More Ksp applications Common Ion Effect l Solution












- Slides: 22

More Ksp applications

Common Ion Effect l Solution contains the ion of the dissolving salt. One of the ions that was in the original Ksp expression is added to the system at equilibrium l Instead of both ions having an initial concentration of 0, one will have a value l

Common Ion Effect l Solution contains the ion of the dissolving salt. Example: You mix Ag 2 Cr. O 4 with a 0. 100 M solution of Ag. NO 3 Ksp of Ag 2 Cr. O 4 = 9. 0 x 10 -12

Common Ion Effect l Example: You mix Ag 2 Cr. O 4 with a 0. 100 M solution of Ag. NO 3 Common ion: Ag+ l Ignore the NO 3 l

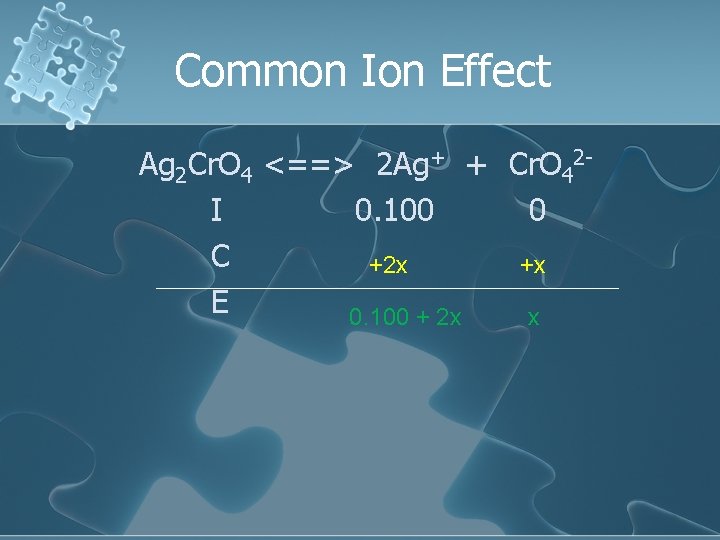
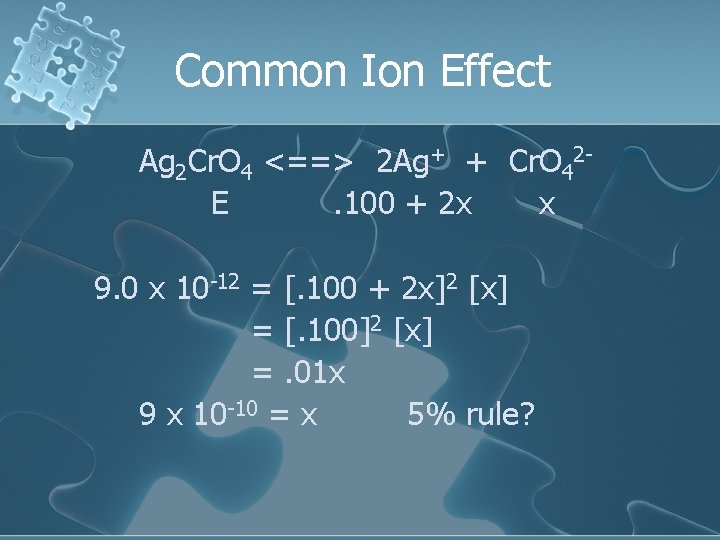
Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 I 0. 100 0 C +2 x +x E 0. 100 + 2 x x

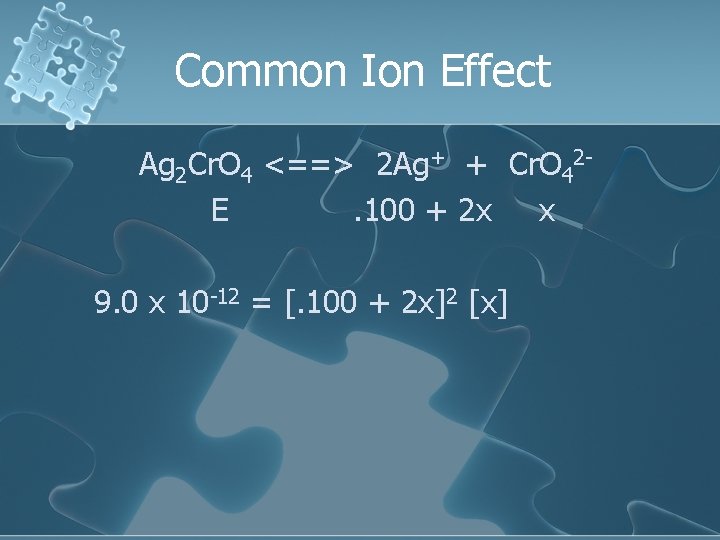
Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 E. 100 + 2 x x 9. 0 x 10 -12 = [. 100 + 2 x]2 [x]

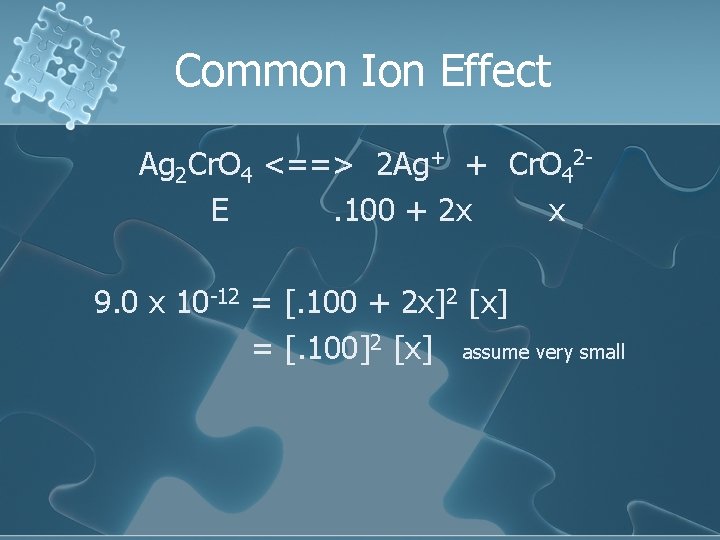
Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 E. 100 + 2 x x 9. 0 x 10 -12 = [. 100 + 2 x]2 [x] = [. 100]2 [x] assume very small

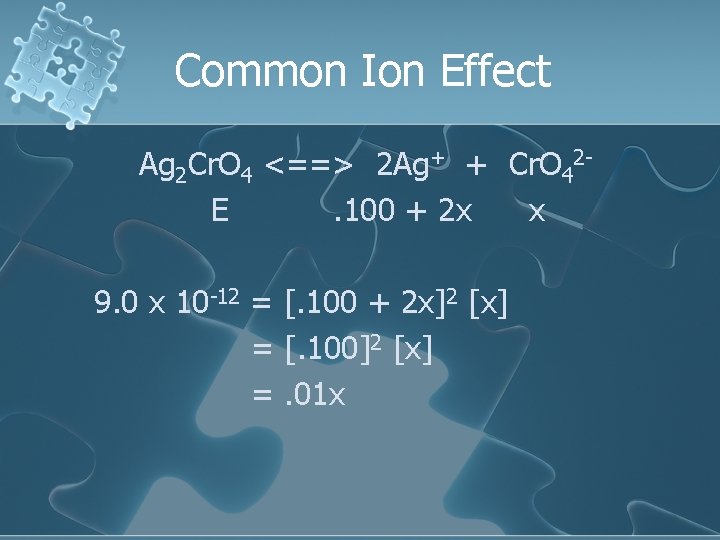
Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 E. 100 + 2 x x 9. 0 x 10 -12 = [. 100 + 2 x]2 [x] = [. 100]2 [x] =. 01 x

Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 E. 100 + 2 x x 9. 0 x 10 -12 = [. 100 + 2 x]2 [x] = [. 100]2 [x] =. 01 x 9 x 10 -10 = x 5% rule?

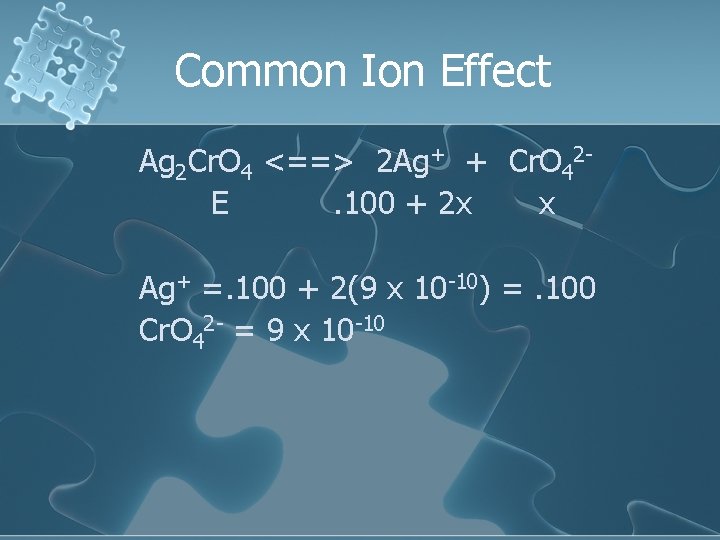
Common Ion Effect Ag 2 Cr. O 4 <==> 2 Ag+ + Cr. O 42 E. 100 + 2 x x Ag+ =. 100 + 2(9 x 10 -10) =. 100 Cr. O 42 - = 9 x 10 -10

Common Ion Effect l What effect does this have according to Le Chatelier’s principle? l l Addition of ion (product) shifts equilibrium to the left So what does a common ion always do to solubility? l LOWER solubility

Common Ions – Your Turn l Al 2(C 2 O 4)3 (s) 2 Al 3+ + 3 C 2 O 42 - l Ksp = 6. 33 x 10 -19 l What is the solubility of aluminum if 0. 250 M sodium oxalate solution is added to the system at equilibrium?

Precipitate Formation? l Will a precipitate form when you mix 2 solutions together?

Precipitate Formation? l Not equilibrium or maybe it is. Not sure, use Q. l Need to find new [ ] after mixing and plug into Q

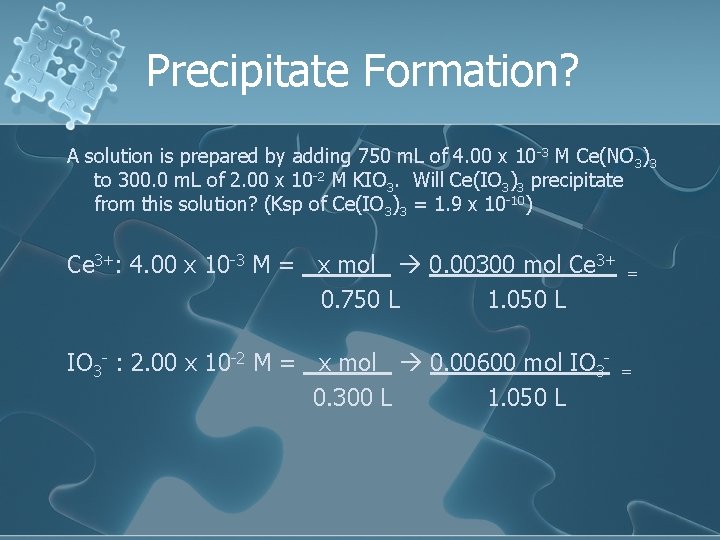
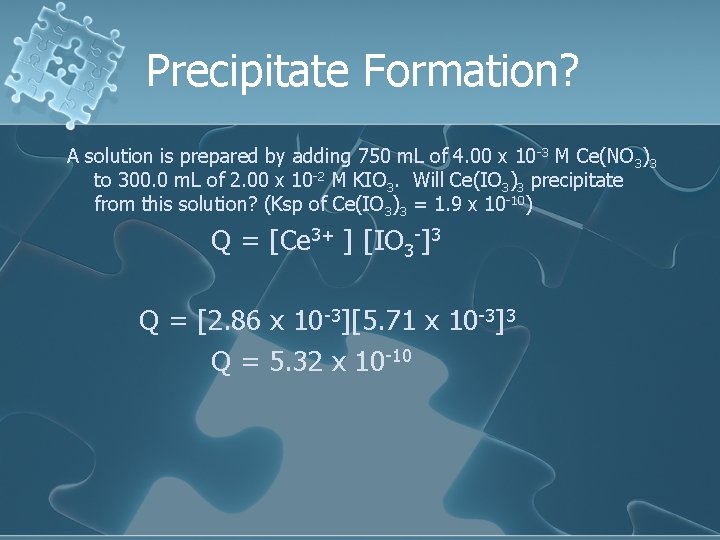
Precipitate Formation? A solution is prepared by adding 750. 0 m. L of 4. 00 x 10 -3 M Ce(NO 3)3 to 300. 0 m. L of 2. 00 x 10 -2 M KIO 3. Will Ce(IO 3)3 precipitate from this solution? (Ksp of Ce(IO 3)3 = 1. 9 x 10 -10)

Precipitate Formation? A solution is prepared by adding 750 m. L of 4. 00 x 10 -3 M Ce(NO 3)3 to 300. 0 m. L of 2. 00 x 10 -2 M KIO 3. Will Ce(IO 3)3 precipitate from this solution? (Ksp of Ce(IO 3)3 = 1. 9 x 10 -10) Ce 3+: 4. 00 x 10 -3 M = x mol 0. 00300 mol Ce 3+ 0. 750 L 1. 050 L IO 3 - : 2. 00 x 10 -2 M = x mol 0. 00600 mol IO 30. 300 L 1. 050 L = =

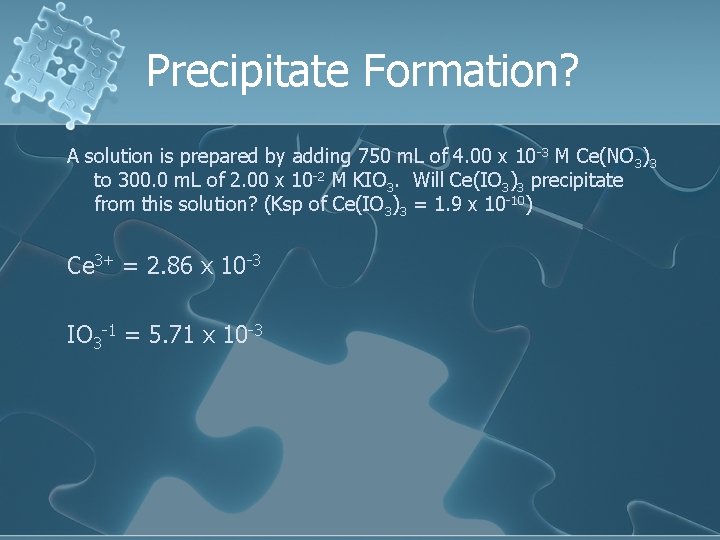
Precipitate Formation? A solution is prepared by adding 750 m. L of 4. 00 x 10 -3 M Ce(NO 3)3 to 300. 0 m. L of 2. 00 x 10 -2 M KIO 3. Will Ce(IO 3)3 precipitate from this solution? (Ksp of Ce(IO 3)3 = 1. 9 x 10 -10) Ce 3+ = 2. 86 x 10 -3 IO 3 -1 = 5. 71 x 10 -3

Precipitate Formation? A solution is prepared by adding 750 m. L of 4. 00 x 10 -3 M Ce(NO 3)3 to 300. 0 m. L of 2. 00 x 10 -2 M KIO 3. Will Ce(IO 3)3 precipitate from this solution? (Ksp of Ce(IO 3)3 = 1. 9 x 10 -10) Q = [Ce 3+ ] [IO 3 -]3 Q = [2. 86 x 10 -3][5. 71 x 10 -3]3 Q = 5. 32 x 10 -10

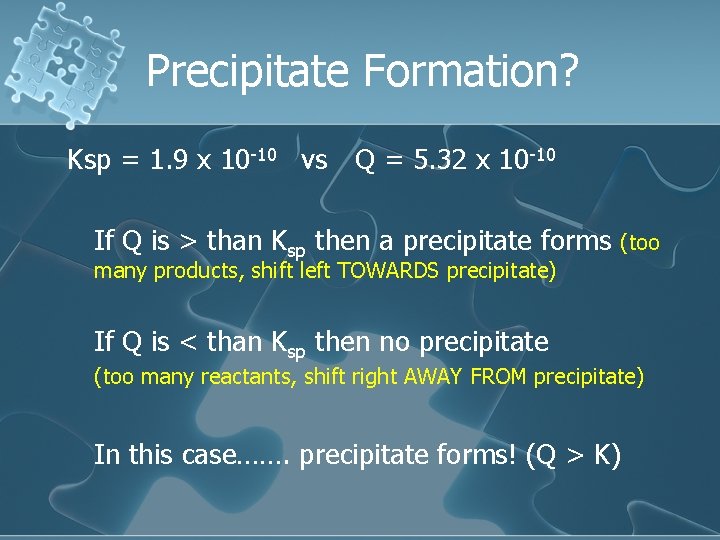
Precipitate Formation? Ksp = 1. 9 x 10 -10 vs Q = 5. 32 x 10 -10 If Q is > than Ksp then a precipitate forms (too many products, shift left TOWARDS precipitate) If Q is < than Ksp then no precipitate (too many reactants, shift right AWAY FROM precipitate) In this case……. precipitate forms! (Q > K)

More Practice l Will a precipitate form when 75. 0 m. L of 0. 020 M Ba. Cl 2 and 125 m. L of 0. 040 M Na 2 SO 4 are mixed together? l Ksp Ba. SO 4 = 1. 48 x 10 -9

Putting Them Together We can now put together a solution stoichiometry problem WITH a Ksp common ion problem!! Yay!!!

Putting Them Together l Calculate the final concentrations of K+, C 2 O 42 -, Ba 2+, and Br- in a solution prepared by adding 0. 100 L of 0. 200 M K 2 C 2 O 4 to 0. 150 L of 0. 250 M Ba. Br 2. l For Ba. C 2 O 4, Ksp = 2. 3 x 10 -8 l Absent? I’ll post a key to this on the website.