Atoms Elements and Molecules Oh My Matter Anything



- Slides: 11

Atoms, Elements, and Molecules…. . Oh My!

Matter • Anything that has mass and takes up space – Mass: – Volume: How much matter is in an object How much space an object takes up

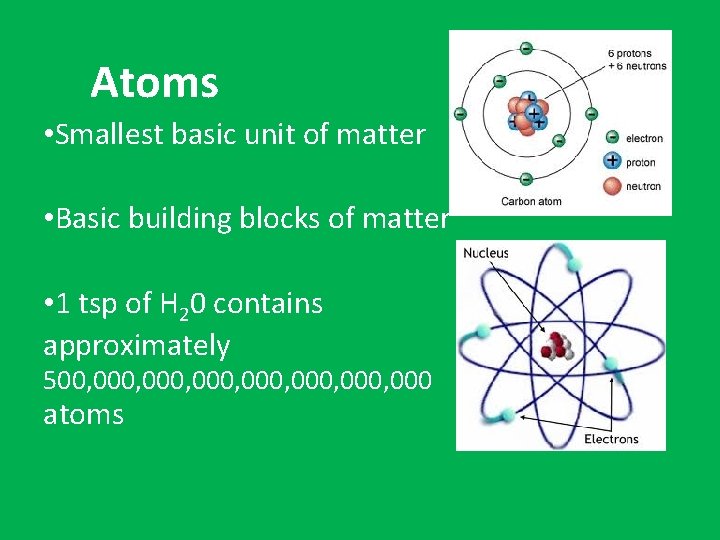
Atoms • Smallest basic unit of matter • Basic building blocks of matter • 1 tsp of H 20 contains approximately 500, 000, 000, 000 atoms

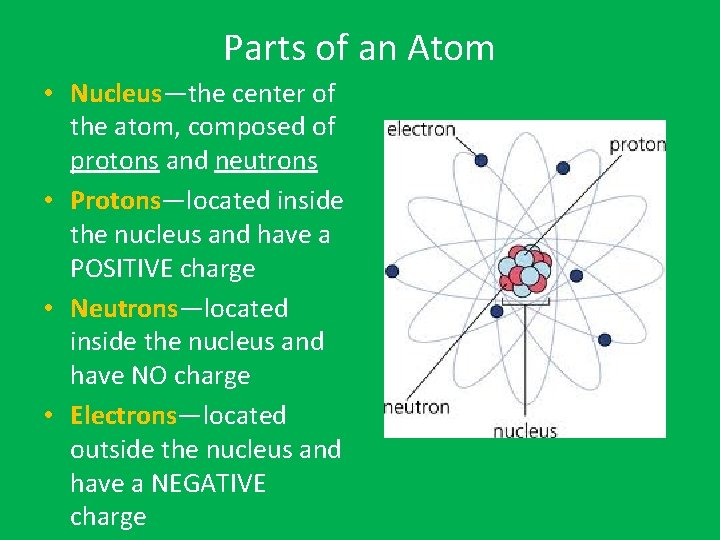
Parts of an Atom • Nucleus—the center of the atom, composed of protons and neutrons • Protons—located inside the nucleus and have a POSITIVE charge • Neutrons—located inside the nucleus and have NO charge • Electrons—located outside the nucleus and have a NEGATIVE charge

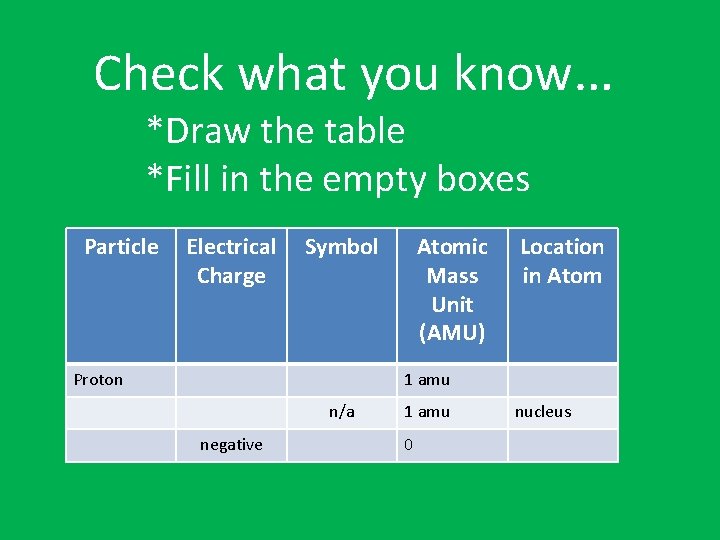
Check what you know… *Draw the table *Fill in the empty boxes Particle Electrical Charge Symbol Proton Atomic Mass Unit (AMU) Location in Atom 1 amu n/a negative 1 amu 0 nucleus

Elements • Characteristics are determined by the structure of its atoms • Each element is made up of atoms that differ from every other element

Element Features 1. Atomic number—describes the number of protons in the nucleus of an atom – No two elements have the same atomic number – Atomic number also equals the number of electrons in an atom 2. Atomic Mass—is equal to the number of protons plus the number of neutrons in the atom’s nucleus 3. Chemical Symbol—is a code composed of one or two letters that represents an element – Each element has its own chemical symbol – Example: C= carbon, Fe = iron


2 Rules 1. Protons + Neutrons = the atomic mass 2. Protons, Electrons, and the Atomic Number are always the same number P + N = AM If you know one of the numbers, you know all 3

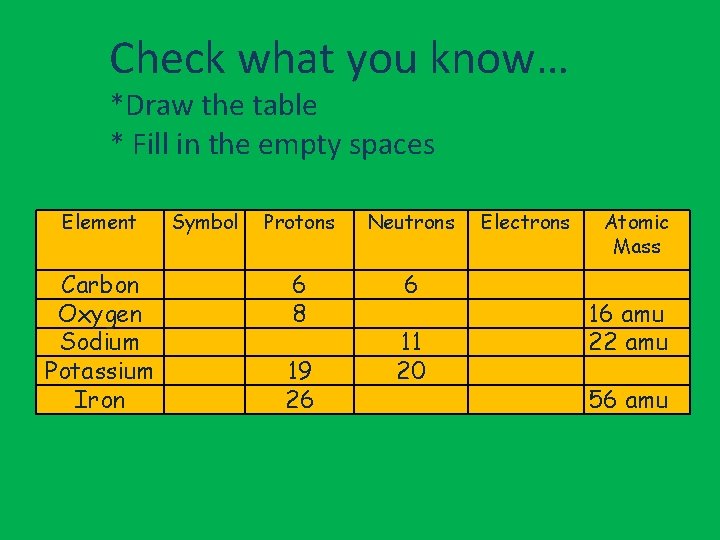
Check what you know… *Draw the table * Fill in the empty spaces Element Carbon Oxygen Sodium Potassium Iron Symbol Protons Neutrons 6 8 6 19 26 11 20 Electrons Atomic Mass 16 amu 22 amu 56 amu

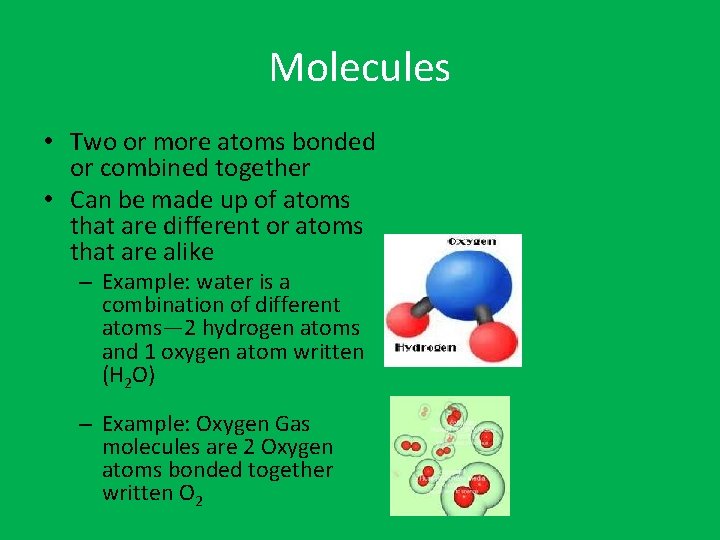
Molecules • Two or more atoms bonded or combined together • Can be made up of atoms that are different or atoms that are alike – Example: water is a combination of different atoms— 2 hydrogen atoms and 1 oxygen atom written (H 2 O) – Example: Oxygen Gas molecules are 2 Oxygen atoms bonded together written O 2