GAS LAWS Get out your NOTES PACKET We

























- Slides: 31

GAS LAWS Get out your NOTES PACKET. We will add some notes to this in a few minutes.

Let’s review what PRESSURE is… • PRESSURE is how much force is exerted on a certain amount of space. • Another way to think of pressure… how hard and how often the particles are hitting each other and the container • Example: dodgeball

DRAW ON YOUR WHITEBOARD: Two containers - one container is twice as big as the other. Both have the same amount of gas particles (5 particles each). Circle the container with greater pressure.

If you increase the VOLUME of a gas (bigger container in previous example), the PRESSURE will decrease. Show what this would look like in the graph below. Pressure Volume

Write IN WORDS what is shown in the picture below about the relationship between PRESSURE and VOLUME of a gas.

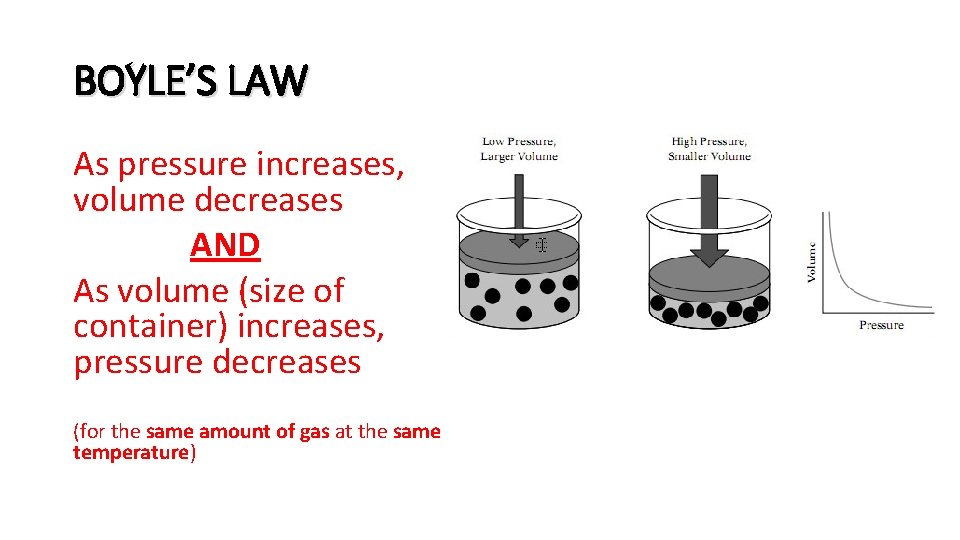
BOYLE’S LAW As pressure increases, volume decreases AND As volume (size of container) increases, pressure decreases (for the same amount of gas at the same temperature)

Real-life examples of BOYLE’S LAW: • The bubbles exhaled by a scuba diver grow as they approach the surface of the ocean. (The pressure from the weight of the water decreases with depth, so the volume of the bubbles increases as they rise. ) • Scuba divers have to be careful about how fast they go up or down because of changes in the volume of gases in their cells and organs. • When you open a can of soda, the gas molecules escape because the “container size” gets bigger, so your soda is less fizzy (less gas molecules with less pressure).

So what is the relationship between TEMPERATURE and VOLUME of a gas? First, let’s look at the relationship between TEMPERATURE and PRESSURE

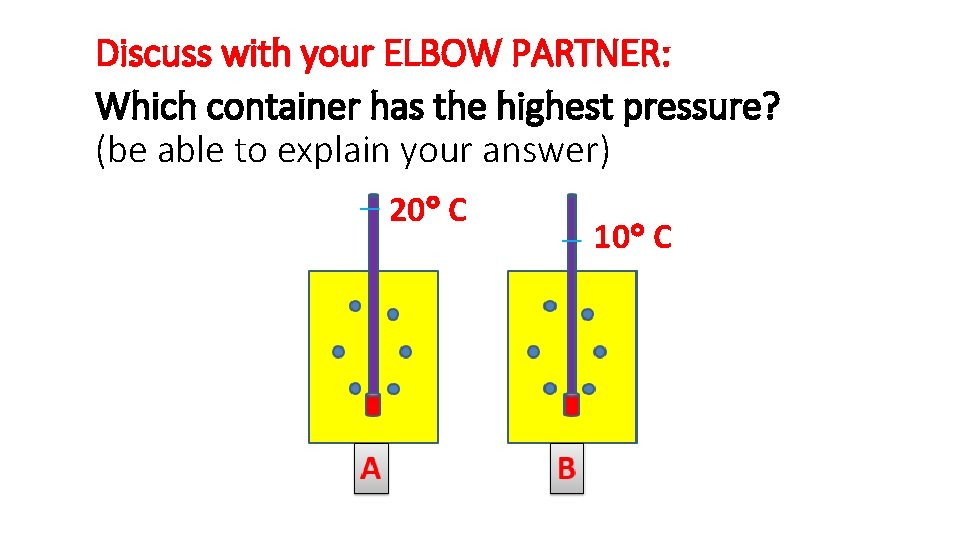
Discuss with your ELBOW PARTNER: Which container has the highest pressure? (be able to explain your answer) 20 C 10 C

Therefore, as the TEMPERATURE of a gas increases, the PRESSURE of a gas increases. Discuss with your ELBOW PARTNER: If the heated gas is not inside a closed container, what would therefore happen to its VOLUME? VOLUME

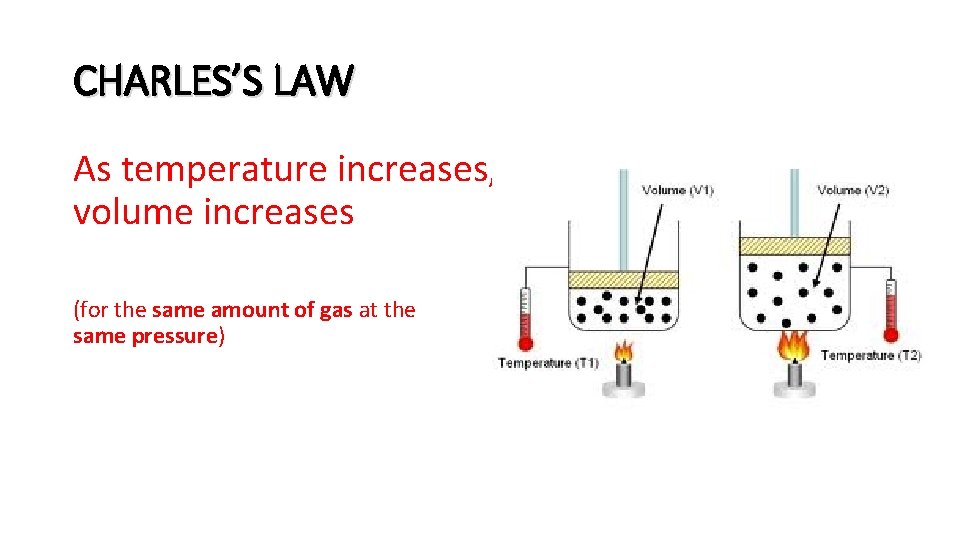
CHARLES’S LAW As temperature increases, volume increases (for the same amount of gas at the same pressure)

Real-life examples of CHARLES’ LAW: • A football inflated inside and then taken outdoors on a winter day shrinks slightly. – Deflate-gate! • It is a good idea not to pump a raft too full of air before it sits in the hot sun. • The plunger on a turkey syringe thermometer pops out when the turkey is done. The volume of air trapped under the plunger increases when the temperature inside the turkey climbs.

BOYLE’S LAW: (stop at 1: 09) https: //www. youtube. com/watch? v=Xto 88 g. Mm. Dzw CHARLES’S LAW: https: //www. youtube. com/watch? v=XBET-a. Ui. JE 8

How can I remember which is which? Charlie Brown was a TV Show (Charles’ Law = Temperature/Volume) Boys play video games (Boyle’s Law = Pressure/Volume)

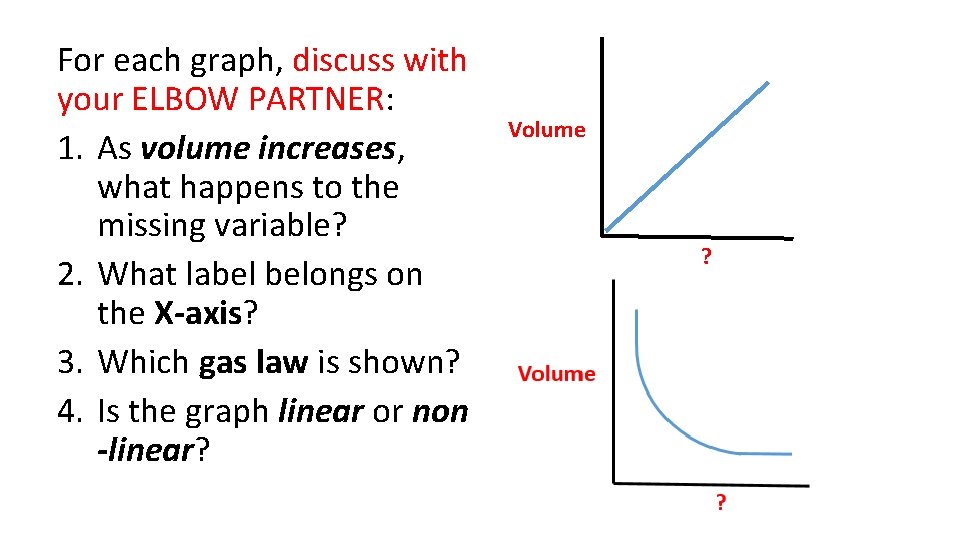
For each graph, discuss with your ELBOW PARTNER: 1. As volume increases, what happens to the missing variable? 2. What label belongs on the X-axis? 3. Which gas law is shown? 4. Is the graph linear or non -linear? Volume ?

Exit Ticket Two balloons are filled with equal volumes of air. One balloon is put into a freezer and the other is put into a hot car. 1. 3 hours later… What would you expect to happen? 2. Which gas law does this illustrate? When you put the plunger of a syringe into the tube, gases from the air are trapped inside the syringe. 3. What would happen to the gases inside if you push the plunger? Why? 4. What would happen to the gases if you pulled the plunger back out? Why? 5. Which gas law does this illustrate?

#1 - Gas Laws Review (2/3/16) 1. Write IN WORDS what Picture A shows. 2. What gas law is this? Picture A 3. Write IN WORDS what Picture B shows. 4. What gas law is this? Picture B



Directions for Part A (Charles’ Law) 1. Get the following materials: 1. From lab station: Gatorade bottle, cup with dish soap, 2 empty cups 2. Get ice from Station 7 in one of your cups 3. Get hot water from me (front of the room) 2. Dip the mouth of your Gatorade bottle into the dish soap solution. You should see a bubble seal over the top. 3. Put the BOTTOM of the Gatorade bottle into the HOT water. Record observations. 4. Now move the Gatorade bottle into the COLD water. Record observations. 5. CLEAN UP (return materials to your lab station).




Explain how gases create pressure. (Hint: think about what the molecules are doing)

Two gases have temperatures of 50 C and 100 C. They have an equal number of gas particles and are in the same size container. Which would have a greater pressure and why?

Explain why a soda left in a super-hot car on a summer day might explode. What gas law does this illustrate?

Why are planes pressurized? What gas law does relate to? (Hint: Think about what happens to atmospheric pressure as you go up/down and what might happen to your cells because of that)

A balloon that is filled up in Medford will change size as you drive up to Mount Ashland. Explain how this relates to the 2 gas laws.

Explain why one small tank of highly pressurized helium gas can be used to fill up hundreds of balloons.


A can full of hot gas will implode if it is cooled off quickly. Explain why in terms of pressure.