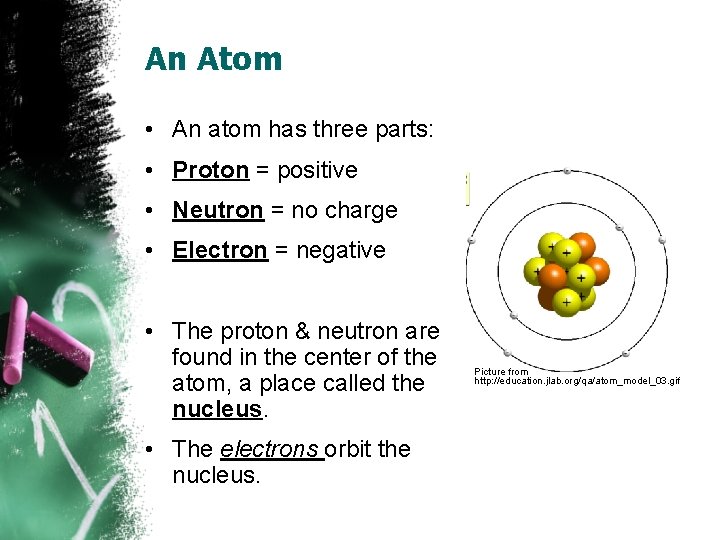
An Atom • An atom has three parts: • Proton = positive • Neutron = no charge • Electron = negative • The proton & neutron are found in the center of the atom, a place called the nucleus. • The electrons orbit the nucleus. Picture from http: //education. jlab. org/qa/atom_model_03. gif

Graphic from http: //education. jlab. org/atomtour/fact 2. html

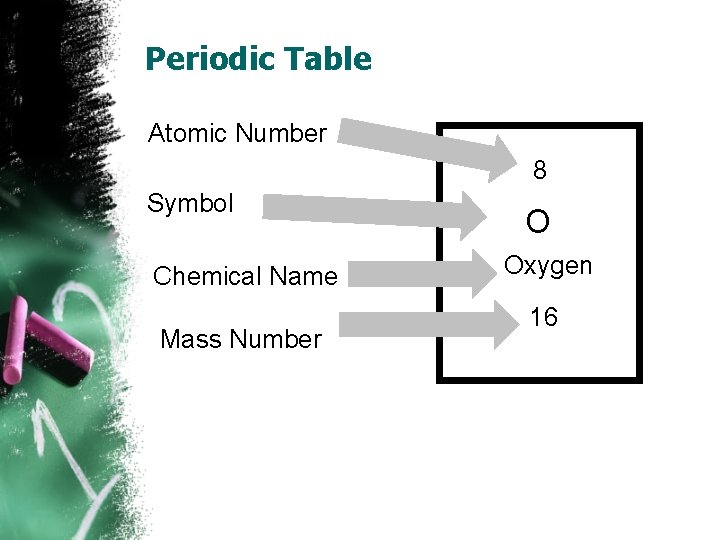
Periodic Table Atomic Number 8 Symbol Chemical Name Mass Number O Oxygen 16

Example • Nitrogen has an atomic number of 7. How many protons and how many electrons are in a neutral nitrogen atom? • Hint: Atomic number = number of protons = number of electrons

Example • Nitrogen has an atomic number of 7. How many protons and how many electrons are in a neutral nitrogen atom? • Hint: Atomic number = number of protons = number of electrons Answer: Protons = 7 Electrons = 7

Ch. 5 - Atomic Structure http: //www. nisd. net/communicationsarts/pages/chem/ind ex. html II. Masses of Atoms ¨ Mass Number ¨ Isotopes ¨ Relative Atomic Mass ¨ Average Atomic Mass

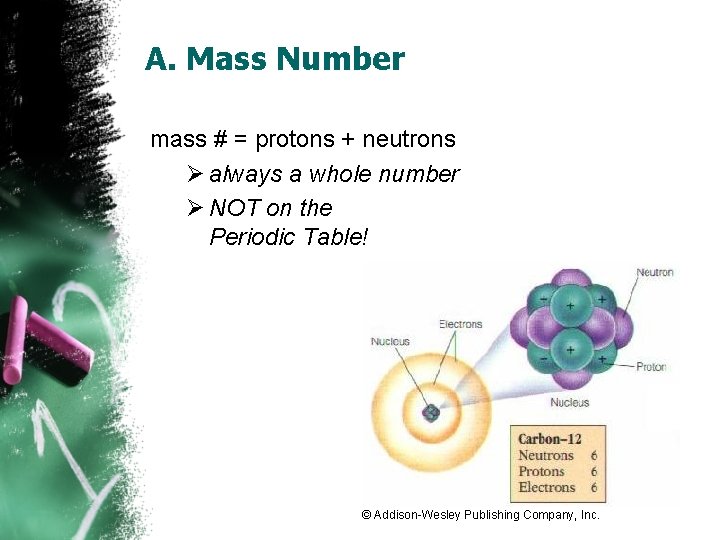
A. Mass Number mass # = protons + neutrons Ø always a whole number Ø NOT on the Periodic Table! © Addison-Wesley Publishing Company, Inc.

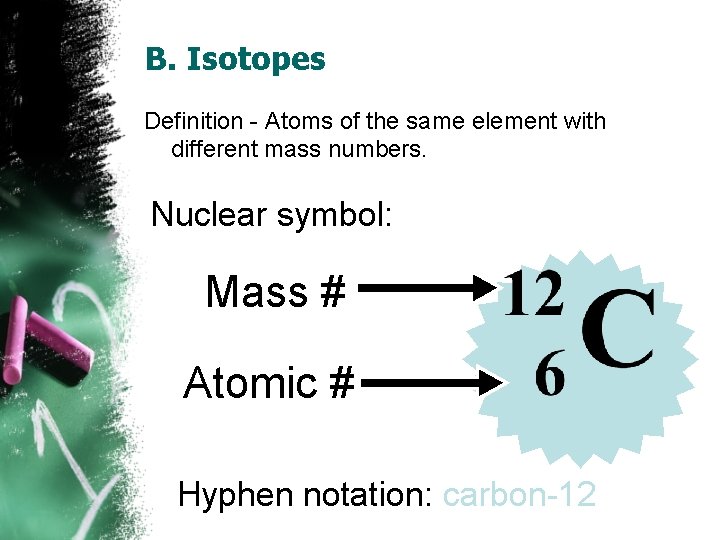
B. Isotopes Definition - Atoms of the same element with different mass numbers. Nuclear symbol: Mass # Atomic # Hyphen notation: carbon-12

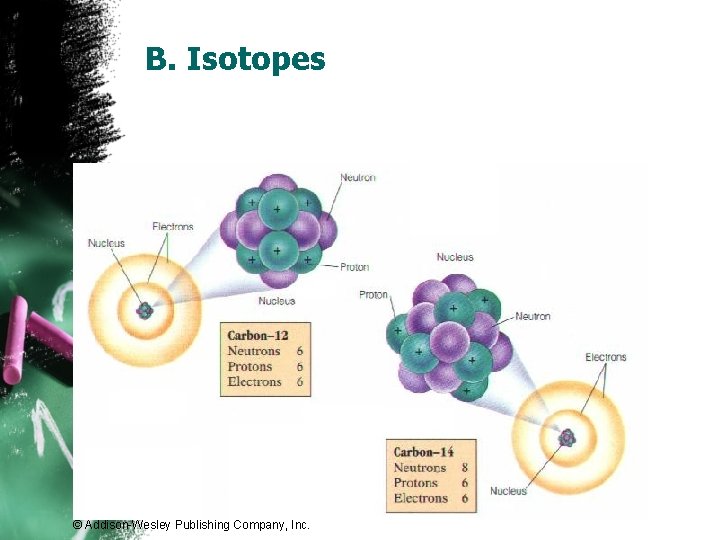
B. Isotopes © Addison-Wesley Publishing Company, Inc.

B. Isotopes • Chlorine-37 Ø atomic #: 17 Ø mass #: 37 Ø # of protons: 17 Ø # of electrons: 17 Ø # of neutrons: 20

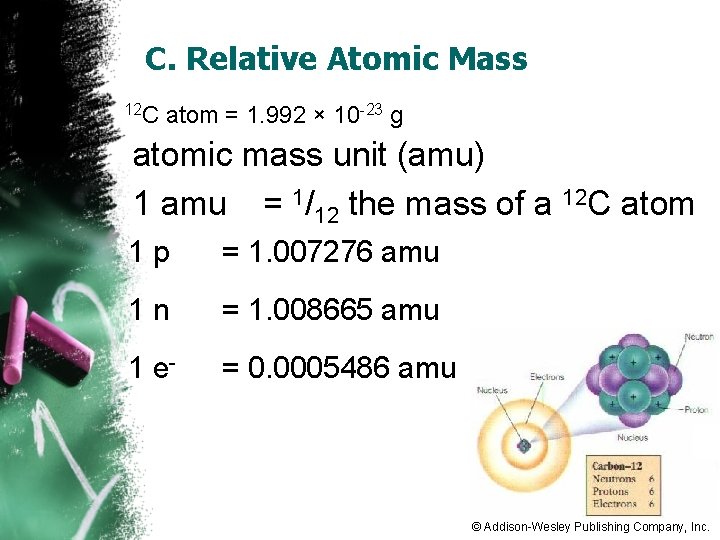
C. Relative Atomic Mass 12 C atom = 1. 992 × 10 -23 g atomic mass unit (amu) 1 amu = 1/12 the mass of a 12 C atom 1 p = 1. 007276 amu 1 n = 1. 008665 amu 1 e- = 0. 0005486 amu © Addison-Wesley Publishing Company, Inc.

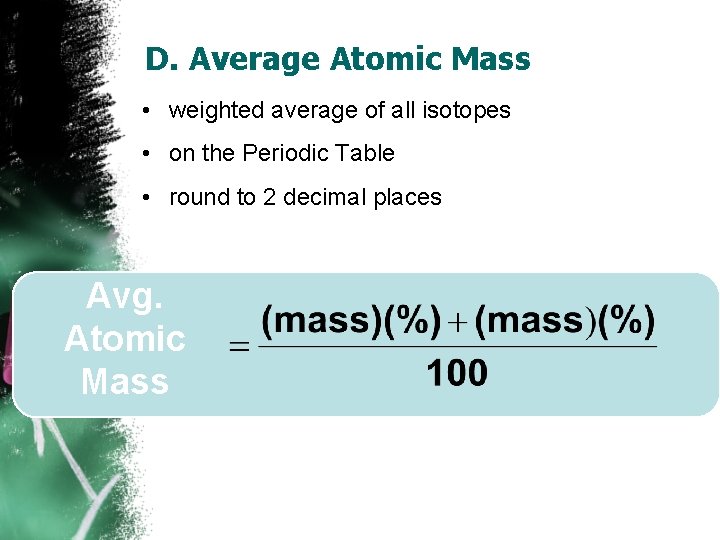
D. Average Atomic Mass • weighted average of all isotopes • on the Periodic Table • round to 2 decimal places Avg. Atomic Mass

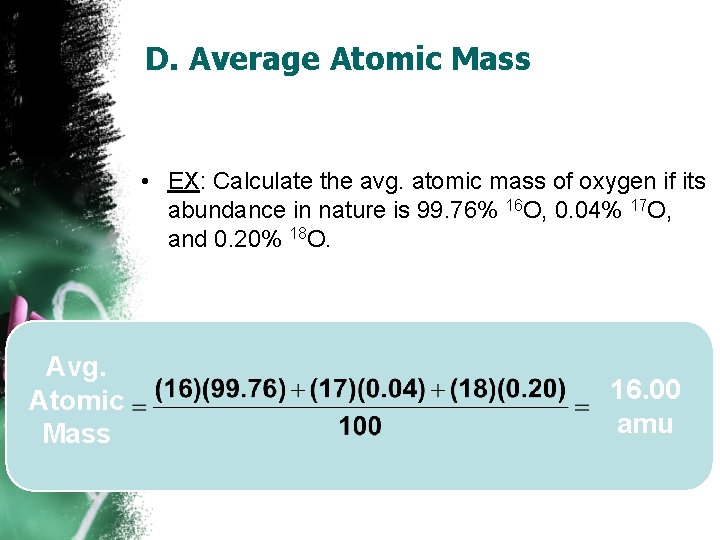
D. Average Atomic Mass • EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. Atomic Mass 16. 00 amu

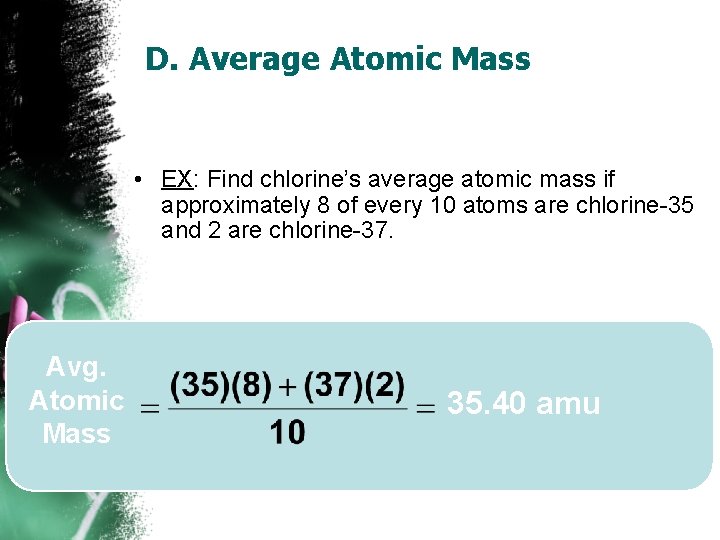
D. Average Atomic Mass • EX: Find chlorine’s average atomic mass if approximately 8 of every 10 atoms are chlorine-35 and 2 are chlorine-37. Avg. Atomic Mass 35. 40 amu

Atomic Mass - End