Compounds And Elements Matter l anything that takes





- Slides: 16

Compounds And Elements

¡ Matter: l anything that takes up space & has a mass Ex. l a desk pizza silver necklace oxygen

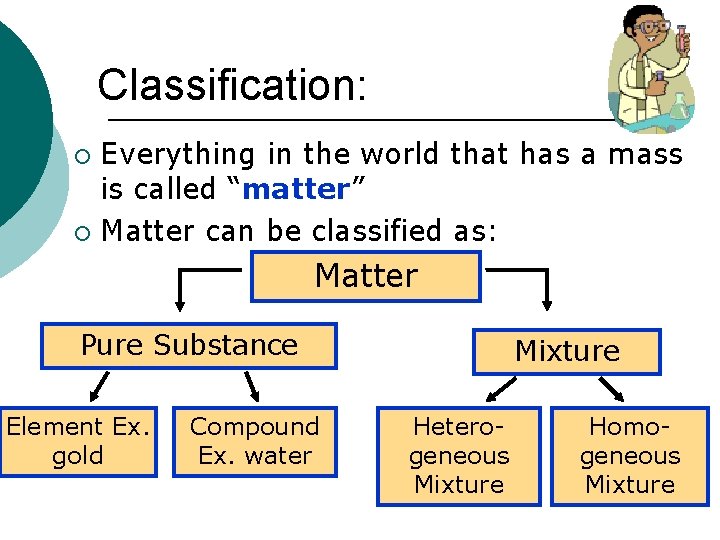
Classification: Everything in the world that has a mass is called “matter” ¡ Matter can be classified as: ¡ Matter Pure Substance Element Ex. gold Compound Ex. water Mixture Heterogeneous Mixture Homogeneous Mixture

¡ Pure Substance: composed of a single substance with the same properties throughout l Ex. water l Ex. table salt l ¡ Mixture: combinations of two or more substances that are not chemically combined (mixed) l Easily separated l Ex. fruit salad l Ex. sugar in coffee l

A pure substance can be further broken down into an element or a compound…… Elements: ¡ ¡ ¡ simplest form of matter cannot be separated or broken down into simpler substance Ex. Ex. Oxygen Aluminum Gold Copper

Recall, a pure substance can be further broken down into an element or a compound Compounds: ¡ ¡ ¡ substance made of two or more elements can be separated into simpler substance Ex. Ex. Water (H 2 O) Carbon dioxide (CO 2) Sugar (C 6 H 12 O 6) Salt (Na. Cl)

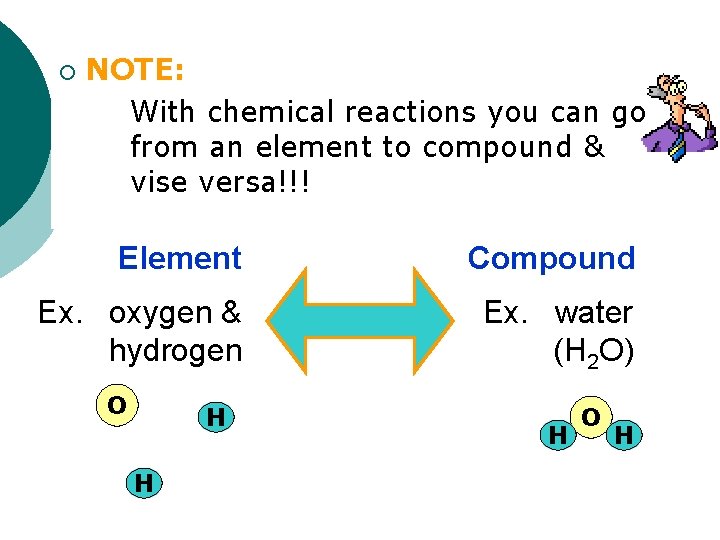
¡ NOTE: With chemical reactions you can go from an element to compound & vise versa!!! Element Compound Ex. oxygen & hydrogen Ex. water (H 2 O) O H H H O H

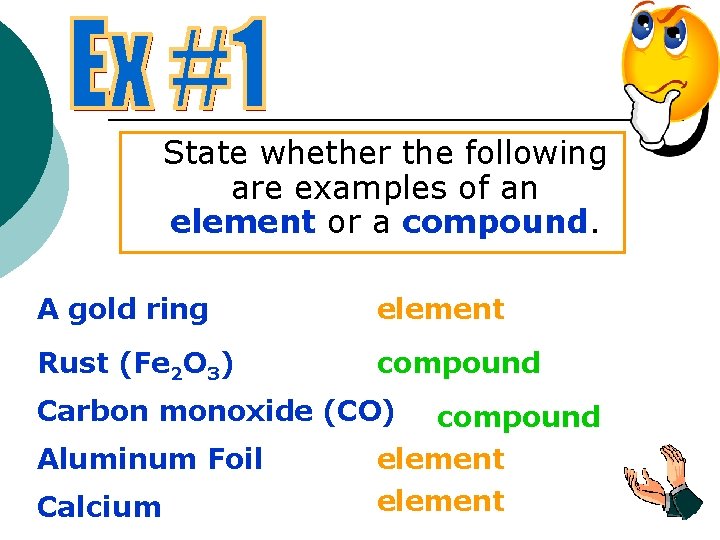
State whether the following are examples of an element or a compound. A gold ring element Rust (Fe 2 O 3) compound Carbon monoxide (CO) Aluminum Foil Calcium compound element

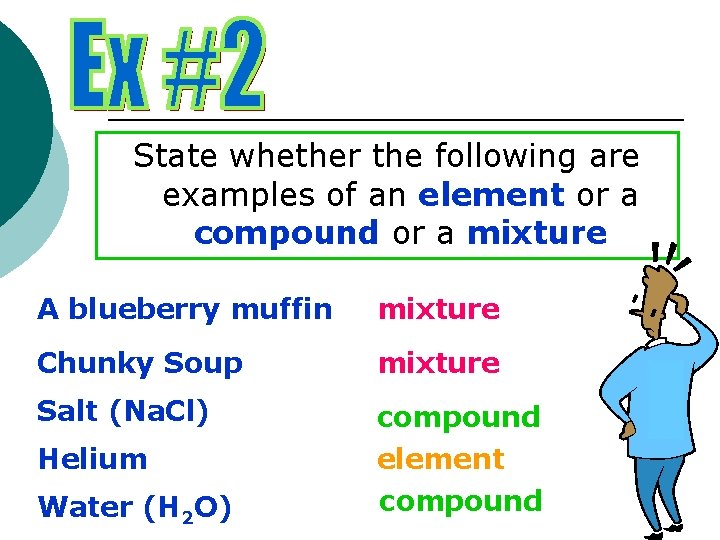
State whether the following are examples of an element or a compound or a mixture A blueberry muffin mixture Chunky Soup mixture Salt (Na. Cl) compound element compound Helium Water (H 2 O)

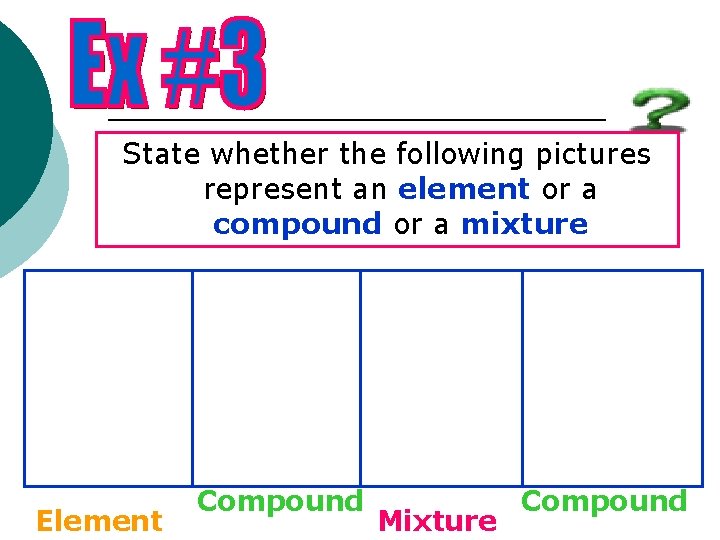
State whether the following pictures represent an element or a compound or a mixture Element Compound Mixture Compound

Which statement best describes a compound. A) A pure substance that has a high melting point. B) A mixture that is formed by two or more elements. C) A pure substance that is formed by two or more elements.

The Molecule Individual atoms can combine to form molecules. ¡ A molecule is a group of two or more chemically bonded atoms. ¡ A molecule is the smallest unit of a compound. ¡ Eg: H 2 O, CO 2, C 6 H 12 O 6

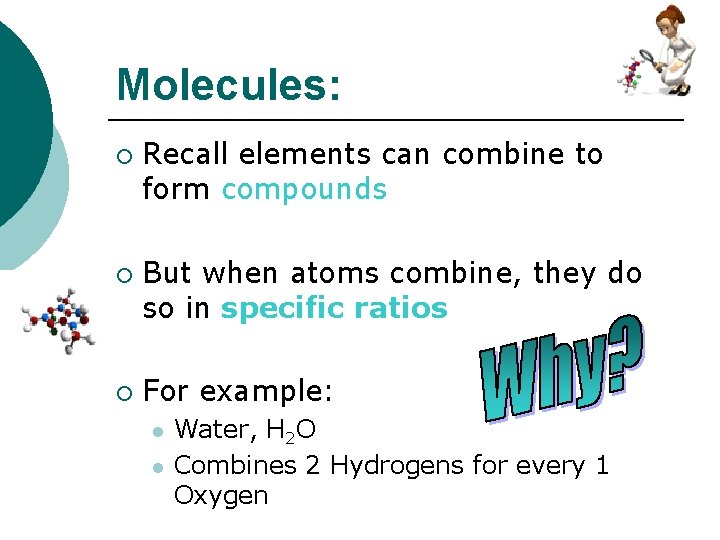
Molecules: ¡ ¡ ¡ Recall elements can combine to form compounds But when atoms combine, they do so in specific ratios For example: l l Water, H 2 O Combines 2 Hydrogens for every 1 Oxygen

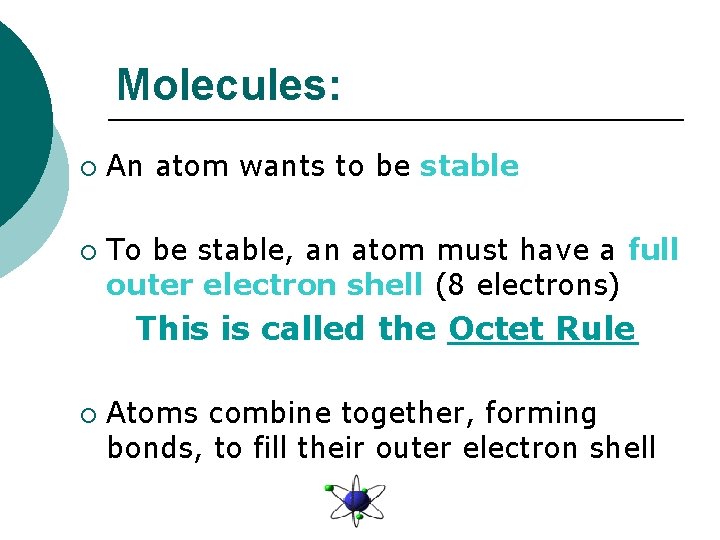
Molecules: ¡ ¡ An atom wants to be stable To be stable, an atom must have a full outer electron shell (8 electrons) This is called the Octet Rule ¡ Atoms combine together, forming bonds, to fill their outer electron shell

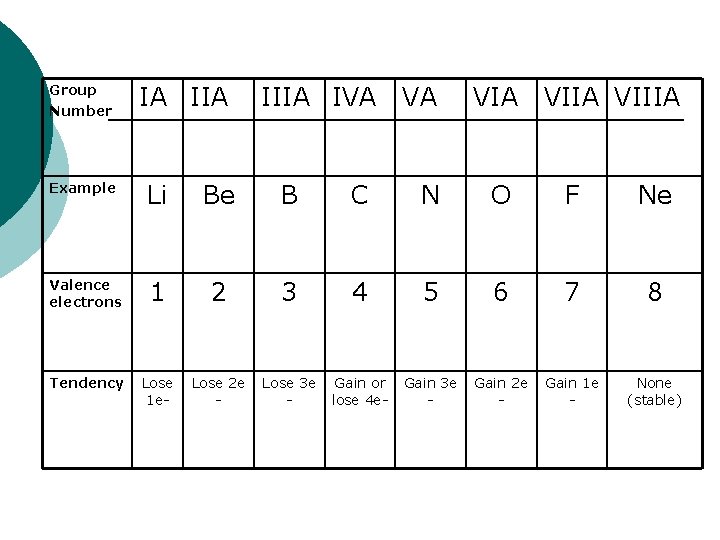
Atoms combine to obtain a complete outer electron shell (8 valence electrons) ¡ Metals tend to lose electrons to achieve this and non-metals tend to gain electrons ¡

Group Number IA IIIA IVA VA VIIA VIIIA Example Li Be B C N O F Ne Valence electrons 1 2 3 4 5 6 7 8 Tendency Lose 1 e- Lose 2 e - Lose 3 e - Gain or lose 4 e- Gain 3 e - Gain 2 e - Gain 1 e - None (stable)