Elements Atoms Matter is anything that takes up









- Slides: 22

Elements & Atoms

• Matter is anything that takes up space and has mass. • All matter is made of atoms Created by G. Baker www. thesciencequeen. net

Elements • Elements are the simplest pure substance. – An element can not be changed into a simpler substance by heating or any chemical process. • The smallest particle of an element that has the properties of that element is called an atom. – An atom is the basic building block of matter. • There are more than one hundred known elements in the universe listed on the periodic table of elements. – These elements combine in such a way to create millions of compounds.

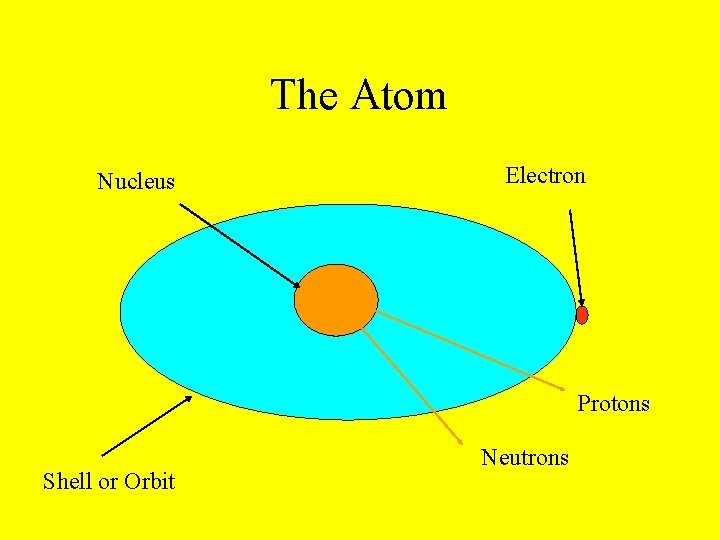
• • An atom has three parts: Proton = positive Neutron = no charge Electron = negative • The proton & neutron are found in the center of the atom, a place called the nucleus. • The electrons orbit the nucleus.

The Atom Nucleus Electron Protons Shell or Orbit Neutrons

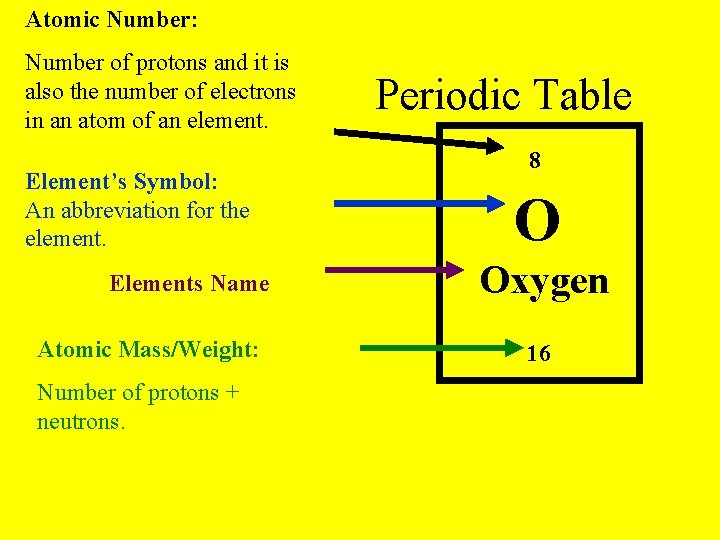
Atomic Number: Number of protons and it is also the number of electrons in an atom of an element. Element’s Symbol: An abbreviation for the element. Elements Name Atomic Mass/Weight: Number of protons + neutrons. Periodic Table 8 O Oxygen 16

Look at Calcium • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Look at Chlorine • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Look at Boron • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Look at Silver • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Look at Gold • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Look at Tin • What is its element symbol? • What is its atomic number? • What is its atomic mass?

Groups • Each column in the table is called a group. Elements in a group share similar properties. Groups are read from top to bottom.

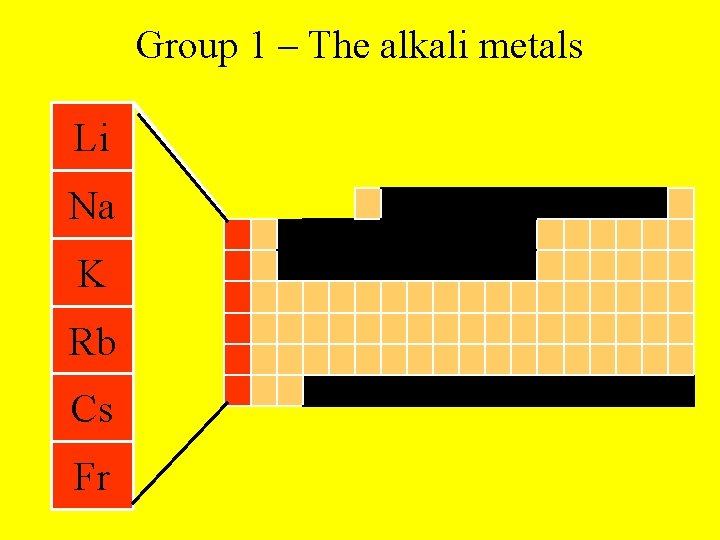
Group 1 – The alkali metals Li Na K Rb Cs Fr

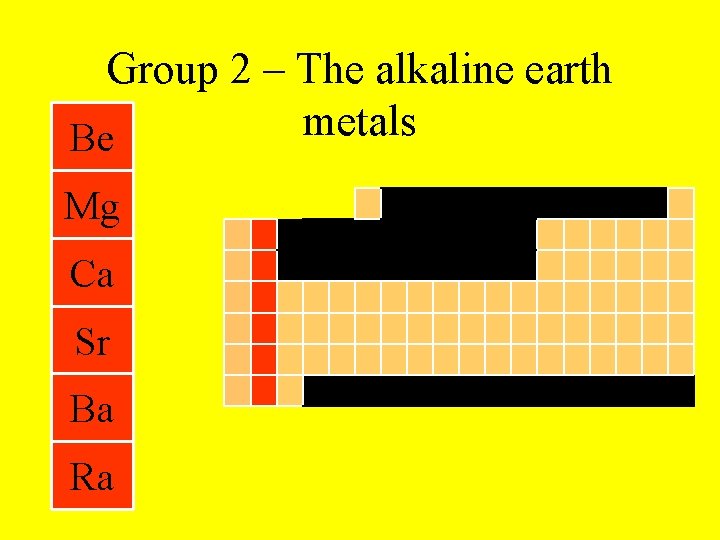
Group 2 – The alkaline earth metals Be Mg Ca Sr Ba Ra

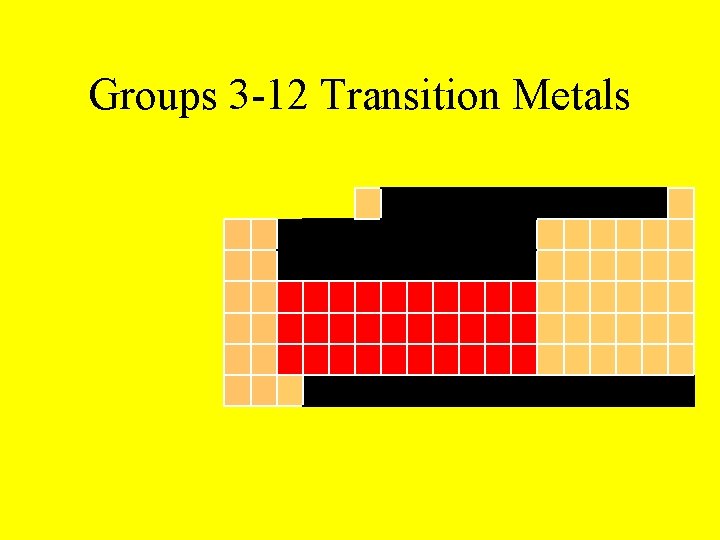
Groups 3 -12 Transition Metals

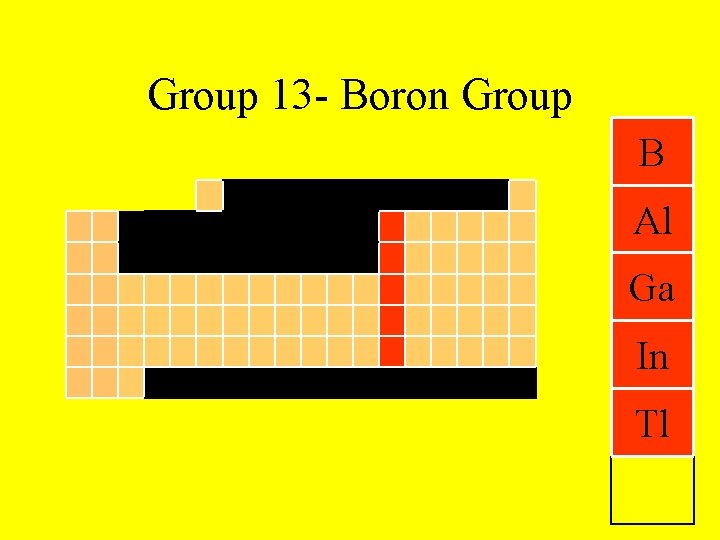
Group 13 - Boron Group B Al Ga In Tl

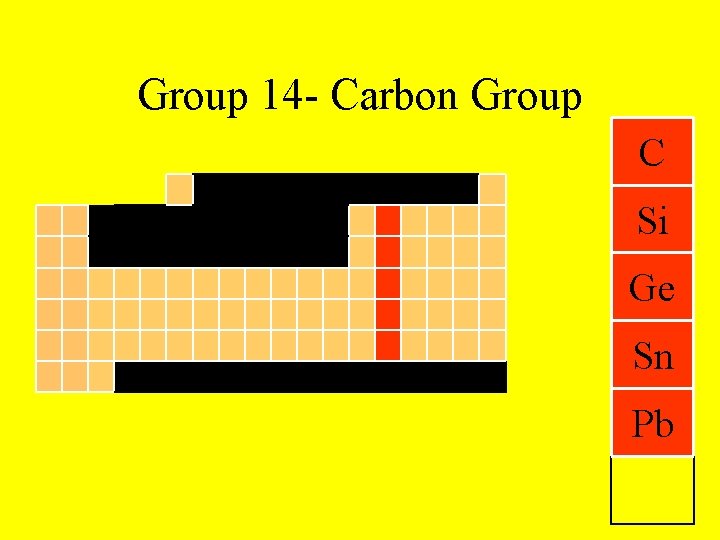
Group 14 - Carbon Group C Si Ge Sn Pb

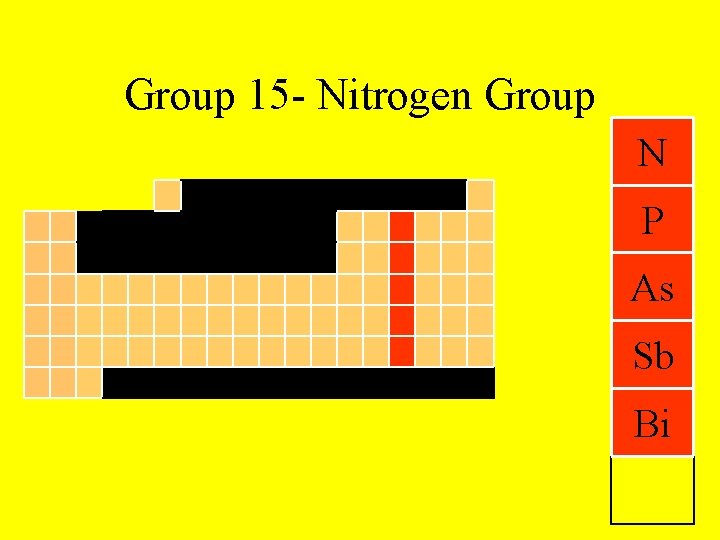
Group 15 - Nitrogen Group N P As Sb Bi

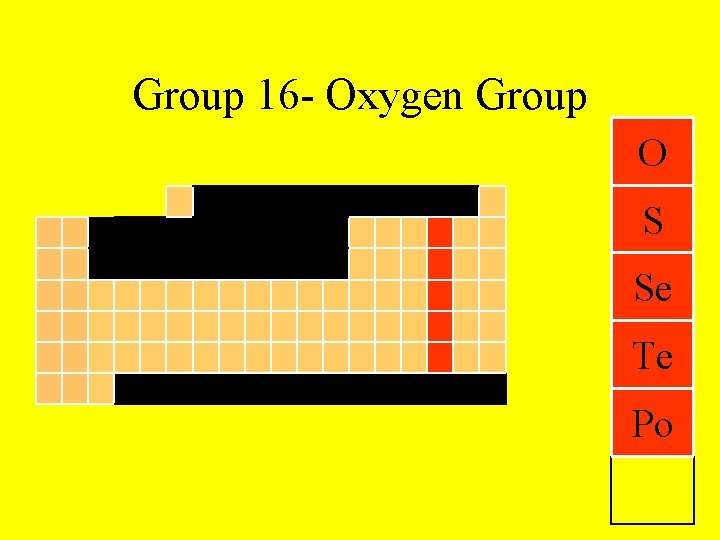
Group 16 - Oxygen Group O S Se Te Po

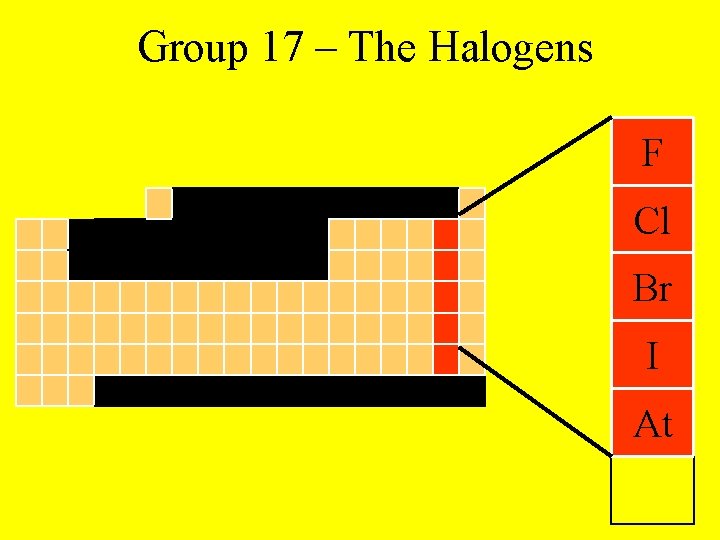
Group 17 – The Halogens F Cl Br I At

Group 18 – The Noble gases He Ne Ar Kr Xe Rn