Chapter 17 Common Ion Effect Drill l Use














![STEP 4: Determine and solve the equilibrium constant expression: l Ka = ([H+][C 2 STEP 4: Determine and solve the equilibrium constant expression: l Ka = ([H+][C 2](https://slidetodoc.com/presentation_image_h2/3f4a058d3aac8eccba1c87cac5f11441/image-17.jpg)



- Slides: 20

Chapter 17 Common Ion Effect

Drill l Use AP Review Drill # 50 -53

Objectives SWBAT l Complete Common Ion Effect calculations. l Explain how the Common Ion Effect is a special case of Le Chatelier’s Principle. l

Common Ion Effect l The Common Ion Effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved substance.

Uses of Common Ion Effect l The common ion effect plays an important role in determining: l the p. H of a solution l and the solubility of a slightly soluble salt. l

The Common Ion Effect When a salt, with the anion of a weak acid, is added to that acid the addition of the salt reverses the dissociation of the acid. l Acid Dissociation: l CH 3 COOH CH 3 COO -1 + H+1 l Salt that is added: l Na. CH 3 COO -1 + Na+1 l

Common Ion Effect l Addition of the salt will increase the concentration of the CH 3 COO-1 ion. l Le Chatelier’s Principle says that the increased ion concentration will reverse the equilibrium reaction. This reversal decreases production of the products and increases production of the reactants.

Equilibrium Special Case l You have probably realized that the Common Ion Effect is just a special case of Le Chatelier’s Principle.

Common Ion Effect Lowers the percent dissociation of the acid. (If the equilibrium shifts left, you produce less products. ) l The same principle applies to salts with the cation of a weak base. l This is an “ICE BOX” problem with an exception. Now you have an initial concentration of the anion. l

Common Ion Effect Example l In this situation, the soluble salt of the weak acid's conjugate base simply provides a source of the conjugate base.

Consider acetic acid in water: l HC 2 H 3 O 2 (aq) ↔ H+ (aq) + C 2 H 3 O 2 - (aq) l Now, add Na. C 2 H 3 O 2, which dissociates completely, we increase the concentration of C 2 H 3 O 2 -1 (aq). l l l Le Chatelier's Principle : the equilibrium will shift to the left (towards the reactants), causing the [H+] to decrease, and therefore the p. H increases!

Common Ion Effect l The common ion effect is the shift in equilibrium that occurs when an ion ALREADY PRESENT in the equilibrium reaction is added.

EXAMPLE l If 0. 100 moles of Na. C 2 H 3 O 2 are added to a 1. 00 L of 0. 100 M solution of acetic acid, HC 2 H 3 O 2, what is the resultant p. H ?

STEP 1: Identify the major species in solution l HC 2 H 3 O 2 (weak acid) l Na+1 (neither acidic nor basic which means it is a SPECTATOR ION) l C 2 H 3 O 2 -1 (conjugate base of weak acid) l H 2 O (very weak acid or base, amphoteric)

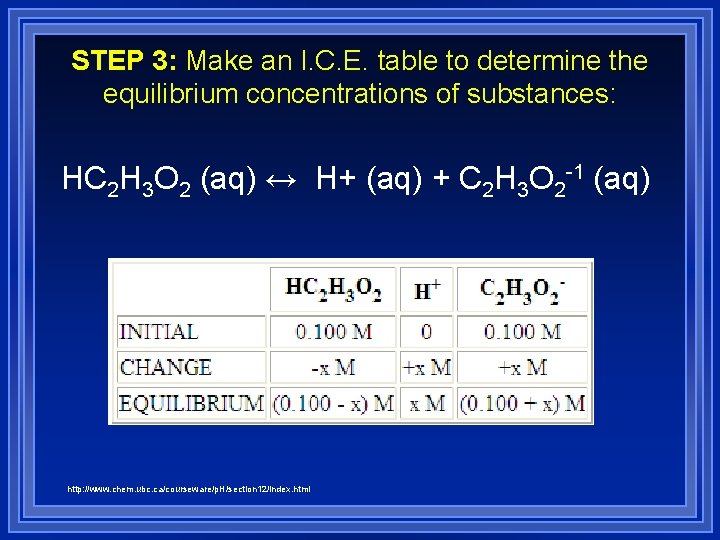
STEP 2: Identify the important equilibrium reaction(s). In this case it is a reaction that involves both the weak acid and its conjugate base: HC 2 H 3 O 2 (aq) ↔ H+ (aq) + C 2 H 3 O 2 -1 (aq)

STEP 3: Make an I. C. E. table to determine the equilibrium concentrations of substances: HC 2 H 3 O 2 (aq) ↔ H+ (aq) + C 2 H 3 O 2 -1 (aq) http: //www. chem. ubc. ca/courseware/p. H/section 12/index. html
![STEP 4 Determine and solve the equilibrium constant expression l Ka HC 2 STEP 4: Determine and solve the equilibrium constant expression: l Ka = ([H+][C 2](https://slidetodoc.com/presentation_image_h2/3f4a058d3aac8eccba1c87cac5f11441/image-17.jpg)
STEP 4: Determine and solve the equilibrium constant expression: l Ka = ([H+][C 2 H 3 O 2 -1] / [HC 2 H 3 O 2] ) l 1. 8 x 10 -5 = ( x(0. 100 + x) / (0. 100 - x) ) l Use the 5% assumption. l 1. 8 x 10 -5 = ( x(0. 100) / (0. 100) ) x = [H+] = 1. 8 x 10 -5 l

STEP 5: Calculate the p. H! l p. H = -log(1. 8 x 10 -5) = 4. 74 l The addition of a common ion can also affect the dissociation of a weak base in a similar manner. l See http: //www. chem. ubc. ca/courseware/p. H/secti on 12/index. html for more information.

Practice Problems l Try B&L #

Wrap Up l How is the Common Ion Effect a special case of Le Chatelier’s Principle?