03102020 Ed Excel Unit P 3 Applications of


















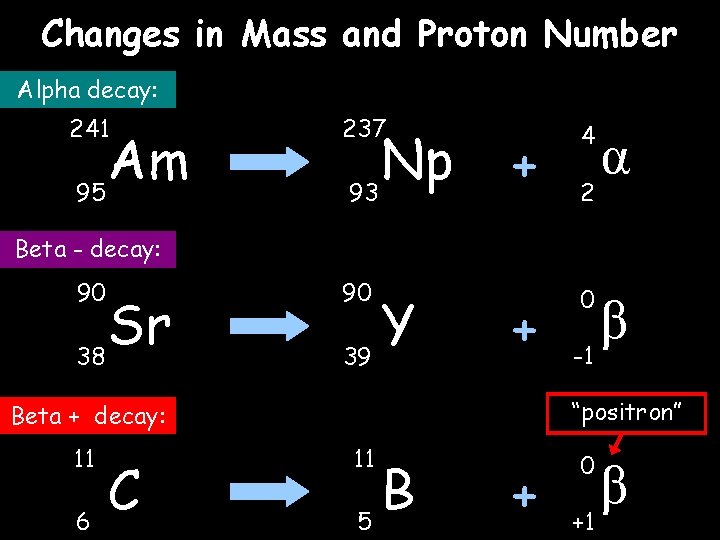
























- Slides: 96

03/10/2020 Ed. Excel Unit P 3 Applications of Physics N Smith St. Aidan’s

Topic 1 – Radiation in Medicine

Medical Physics “Medical Physics” is a big branch of science and refers to using physics to investigate medical issues. Some examples: CAT scans Endoscopes Ultrasound Radiotherapy

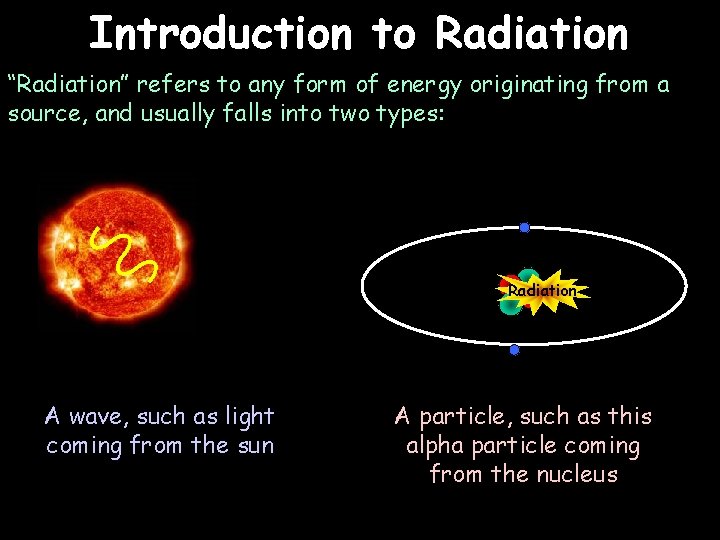
Introduction to Radiation “Radiation” refers to any form of energy originating from a source, and usually falls into two types: Radiation A wave, such as light coming from the sun A particle, such as this alpha particle coming from the nucleus

Intensity Clearly, the intensity of radiation received by an object decreases the further out the object is. This is due to two things: 1) The radiation “spreads out” in a circle 2) It is also absorbed by the medium it travels through

Intensity 03/10/2020 Definition: “Intensity” means the strength of light arriving at a certain point, and can also be called “Radiation flux density” Energy dissipation Clearly, a wave will get weaker the further it travels. Assuming the wave comes from a point source and travels out equally in all directions we can say: Intensity = Power (in W) (in Wm-2) Area (in m 2) I= P 4πr 2 An “inverse square law”

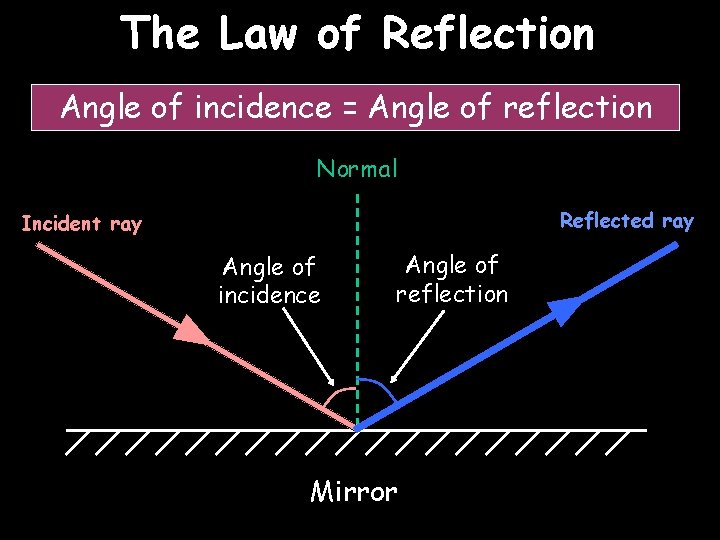
The Law of Reflection Angle of incidence = Angle of reflection Normal Reflected ray Incident ray Angle of incidence Angle of reflection Mirror

Refraction

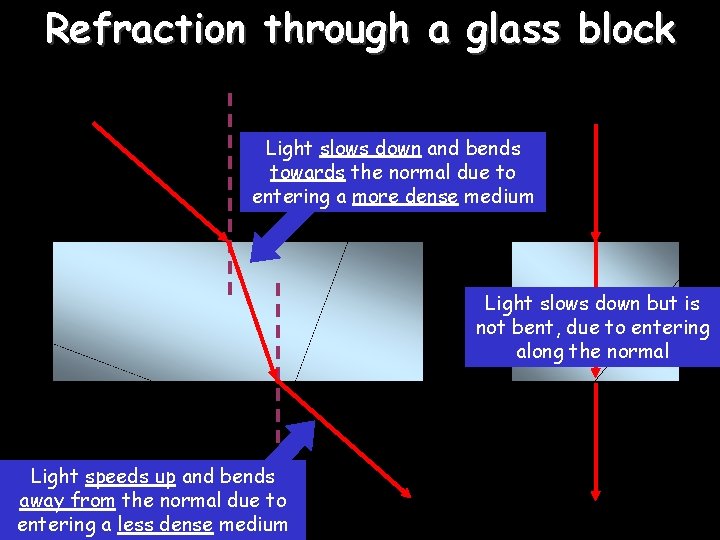
Refraction through a glass block Light slows down and bends towards the normal due to entering a more dense medium Light slows down but is not bent, due to entering along the normal Light speeds up and bends away from the normal due to entering a less dense medium

Lenses use the idea of refraction: When light enters a MORE DENSE medium it slows down… A prism uses this idea to split light. This happens because purple light is refracted more than red light

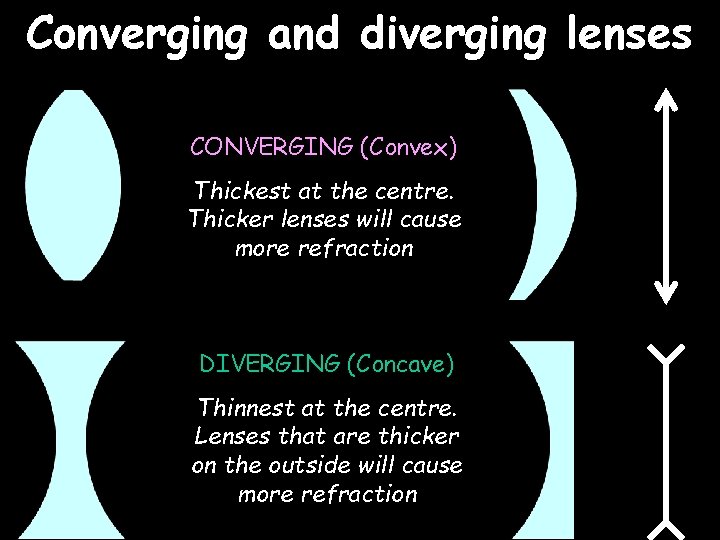
Converging and diverging lenses CONVERGING (Convex) Thickest at the centre. Thicker lenses will cause more refraction DIVERGING (Concave) Thinnest at the centre. Lenses that are thicker on the outside will cause more refraction

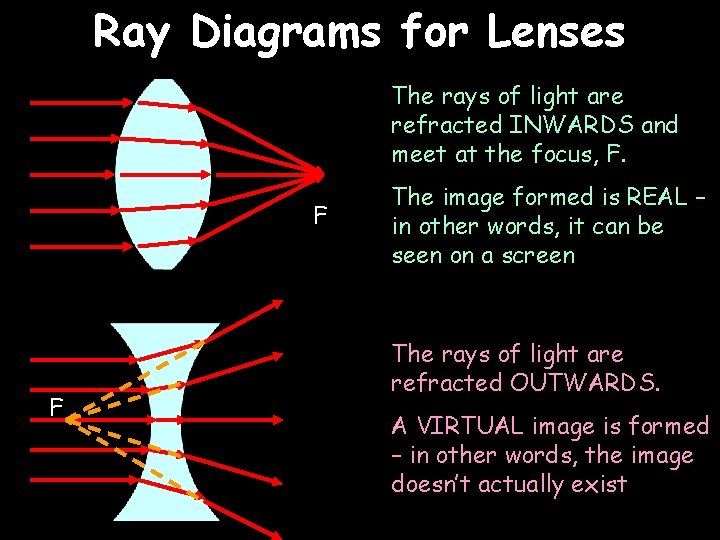
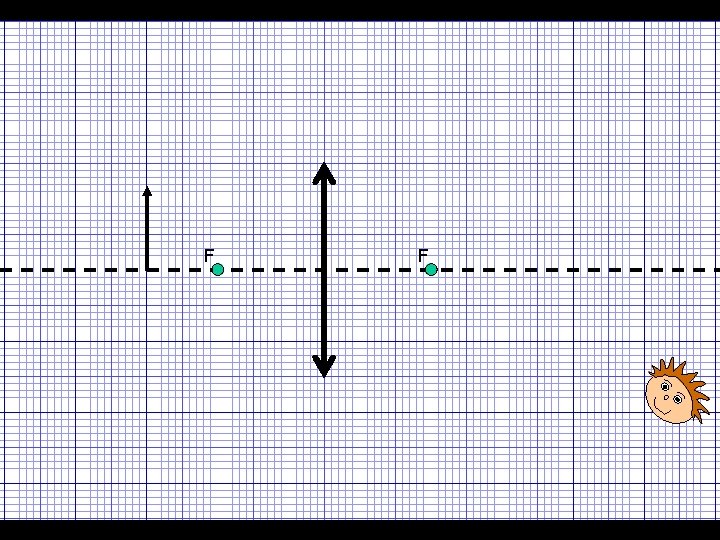
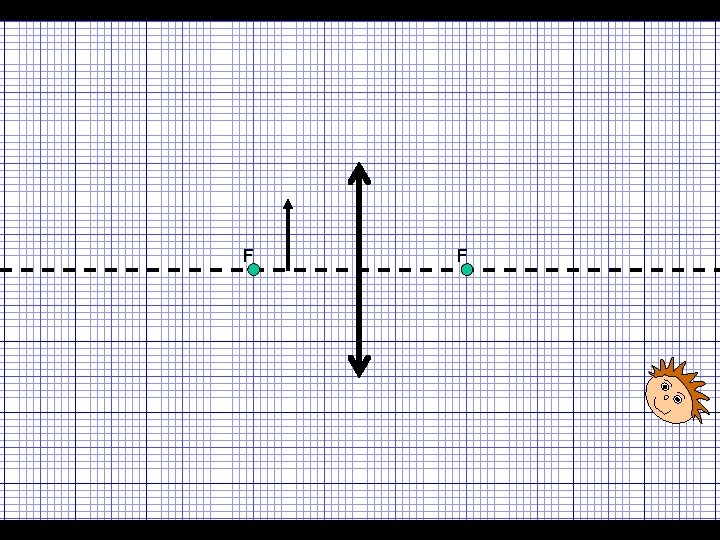
Ray Diagrams for Lenses The rays of light are refracted INWARDS and meet at the focus, F. F F The image formed is REAL – in other words, it can be seen on a screen The rays of light are refracted OUTWARDS. A VIRTUAL image is formed – in other words, the image doesn’t actually exist

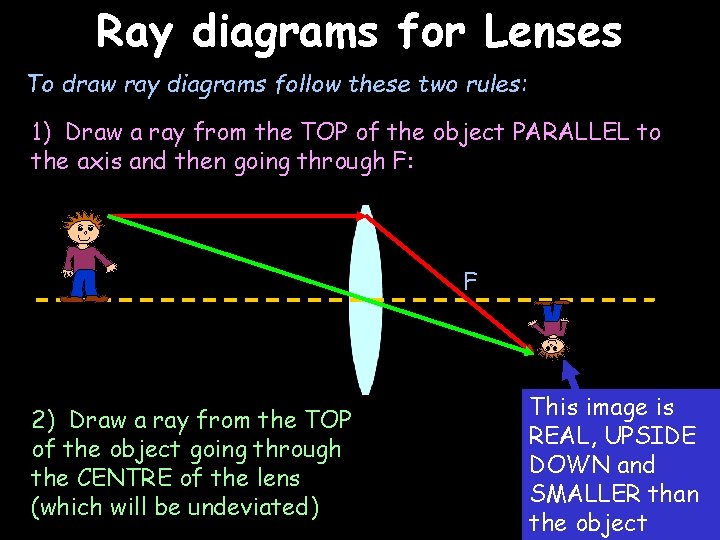
Ray diagrams for Lenses To draw ray diagrams follow these two rules: 1) Draw a ray from the TOP of the object PARALLEL to the axis and then going through F: F 2) Draw a ray from the TOP of the object going through the CENTRE of the lens (which will be undeviated) This image is REAL, UPSIDE DOWN and SMALLER than the object

F F

F F

F F

F F

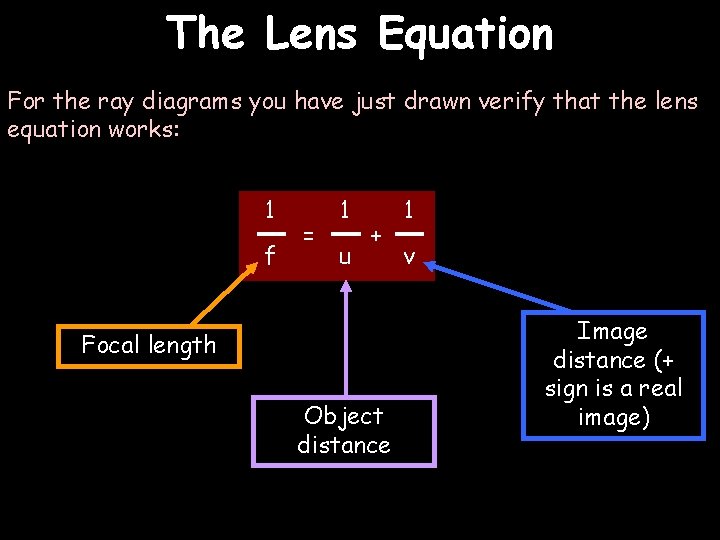
The Lens Equation For the ray diagrams you have just drawn verify that the lens equation works: 1 f = 1 u + Focal length Object distance 1 v Image distance (+ sign is a real image)

The Eye

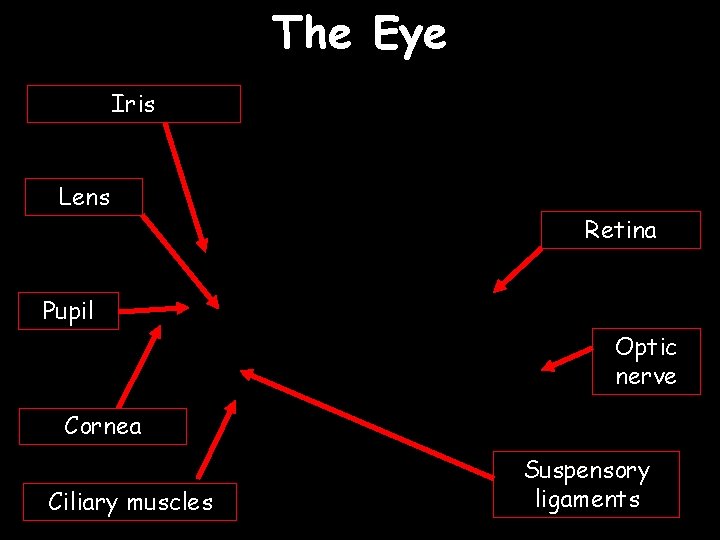
The Eye Iris Lens Retina Pupil Optic nerve Cornea Ciliary muscles Suspensory ligaments

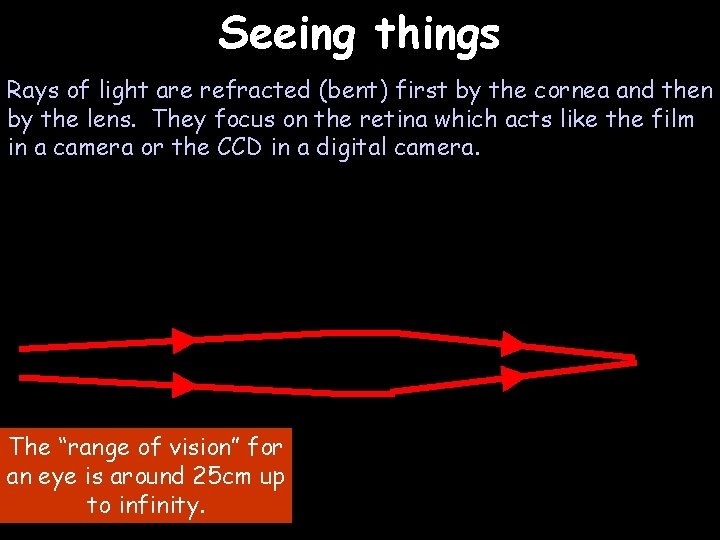
Seeing things Rays of light are refracted (bent) first by the cornea and then by the lens. They focus on the retina which acts like the film in a camera or the CCD in a digital camera. The “range of vision” for an eye is around 25 cm up to infinity.

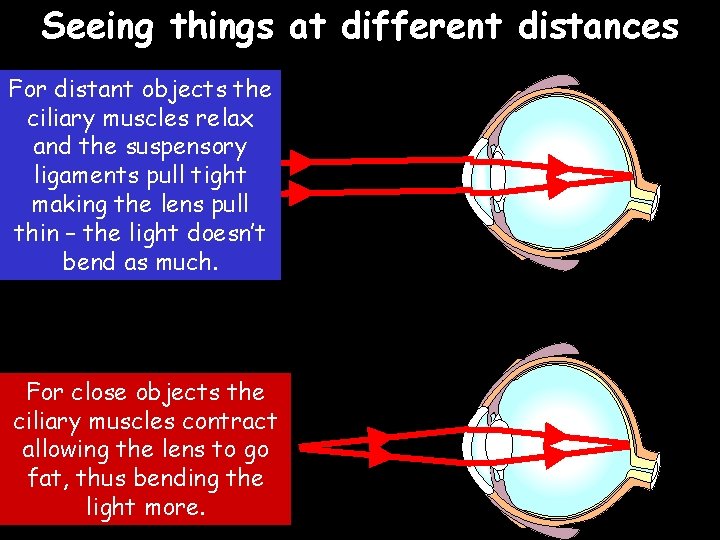
Seeing things at different distances For distant objects the ciliary muscles relax and the suspensory ligaments pull tight making the lens pull thin – the light doesn’t bend as much. For close objects the ciliary muscles contract allowing the lens to go fat, thus bending the light more.

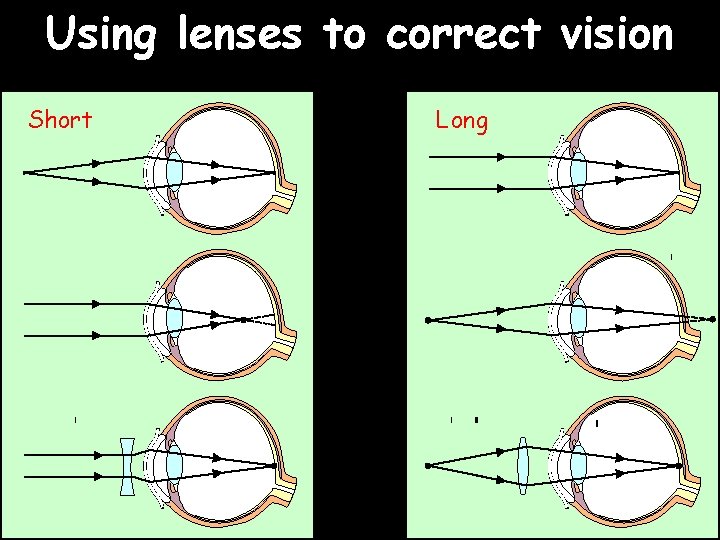
Using lenses to correct vision Short Long

More about lenses Compare thin and thick lenses: Lenses are measured in units called “dioptres”: Power in dioptres = 1 focal length in m …where converging lenses (for long sighted people) have positive values and diverging lenses (for short sighted people) have negative values. Notice that these glasses have got a large curvature. How would you make strong glasses but also make them thinner and with less curvature?

Using Lasers in Surgery Lasers are being increasingly used in laser eye surgery: What are the advantages and disadvantages of this treatment compared to using glasses or contact lenses?

Refractive Index 03/10/2020 The Refractive Index of a material is a measure of the factor by which the material will bend light: Snell’s Law: Refractive index = sin i sin r Willebrord Snellius, 1580 -1626

Example questions 03/10/2020 Here’s my law again: Refractive index = sin i sin r 1) Light passes from air into crystal with a refractive index of 1. 5. Calculate the angles of refraction for light incident at 20 O, 30 O, 40 O and 50 O. 2) A ray of light travels through a vacuum and is incident upon a glass block (of refractive index 1. 5) at an angle of 30 O. The ray then passes into water. Draw an accurate diagram to show the path of this light as it travels from the vacuum through the glass and into the water.

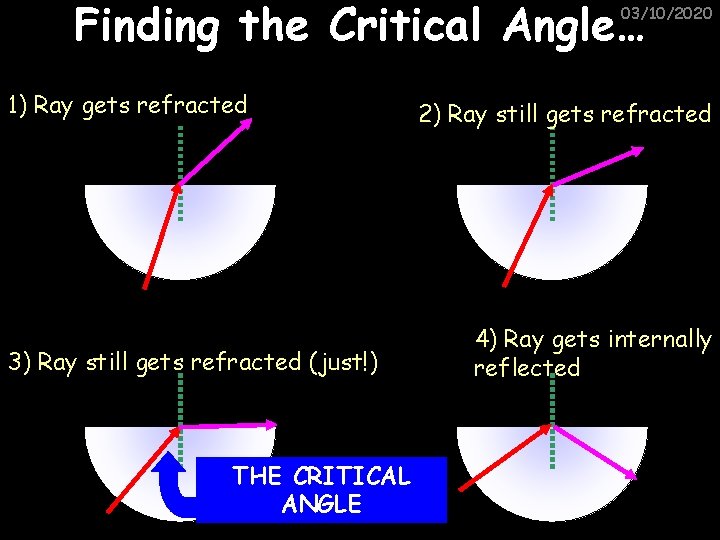
Finding the Critical Angle… 03/10/2020 1) Ray gets refracted 3) Ray still gets refracted (just!) THE CRITICAL ANGLE 2) Ray still gets refracted 4) Ray gets internally reflected

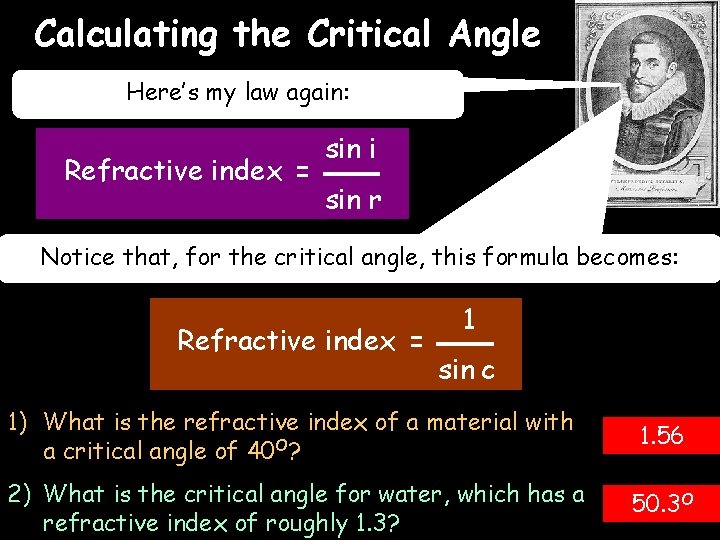
Calculating the Critical Angle 03/10/2020 Here’s my law again: Refractive index = sin i sin r Notice that, for the critical angle, this formula becomes: Refractive index = 1 sin c 1) What is the refractive index of a material with a critical angle of 40 O? 1. 56 2) What is the critical angle for water, which has a refractive index of roughly 1. 3? 50. 3 O

03/10/2020 Uses of Total Internal Reflection Optical fibres: An optical fibre is a long, thin, _______ rod made of glass or plastic. Light is _______ reflected from one end to the other, making it possible to send ____ chunks of information Optical fibres can be used for _____ by sending electrical signals through the cable. The main advantage of this is a reduced ______ loss and endoscopes use this principle: Words – communications, internally, large, transparent, signal

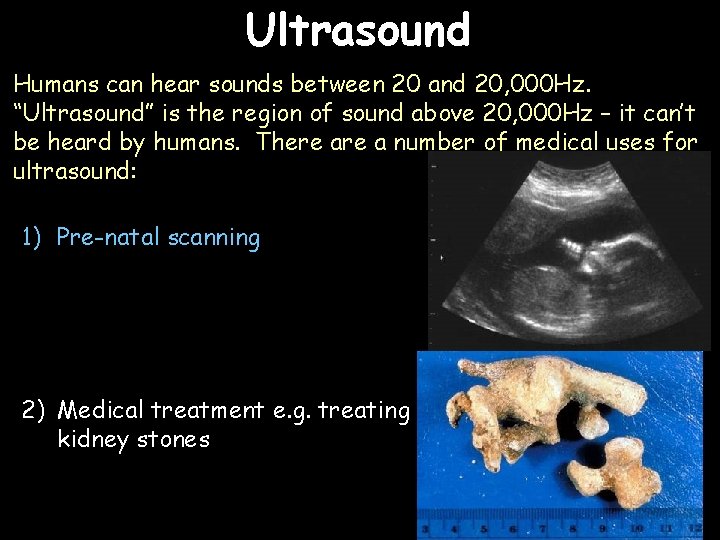
Ultrasound Humans can hear sounds between 20 and 20, 000 Hz. “Ultrasound” is the region of sound above 20, 000 Hz – it can’t be heard by humans. There a number of medical uses for ultrasound: 1) Pre-natal scanning 2) Medical treatment e. g. treating kidney stones

03/10/2020 Calculating distances with ultrasound The echo takes 0. 8 seconds to return and the speed of sound in water is 1500 ms-1. How deep is the water? 25 50 75 100 125 150 175 200 t/μs Hard question! Use the ultrasound scan to determine the width of the amniotic sac and the width of the baby’s body. The speed of sound in the fluid is 1500 ms-1 and in soft tissue the speed is 1560 ms-1.

Using an oscilloscope with ultrasound Consider a block of metal with a flaw: 20 ms/div Q. If the speed of the ultrasonic wave is 3, 000 m/s how far away is the flaw from the detector?

Topic 2 – X-rays and ECGs

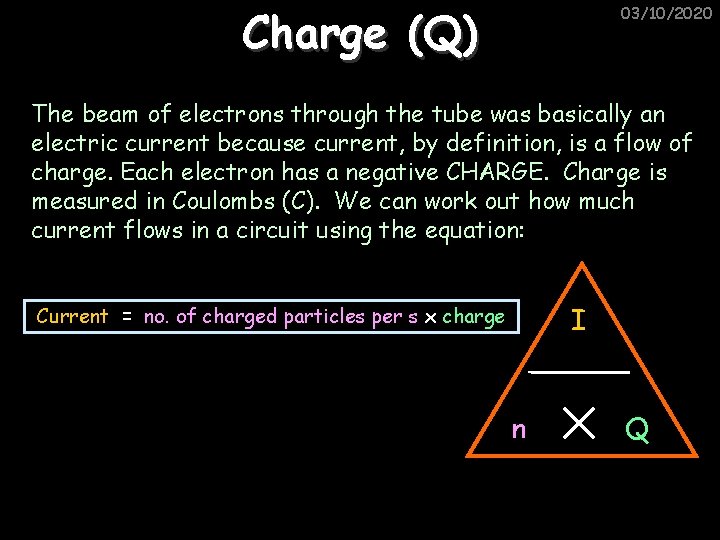
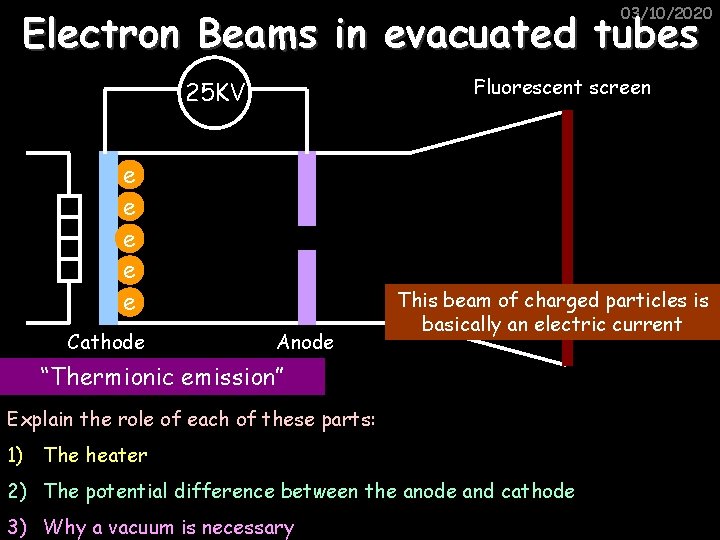
Charge (Q) 03/10/2020 The beam of electrons through the tube was basically an electric current because current, by definition, is a flow of charge. Each electron has a negative CHARGE. Charge is measured in Coulombs (C). We can work out how much current flows in a circuit using the equation: I Current = no. of charged particles per s x charge n Q

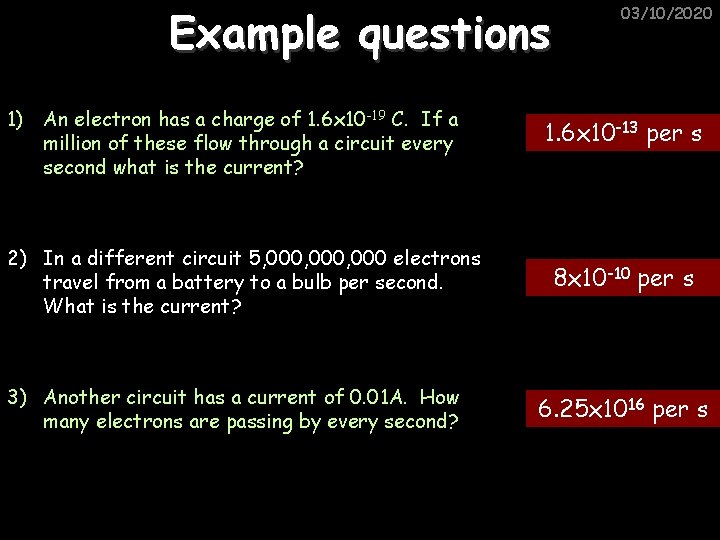
Example questions 1) An electron has a charge of 1. 6 x 10 -19 C. If a million of these flow through a circuit every second what is the current? 2) In a different circuit 5, 000, 000 electrons travel from a battery to a bulb per second. What is the current? 3) Another circuit has a current of 0. 01 A. How many electrons are passing by every second? 03/10/2020 1. 6 x 10 -13 per s 8 x 10 -10 per s 6. 25 x 1016 per s

03/10/2020 Electron Beams in evacuated tubes Fluorescent screen 25 KV e e e Cathode Anode This beam of charged particles is basically an electric current “Thermionic emission” Explain the role of each of these parts: 1) The heater 2) The potential difference between the anode and cathode 3) Why a vacuum is necessary

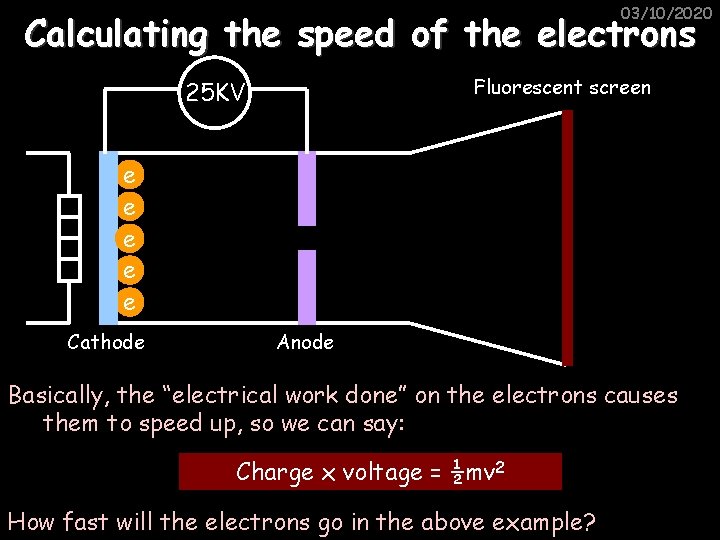
03/10/2020 Calculating the speed of the electrons Fluorescent screen 25 KV e e e Cathode Anode Basically, the “electrical work done” on the electrons causes them to speed up, so we can say: Charge x voltage = ½mv 2 How fast will the electrons go in the above example?

X-Rays X-rays are part of the electromagnetic spectrum with a high frequency and therefore high energy. They cause ionisation (which could cause cancer). X-ray images are possible because they are absorbed by thick, dense tissue like bone but transmitted by soft tissue. X-rays are formed through collisions with metal targets.

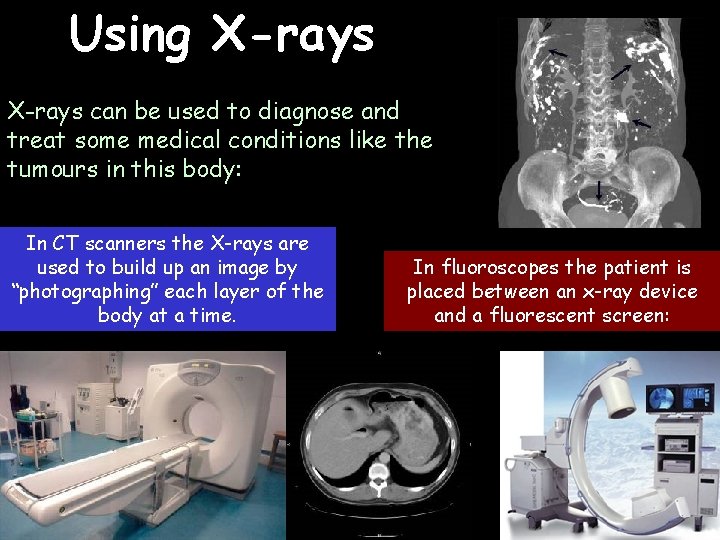
Using X-rays can be used to diagnose and treat some medical conditions like the tumours in this body: In CT scanners the X-rays are used to build up an image by “photographing” each layer of the body at a time. In fluoroscopes the patient is placed between an x-ray device and a fluorescent screen:

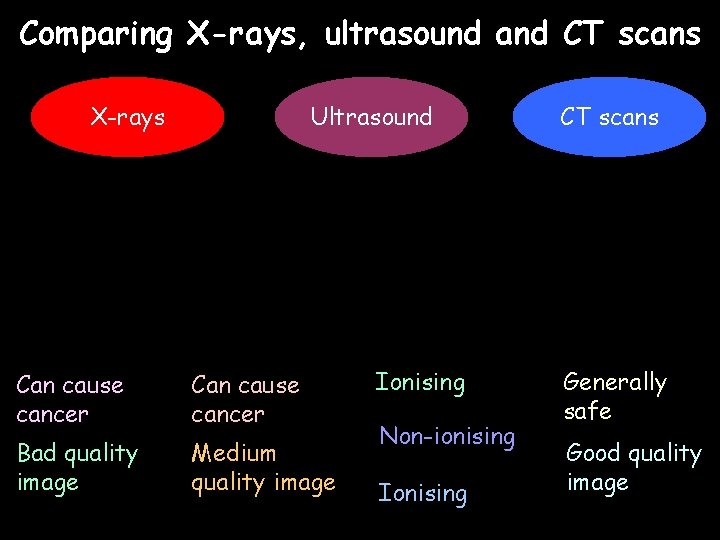
Comparing X-rays, ultrasound and CT scans X-rays Ultrasound Can cause cancer Bad quality image Medium quality image Ionising Non-ionising Ionising CT scans Generally safe Good quality image

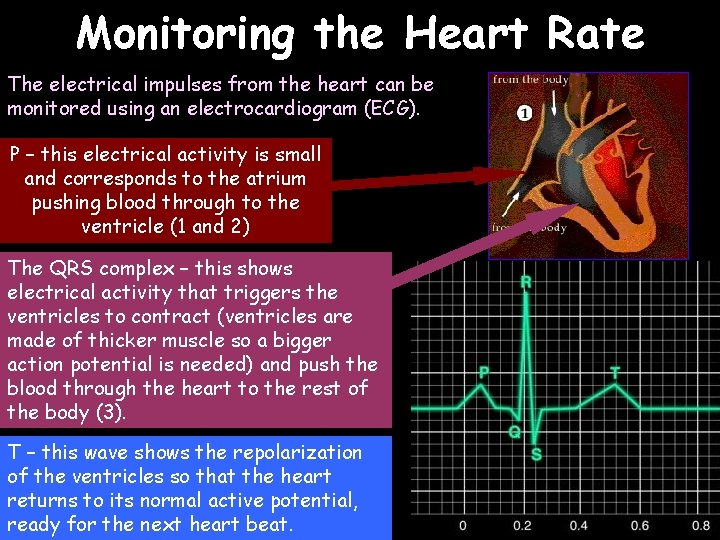
Monitoring the Heart Rate The electrical impulses from the heart can be monitored using an electrocardiogram (ECG). P – this electrical activity is small and corresponds to the atrium pushing blood through to the ventricle (1 and 2) The QRS complex – this shows electrical activity that triggers the ventricles to contract (ventricles are made of thicker muscle so a bigger action potential is needed) and push the blood through the heart to the rest of the body (3). T – this wave shows the repolarization of the ventricles so that the heart returns to its normal active potential, ready for the next heart beat.

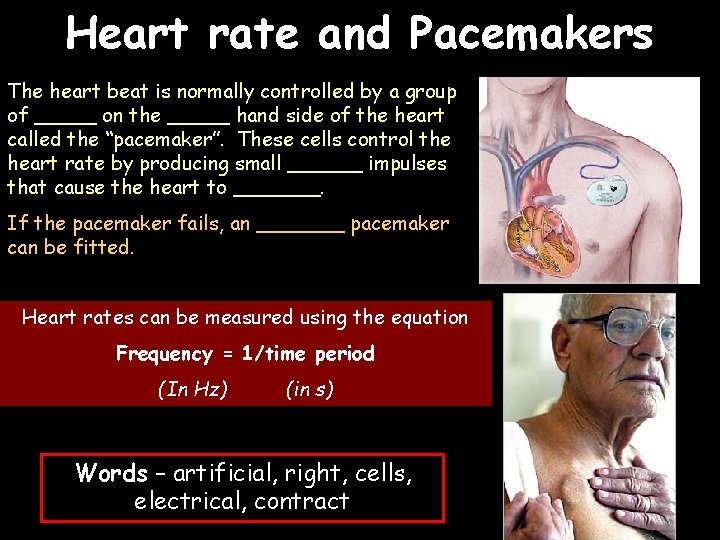
Heart rate and Pacemakers The heart beat is normally controlled by a group of _____ on the _____ hand side of the heart called the “pacemaker”. These cells control the heart rate by producing small ______ impulses that cause the heart to _______. If the pacemaker fails, an _______ pacemaker can be fitted. Heart rates can be measured using the equation Frequency = 1/time period (In Hz) (in s) Words – artificial, right, cells, electrical, contract

Pulse Oximetry A pulse oximeter is a device used to measure the blood oxygen level and heart rate through non-invasive methods. It works by basically sending two different _______ of light through the _____ and the ______ of each of these wavelengths is measured by a _____. The absorbance of each wavelength is then turned into a blood-oxygen level by the attached ____. Words – photodetector, wavelengths, computer, finger, absorbance

Topic 3 – Ionising Radiation

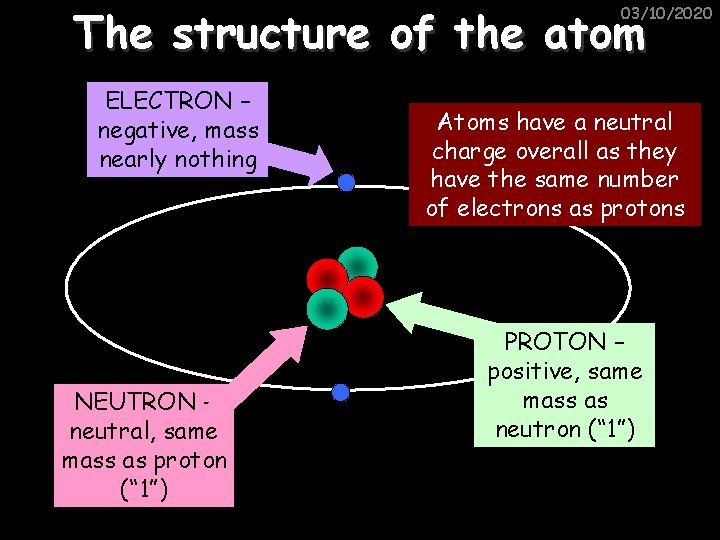
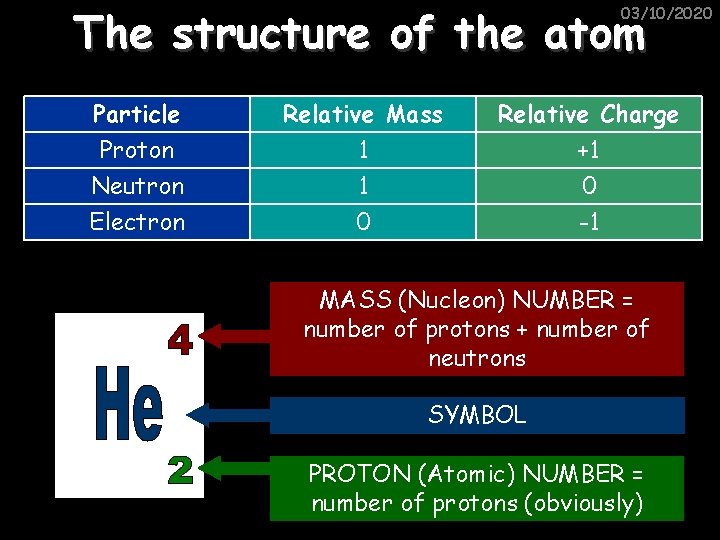
The structure of the atom 03/10/2020 ELECTRON – negative, mass nearly nothing NEUTRON – neutral, same mass as proton (“ 1”) Atoms have a neutral charge overall as they have the same number of electrons as protons PROTON – positive, same mass as neutron (“ 1”)

The structure of the atom 03/10/2020 Particle Proton Neutron Electron Relative Mass 1 1 0 Relative Charge +1 0 -1 MASS (Nucleon) NUMBER = number of protons + number of neutrons SYMBOL PROTON (Atomic) NUMBER = number of protons (obviously)

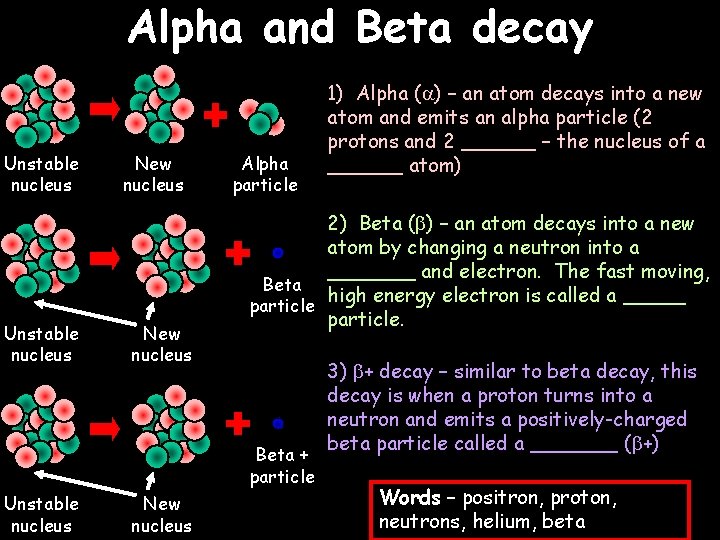
Alpha and Beta decay Unstable nucleus New nucleus Alpha particle 2) Beta ( ) – an atom decays into a new atom by changing a neutron into a _______ and electron. The fast moving, Beta high energy electron is called a _____ particle. Beta + particle Unstable nucleus New nucleus 1) Alpha ( ) – an atom decays into a new atom and emits an alpha particle (2 protons and 2 ______ – the nucleus of a ______ atom) 3) + decay – similar to beta decay, this decay is when a proton turns into a neutron and emits a positively-charged beta particle called a _______ ( +) Words – positron, proton, neutrons, helium, beta
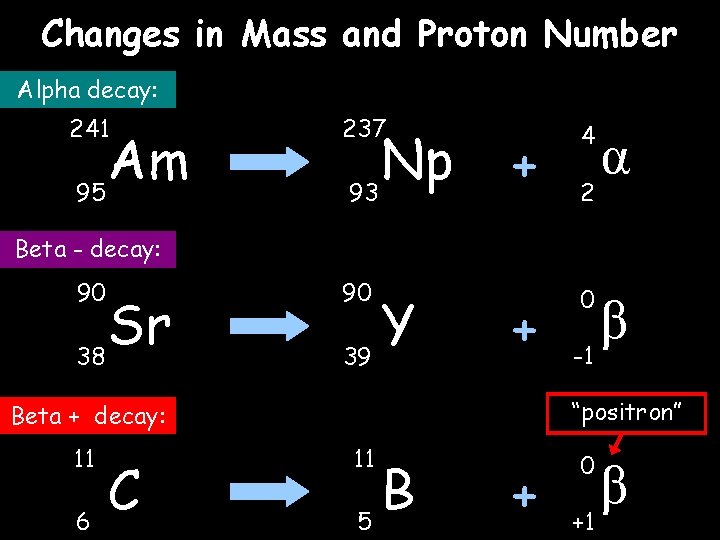
Changes in Mass and Proton Number Alpha decay: 241 Am 95 237 Np 93 + 4 + 0 2 α Beta - decay: 90 Sr 38 90 Y 39 “positron” Beta + decay: 11 6 C β -1 11 B 5 + 0 +1 β

N-Z Curve An N-Z graph plots neutron number (N) against proton number (Z). The graph looks like this: Beta – emitters would be this side of the line: No. of neutrons (N) 120 Alpha emitters would be up here (above 82 protons): 100 80 60 40 20 40 60 80 Proton number (Z) 100 Beta + emitters would be this side of the line:

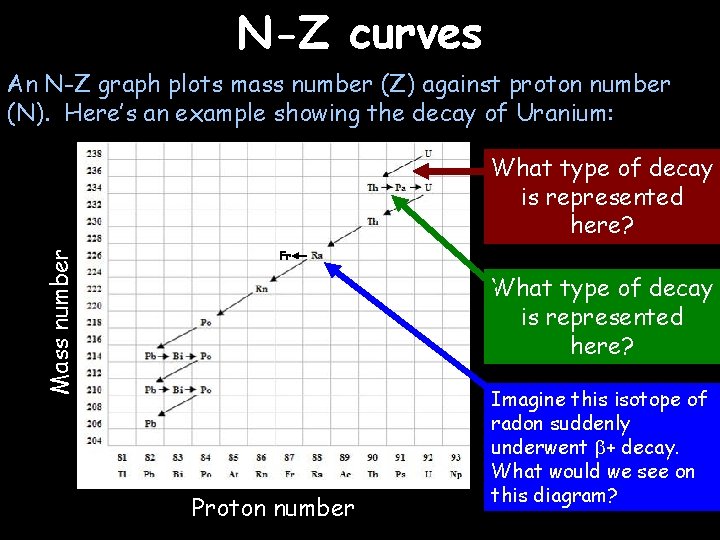
N-Z curves An N-Z graph plots mass number (Z) against proton number (N). Here’s an example showing the decay of Uranium: Mass number What type of decay is represented here? Fr What type of decay is represented here? Proton number Imagine this isotope of radon suddenly underwent + decay. What would we see on this diagram?

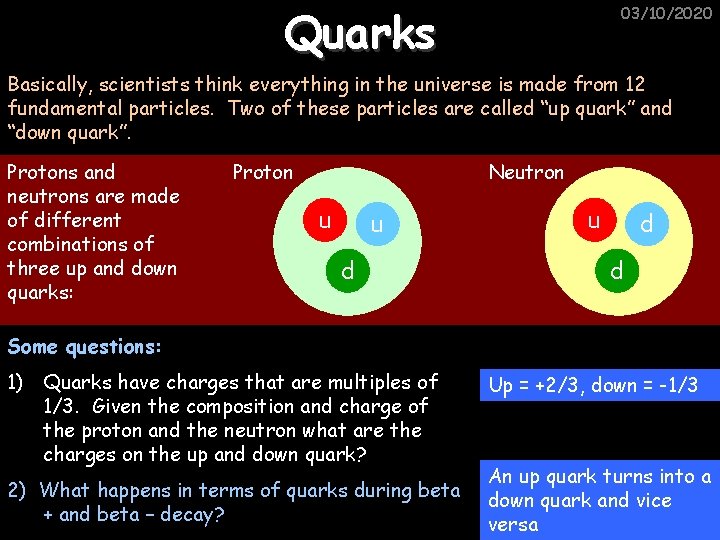
Quarks 03/10/2020 Basically, scientists think everything in the universe is made from 12 fundamental particles. Two of these particles are called “up quark” and “down quark”. Protons and neutrons are made of different combinations of three up and down quarks: Proton Neutron u u d d Some questions: 1) Quarks have charges that are multiples of 1/3. Given the composition and charge of the proton and the neutron what are the charges on the up and down quark? 2) What happens in terms of quarks during beta + and beta – decay? Up = +2/3, down = -1/3 An up quark turns into a down quark and vice versa

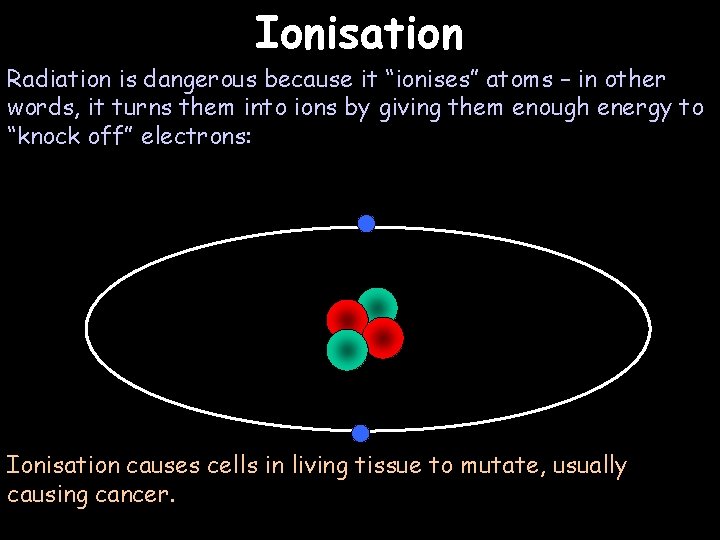
Ionisation Radiation is dangerous because it “ionises” atoms – in other words, it turns them into ions by giving them enough energy to “knock off” electrons: Ionisation causes cells in living tissue to mutate, usually causing cancer.

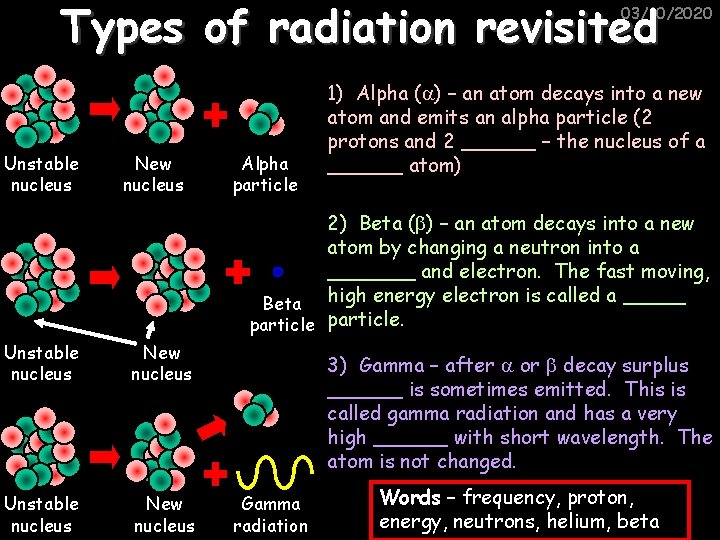
Types of radiation revisited 03/10/2020 Unstable nucleus New nucleus Alpha particle 1) Alpha ( ) – an atom decays into a new atom and emits an alpha particle (2 protons and 2 ______ – the nucleus of a ______ atom) 2) Beta ( ) – an atom decays into a new atom by changing a neutron into a _______ and electron. The fast moving, Beta high energy electron is called a _____ particle. Unstable nucleus New nucleus 3) Gamma – after or decay surplus ______ is sometimes emitted. This is called gamma radiation and has a very high ______ with short wavelength. The atom is not changed. Gamma radiation Words – frequency, proton, energy, neutrons, helium, beta

Summary Property Charge Mass Penetration ability Range in air What is it? Ionising ability Alpha Beta Gamma Positron Neutron

Exposure to Radiation People like me work with radiation a lot so we need to wear a “dosimeter” to record our exposure to radiation: Radiation dose is measured in units called “sieverts” (Sv).

How we are exposed to Radiation We can be exposed to radiation by “irradiation” or by “contamination”: Irradiation Contamination Gamma “Irradiation” is when radiation “hits” us from the outside, like background radiation. “Contamination” is when we take radioactive sources in, like the case of Alexander Litvinenko

03/10/2020 Using Radioactivity in Medicine - Tracers A tracer is a small amount of radioactive material used to detect things, e. g. a leak in a pipe: Gamma source The radiation from the radioactive source is picked up above the ground, enabling the leak in the pipe to be detected. Tracers can also be used in medicine to detect tumours: For medicinal tracers, you would probably use a beta or gamma source with a short half life – why?

PET scanners work by basically building up a 3 -D image of something in the body by detecting radioactive emissions from a tracer. The radioactive source would need to be produced nearby. Why is this? Radiation can also be used in palliative care to relieve the symptoms caused by tumours.

Topic 4 – Motion of Particles

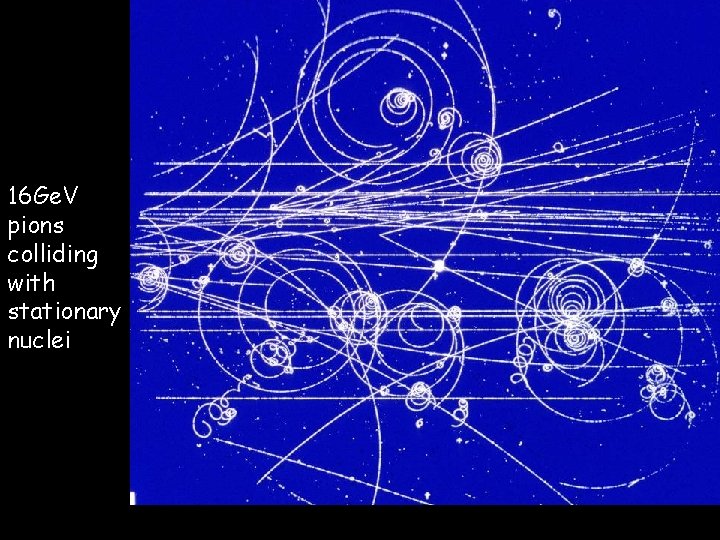
Particle Accelerators 03/10/2020 The CERN lab in Geneva is basically a huge particle accelerator (27 km circumference). Scientists from around the world work there every day to analyse pictures like the following in order to understand our world better…

03/10/2020

03/10/2020

03/10/2020 16 Ge. V pions colliding with stationary nuclei

Circular Motion For any object to travel in a circle there must be a “centripetal force” acting on it: In this case the force is gravity. 03/10/2020

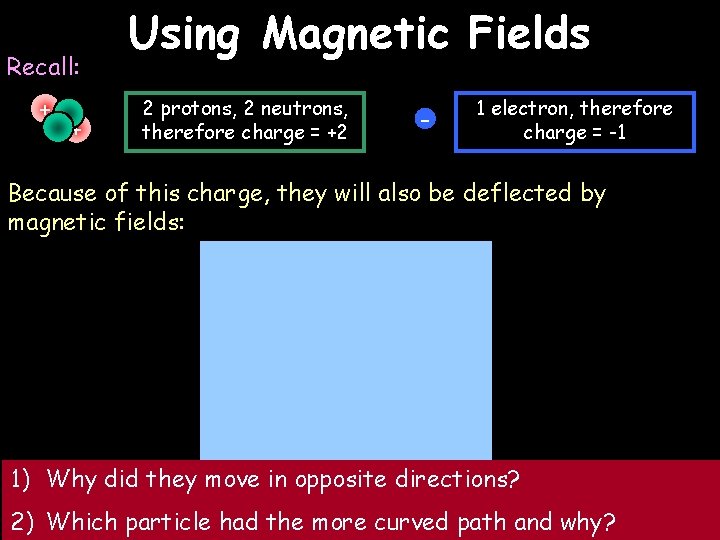
Recall: + + Using Magnetic Fields 2 protons, 2 neutrons, therefore charge = +2 - 1 electron, therefore charge = -1 Because of this charge, they will also be deflected by magnetic fields: 1) Why did they move in opposite directions? Region of magnetic field 2) Which particle had the more curved path and why?

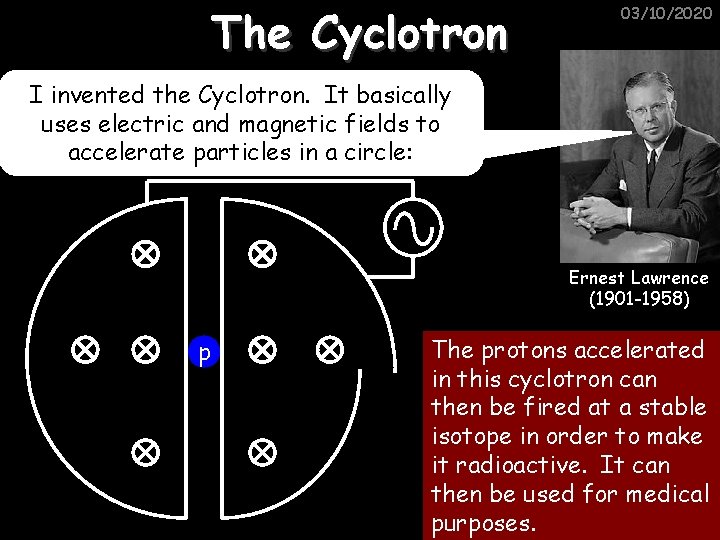
The Cyclotron 03/10/2020 I invented the Cyclotron. It basically uses electric and magnetic fields to accelerate particles in a circle: Ernest Lawrence (1901 -1958) p The protons accelerated in this cyclotron can then be fired at a stable isotope in order to make it radioactive. It can then be used for medical purposes.

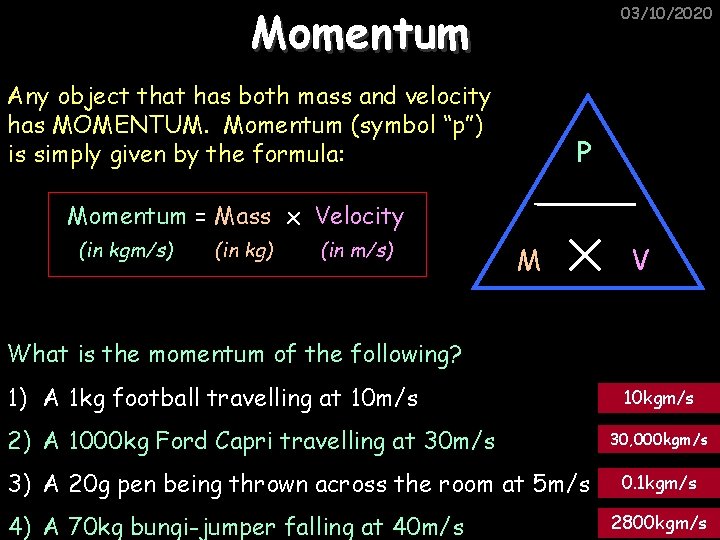
Momentum 03/10/2020 Any object that has both mass and velocity has MOMENTUM. Momentum (symbol “p”) is simply given by the formula: P Momentum = Mass x Velocity (in kgm/s) (in kg) (in m/s) M V What is the momentum of the following? 1) A 1 kg football travelling at 10 m/s 2) A 1000 kg Ford Capri travelling at 30 m/s 3) A 20 g pen being thrown across the room at 5 m/s 4) A 70 kg bungi-jumper falling at 40 m/s 10 kgm/s 30, 000 kgm/s 0. 1 kgm/s 2800 kgm/s

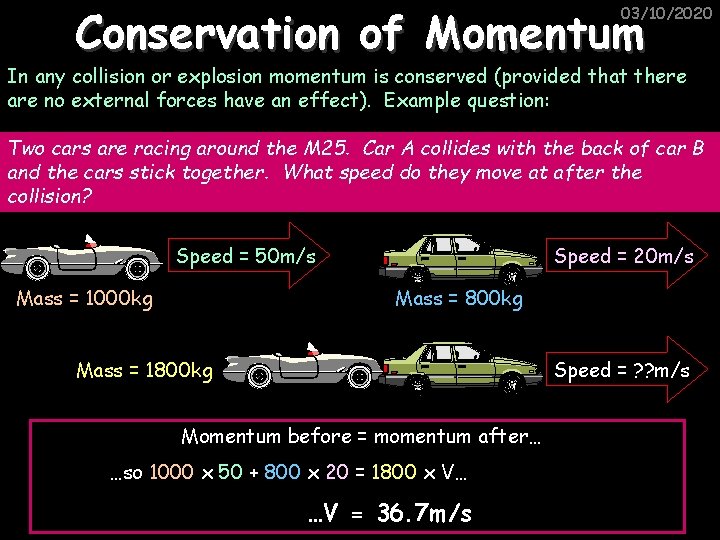
Conservation of Momentum 03/10/2020 In any collision or explosion momentum is conserved (provided that there are no external forces have an effect). Example question: Two cars are racing around the M 25. Car A collides with the back of car B and the cars stick together. What speed do they move at after the collision? Speed = 50 m/s Mass = 1000 kg Speed = 20 m/s Mass = 800 kg Mass = 1800 kg Speed = ? ? m/s Momentum before = momentum after… …so 1000 x 50 + 800 x 20 = 1800 x V… …V = 36. 7 m/s

03/10/2020 Momentum in different directions What happens if the bodies are moving in opposite directions? Speed = 50 m/s Mass = 1000 kg Speed = 20 m/s Mass = 800 kg Momentum is a VECTOR quantity, so the momentum of the second car is negative… Total momentum = 1000 x 50 – 800 x 20 = 34000 kgm/s Speed after collision = 34000 kgm/s / 1800 = 18. 9 m/s

Another example 03/10/2020 Consider the nuclear decay of Americium-241: 237 93 Np 241 95 Am If the new neptunium atom moves away at a speed of 5 x 105 m/s what was the speed of the alpha particle? 2. 96 x 107 m/s 4 2 α

More questions… 1. A car of mass 1000 kg heading up the M 1 at 50 m/s collides with a stationary truck of mass 8000 kg and sticks to it. What velocity does the wreckage move forward at? 2. A defender running away from a goalkeeper at 5 m/s is hit in the back of his head by the goal kick. The ball stops dead and the player’s speed increases to 5. 5 m/s. If the ball had a mass of 500 g and the player had a mass of 70 kg how fast was the ball moving? 3. A white snooker ball moving at 5 m/s strikes a red ball and pots it. Both balls have a mass of 1 kg. If the white ball continued in the same direction at 2 m/s what was the velocity of the red ball? 4. A gun has a recoil speed of 2 m/s when firing. If the gun has a mass of 2 kg and the bullet has a mass of 10 g what speed does the bullet come out at? 03/10/2020 5. 6 m/s 70 m/s 3 m/s 400 m/s

Recap question on momentum 03/10/2020 1. Matt and Dan are racing against each other over 400 m at Sports Day. Matt is running at 8 m/s and catches up with Dan who is running at 6 m/s. After the collision Matt stops and Dan moves slightly faster. If Matt’s mass is 60 kg and Dan’s is 70 kg calculate how fast Dan moves after the collision. 12. 9 m/s 2. Bobbie is driving her 5 kg toy car around. It is travelling at 10 m/s when it hits the back of Heather’s (stationary) leg and sticks to it. Assuming Heather’s leg can move freely and has a mass of 10 kg calculate how fast it will move after the collision. 3. 3 m/s

Energy loss in collisions In previous work we looked at how to calculate an object’s kinetic energy: Kinetic energy = ½ x mass x velocity squared in J in kg in ms -1 We’ve also said that in a collision momentum is conserved (unless an external force acts). The same cannot usually be said for kinetic energy… For example, consider the following collision. How much kinetic energy is lost? Before Speed = 50 ms-1 Speed = 20 ms-1 Mass = 1000 kg Mass = 800 kg After Mass = 1000 kg Speed = 20 ms-1 Mass = 800 kg Speed = 30 ms-1

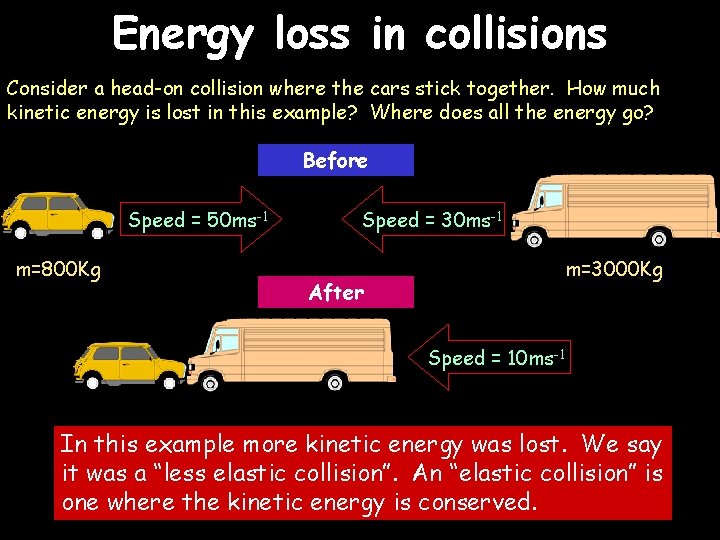
Energy loss in collisions Consider a head-on collision where the cars stick together. How much kinetic energy is lost in this example? Where does all the energy go? Before Speed = 50 ms-1 m=800 Kg Speed = 30 ms-1 m=3000 Kg After Speed = 10 ms-1 In this example more kinetic energy was lost. We say it was a “less elastic collision”. An “elastic collision” is one where the kinetic energy is conserved.

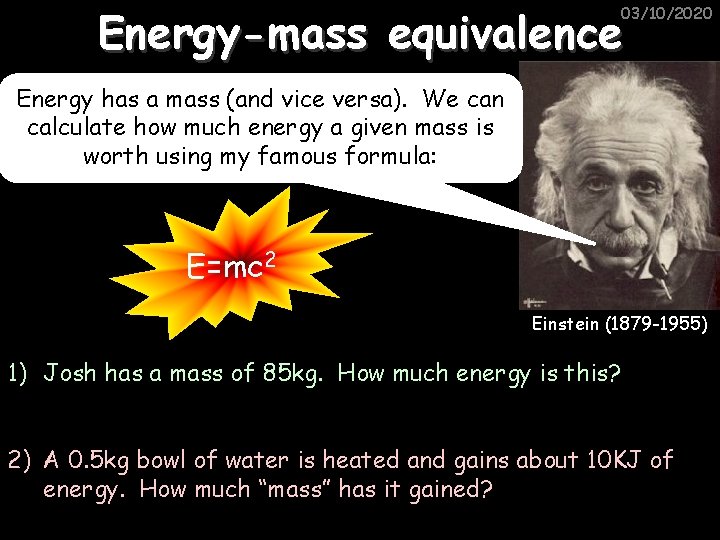
Energy-mass equivalence 03/10/2020 Energy has a mass (and vice versa). We can calculate how much energy a given mass is worth using my famous formula: E=mc 2 Einstein (1879 -1955) 1) Josh has a mass of 85 kg. How much energy is this? 2) A 0. 5 kg bowl of water is heated and gains about 10 KJ of energy. How much “mass” has it gained?

Electron-Positron Annihilation 03/10/2020 We can also apply my equation to situations where electrons and positrons annihilate each other Example question: An electron and positron collide with each other and annihilate to produce a gamma ray. What happens to charge and mass during this process? Einstein (1879 -1955) Charge – the positron has a charge of +1, the electron has a charge of -1 and the gamma ray has no charge so charge is conserved. Mass – If the electron and the positron both have a mass of 9. 11 x 10 -31 kg how much energy is released?

Topic 5 – Kinetic Theory and Gases

Particle theory revision Particle theory is all about explaining the properties of solids, liquids and gases by looking at what the particles do. SOLIDS In a solid the particles ______ around a _____ position. There is a ______ force of attraction between each particle and they are very _____ together Words – strong, close, vibrate, fixed

LIQUIDS In a liquid the particles are _____ together but can move in any direction. They won’t keep a _____ shape like _____ do. GASES In a gas the particles are very far apart and move _____ in all directions. They often ______ with each other and because they are far apart they can be easily _______. Words – fixed, collide, quickly, close, squashed, solids

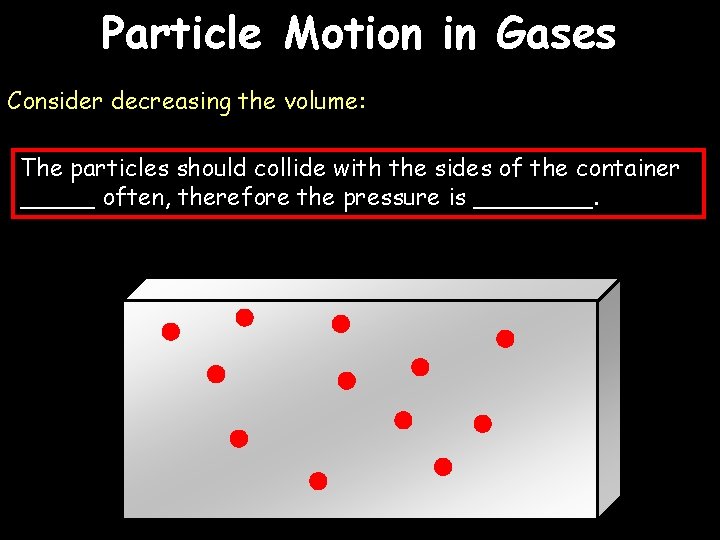
Particle Motion in Gases Gas pressure is caused by particles hitting the side of a container. Anything we do that increases those collisions will increase the pressure:

Particle Motion in Gases Consider increasing the temperature: The particles should collide with the sides of the container _____ often, therefore the pressure is ____. This could cause the container to ______.

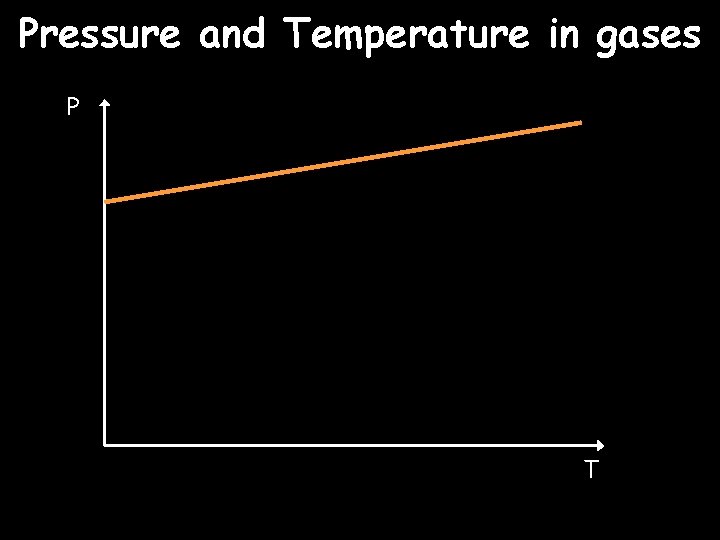
Pressure and Temperature in gases P T

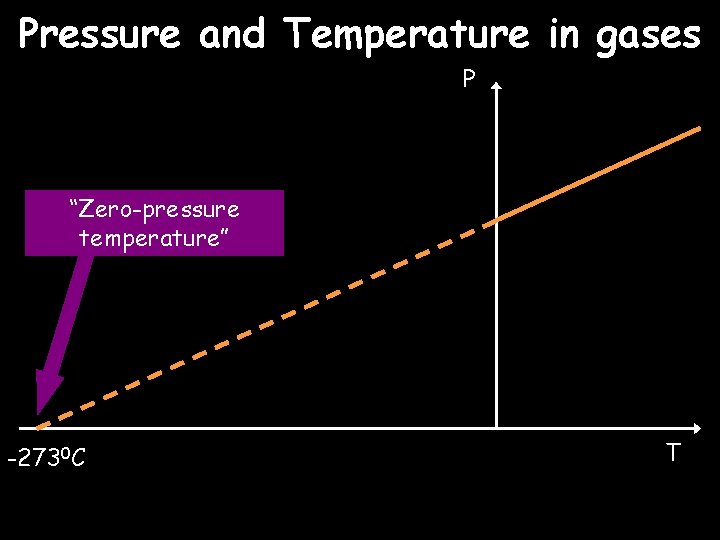
Pressure and Temperature in gases P “Zero-pressure temperature” -2730 C T

Absolute Temperature “Absolute Temperature” starts at 0 K and represents the temperature at which particles have zero kinetic energy. It goes up in the same steps as OC. For example: 1) The freezing point of water is 273 K 2) The boiling point of water is 373 K 3) Room temperature is around 293 K Lord Kelvin, 1824 -1907

Kinetic Energy and Temperature in gases KE T (in K)

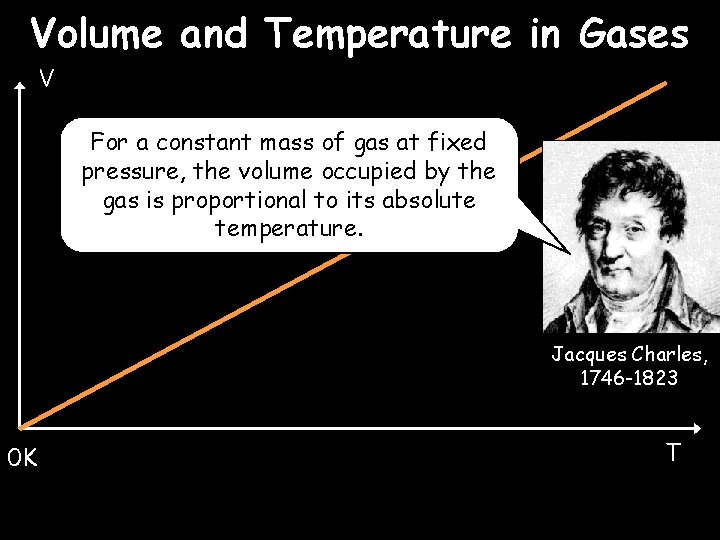
Volume and Temperature in Gases V For a constant mass of gas at fixed pressure, the volume occupied by the gas is proportional to its absolute temperature. Jacques Charles, 1746 -1823 0 K T

Volume and Temperature in Gases Provided the pressure of a gas stays the same we can use this relationship to calculate the volume of a gas: V 1 = V 2 T 1 T 2 Jacques Charles, 1746 -1823 1) A gas changes in temperature from 200 K to 300 K. If its original volume was 2 m 3 what is the new volume? 3 m 3 2) Another gas is halved in volume. What will happen to its temperature? It will halve 3) A third gas is kept at constant pressure while being compressed from 20 litres to 15 litres. If its new temperature is 275 K what was its original temperature? 367 K

Pressure and Volume in gases Pressure Volume Pressure x volume

Particle Motion in Gases Consider decreasing the volume: The particles should collide with the sides of the container _____ often, therefore the pressure is ____.

Pressure and Volume in gases Conclusion When we multiplied the pressure of a gas by its volume we found that the answer was always __ _______. In other words, if you DECREASE the volume you _______ the pressure and so on. “One goes up, the other goes down”

Boyle’s Law “For a fixed mass of gas at constant temperature (“isothermal”), the product of the pressure and volume is constant. ” Robert Boyle 1627 -1691 Higher temperature? Let’s draw this… P P V 1/V

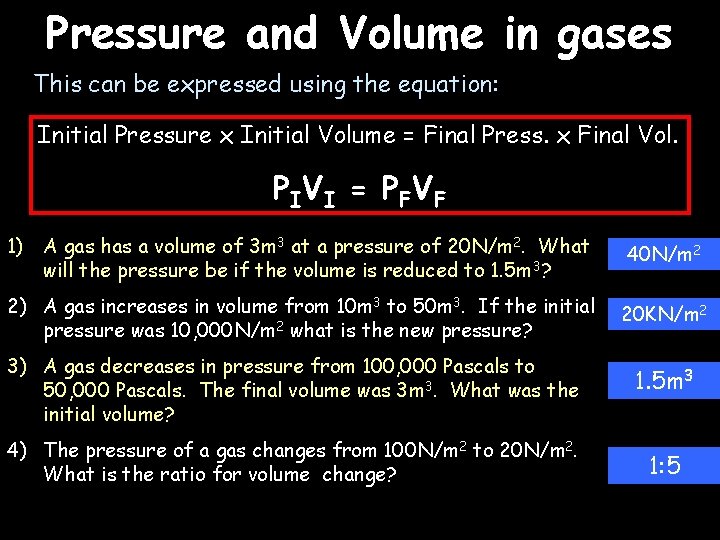
Pressure and Volume in gases This can be expressed using the equation: Initial Pressure x Initial Volume = Final Press. x Final Vol. P IV I = P F V F 1) A gas has a volume of 3 m 3 at a pressure of 20 N/m 2. What will the pressure be if the volume is reduced to 1. 5 m 3? 40 N/m 2 2) A gas increases in volume from 10 m 3 to 50 m 3. If the initial pressure was 10, 000 N/m 2 what is the new pressure? 20 KN/m 2 3) A gas decreases in pressure from 100, 000 Pascals to 50, 000 Pascals. The final volume was 3 m 3. What was the initial volume? 4) The pressure of a gas changes from 100 N/m 2 to 20 N/m 2. What is the ratio for volume change? 1. 5 m 3 1: 5

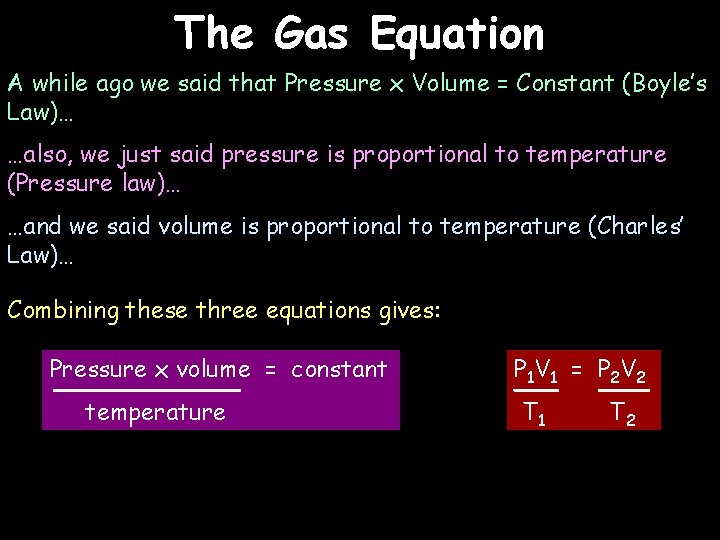
The Gas Equation A while ago we said that Pressure x Volume = Constant (Boyle’s Law)… …also, we just said pressure is proportional to temperature (Pressure law)… …and we said volume is proportional to temperature (Charles’ Law)… Combining these three equations gives: Pressure x volume = constant temperature P 1 V 1 = P 2 V 2 T 1 T 2

Some example questions 1) An ideal gas has a volume of 2 m 3 and a pressure of 101 KPa (101, 000 N/m 2) at a temperature of 300 K. The gas is then increased in temperature to 400 K but kept at constant volume. Calculate the new pressure. 2) The same gas is then allowed to cool to 200 K while being kept at constant pressure. Calculate the new volume. 3) Another gas at 300 K and 101 KPa is allowed to halve in volume while being kept at the same pressure. What is the new temperature? 135 KPa 1 m 3 150 K

Using this in Medicine These principles are used in medicine in bottled gas canisters. The gas is kept at a higher pressure than atmospheric pressure.