Announcements n Homework n Chapter 4 n n




















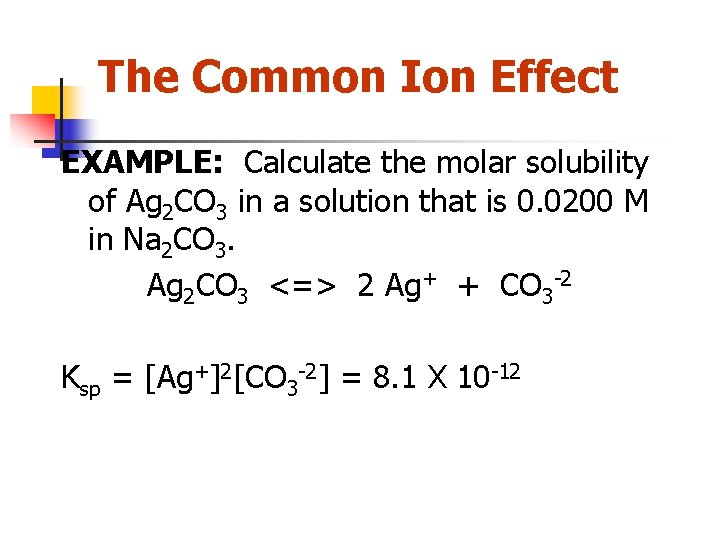




![EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-46.jpg)
![EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-47.jpg)
![EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1. EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1.](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-48.jpg)


![EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1. EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1.](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-51.jpg)
![EXAMPLE: Separate Iron and Magnesium? What [OH-] is required to begin the precipitation of EXAMPLE: Separate Iron and Magnesium? What [OH-] is required to begin the precipitation of](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-52.jpg)
![EXAMPLE: Separate Iron and Magnesium? @ equilibrium [OH-] to ‘completely’ remove Fe 3+ ^ EXAMPLE: Separate Iron and Magnesium? @ equilibrium [OH-] to ‘completely’ remove Fe 3+ ^](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-53.jpg)
- Slides: 53

Announcements n Homework – n Chapter 4 n n 8, 11, 13, 17, 19, 22 Chapter 6 n 6, 9, 14, 15 n Exam n Thursday

4 -8 n Meaning of Confidence interval? n Is an interval around the experimental mean that most likely contains the true mean (m).

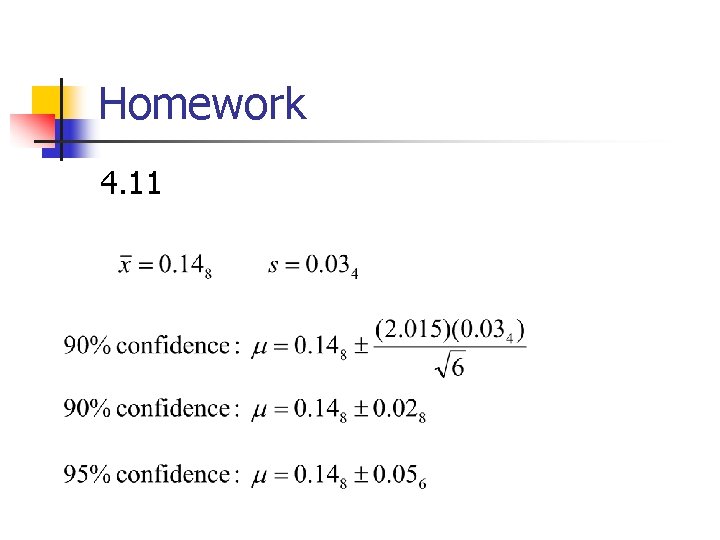
Homework 4. 11

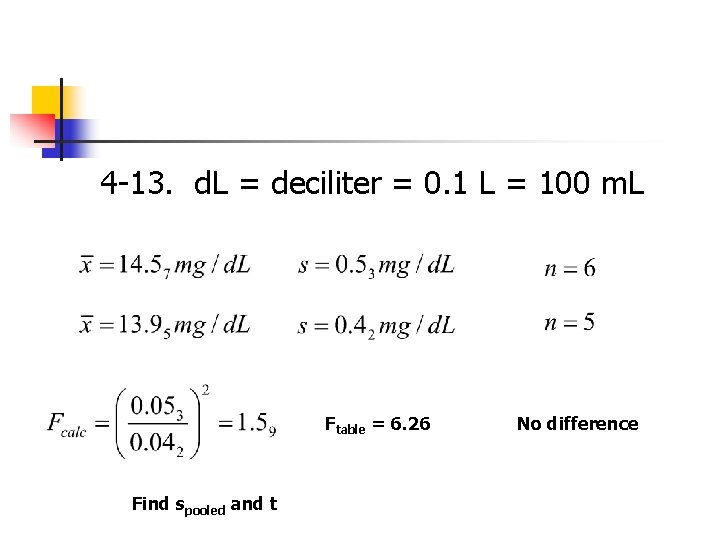
Question 4 -13. A trainee in a medical lab will be released to work on her own when her results agree with those of an experienced worker at the 95% confidence interval. Results for a blood urea nitrogen analysis are shown …. a) What does abbreviation d. L refer to? d. L = deciliter = 0. 1 L = 100 m. L b) Should the trainee work alone?

Comparison of Means with Student’s t Is there a significant difference? First you must ask, is there a significant difference in their standard deviations? NO YES f-test

4 -13. d. L = deciliter = 0. 1 L = 100 m. L Ftable = 6. 26 Find spooled and t No difference

ttable = 2. 262 No significant difference between two workers … Therefore trainee should be “Released”

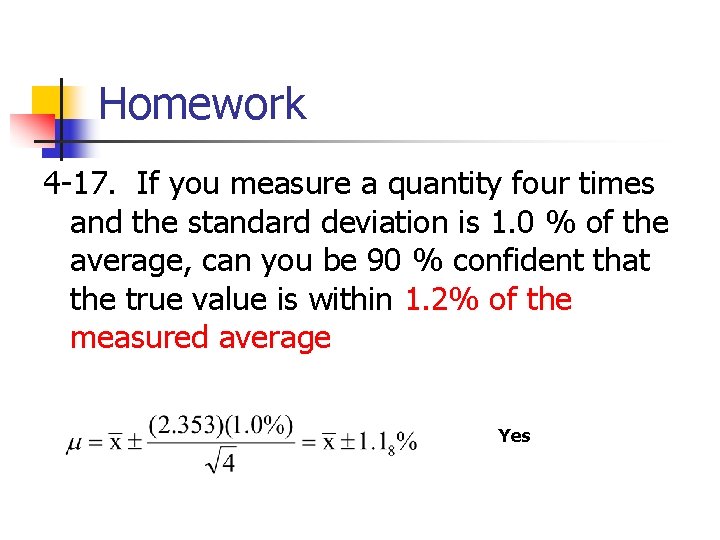
Homework 4 -17. If you measure a quantity four times and the standard deviation is 1. 0 % of the average, can you be 90 % confident that the true value is within 1. 2% of the measured average Yes

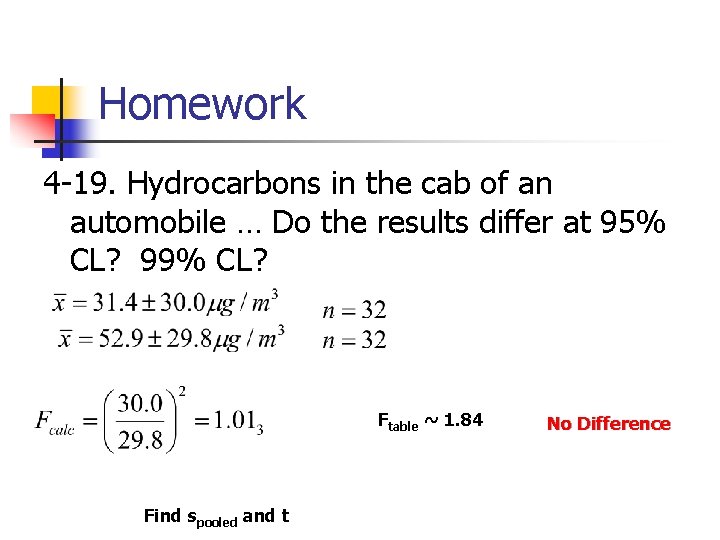
Homework 4 -19. Hydrocarbons in the cab of an automobile … Do the results differ at 95% CL? 99% CL? Ftable ~ 1. 84 Find spooled and t No Difference

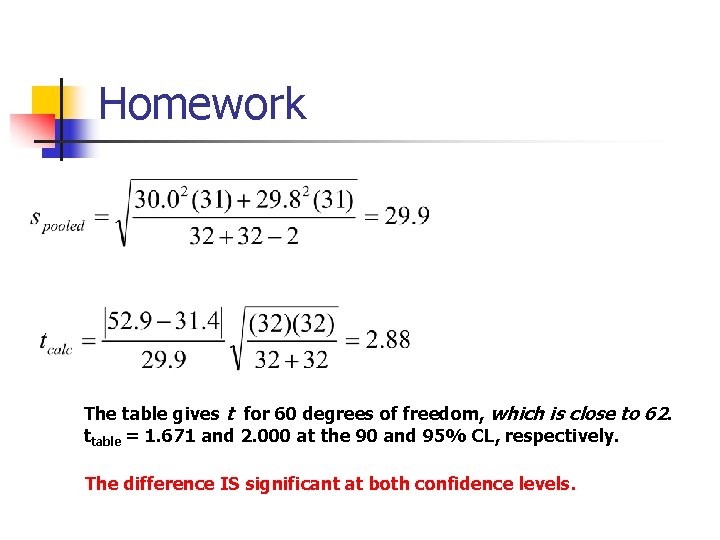
Homework The table gives t for 60 degrees of freedom, which is close to 62. ttable = 1. 671 and 2. 000 at the 90 and 95% CL, respectively. The difference IS significant at both confidence levels.

n 4 -22. Q-test, Is 216 rejectable? n 192, 216, 202, 195, 204 Qtable = 0. 64 Retain the “outlier” 216

Chapter 6 Chemical Equilibrium

Chemical Equilibrium n n Equilibrium Constant Equilibrium and Thermodynamics n n n n Enthalpy Entropy Free Energy Le Chatelier’s Principle Solubility product (Ksp) Common Ion Effect Separation by precipitation Complex formation

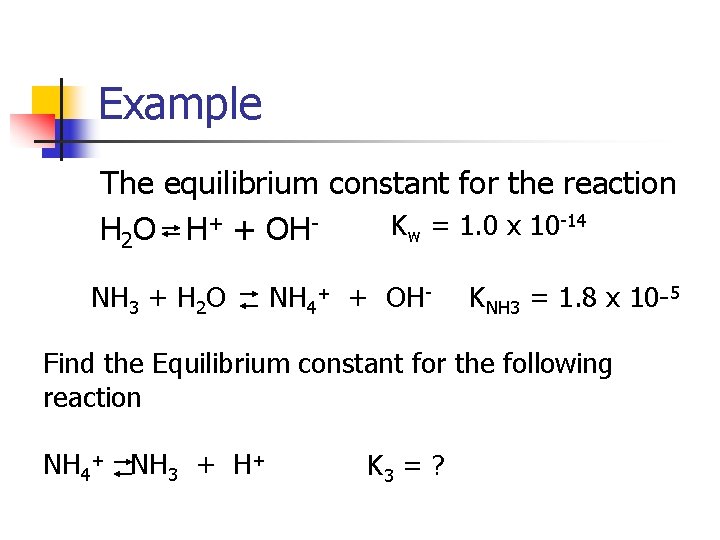
Example The equilibrium constant for the reaction -14 + K = 1. 0 x 10 H 2 O H + OH w NH 3 + H 2 O NH 4+ + OH- KNH 3 = 1. 8 x 10 -5 Find the Equilibrium constant for the following reaction NH 4+ NH 3 + H+ K 3 = ?

Equilibrium and Thermodynamics A brief review …

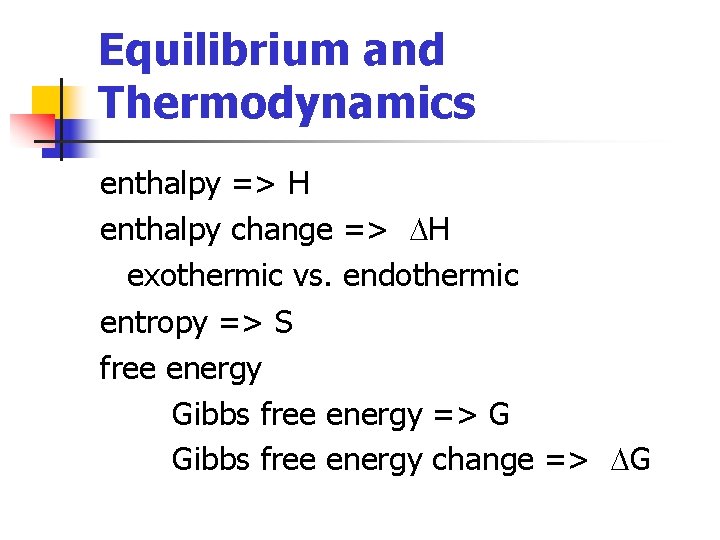
Equilibrium and Thermodynamics enthalpy => H enthalpy change => DH exothermic vs. endothermic entropy => S free energy Gibbs free energy => G Gibbs free energy change => DG

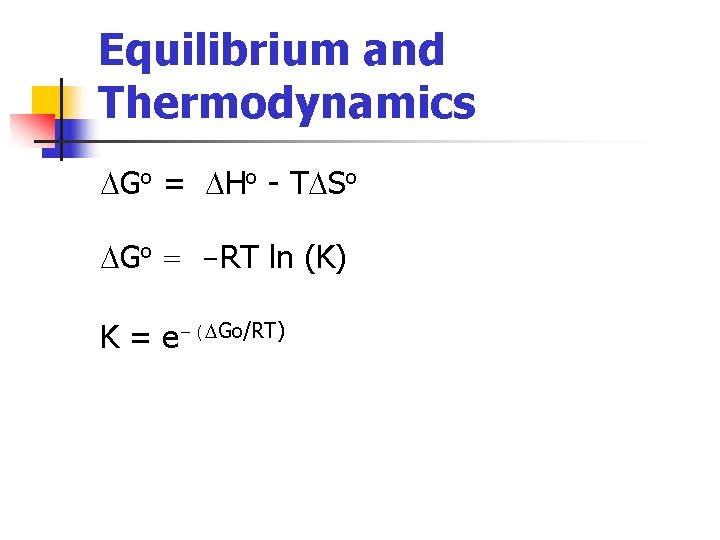
Equilibrium and Thermodynamics DGo = DHo - TDSo DGo = -RT ln (K) K = e-(DGo/RT)

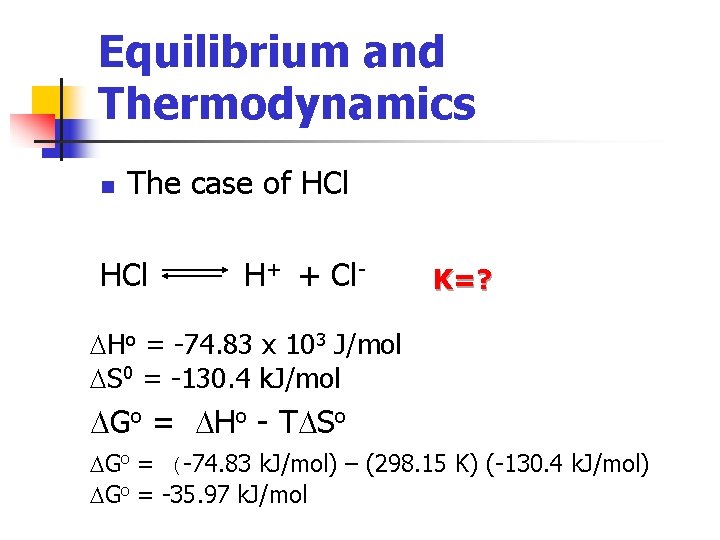
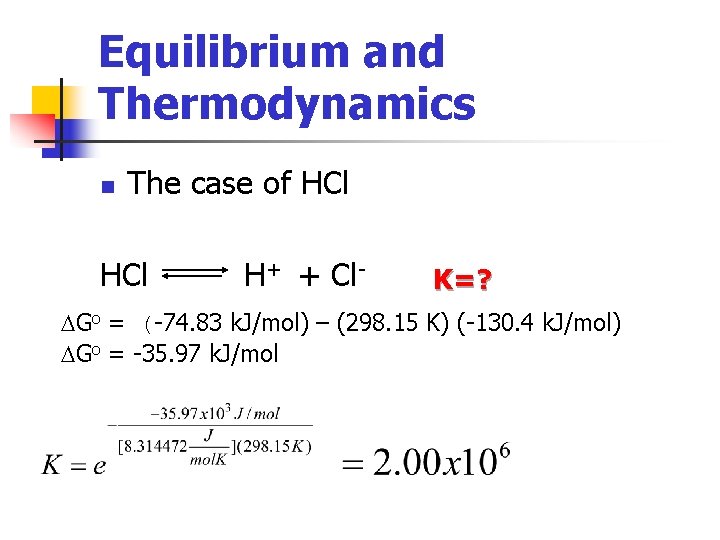
Equilibrium and Thermodynamics n The case of HCl H+ + Cl- K=? DHo = -74. 83 x 103 J/mol DS 0 = -130. 4 k. J/mol DGo = DHo - TDSo DGo = (-74. 83 k. J/mol) – (298. 15 K) (-130. 4 k. J/mol) DGo = -35. 97 k. J/mol

Equilibrium and Thermodynamics n The case of HCl H+ + Cl- K=? DGo = (-74. 83 k. J/mol) – (298. 15 K) (-130. 4 k. J/mol) DGo = -35. 97 k. J/mol

Predicting the direction in which an equilibrium will initially move Le. Chatelier’s Principle and Reaction Quotient

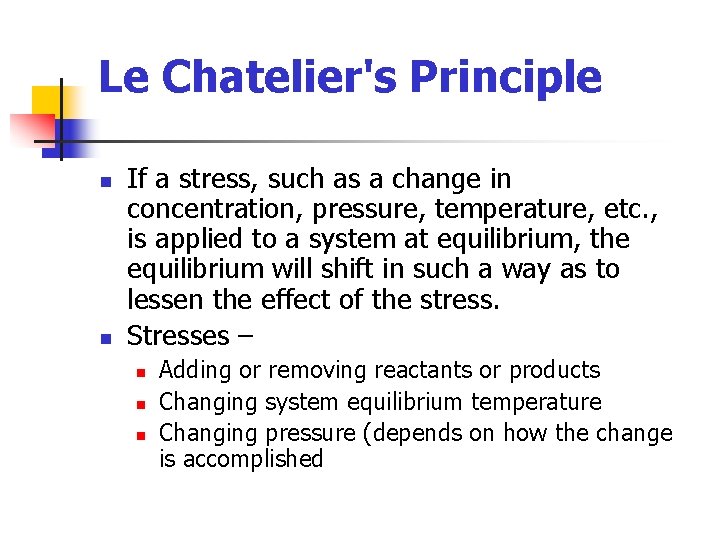
Le Chatelier's Principle n n If a stress, such as a change in concentration, pressure, temperature, etc. , is applied to a system at equilibrium, the equilibrium will shift in such a way as to lessen the effect of the stress. Stresses – n n n Adding or removing reactants or products Changing system equilibrium temperature Changing pressure (depends on how the change is accomplished

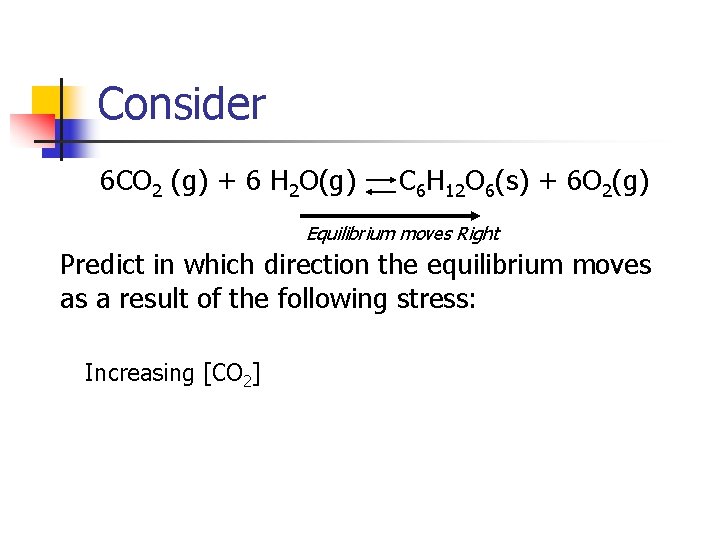
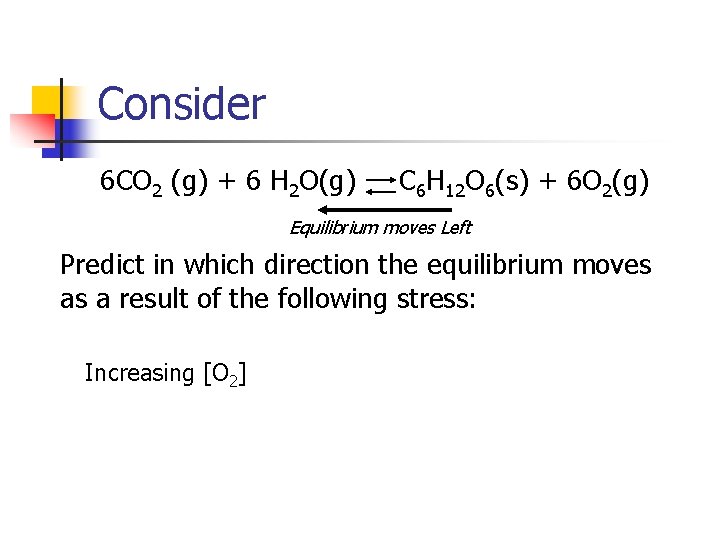
Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) Equilibrium moves Right Predict in which direction the equilibrium moves as a result of the following stress: Increasing [CO 2]

Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) Equilibrium moves Left Predict in which direction the equilibrium moves as a result of the following stress: Increasing [O 2]

Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) Equilibrium moves Left Predict in which direction the equilibrium moves as a result of the following stress: Decreasing [H 2 O]

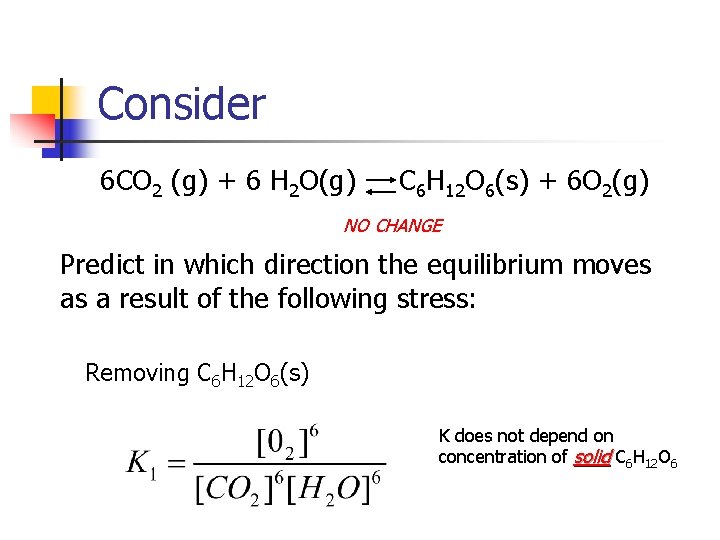
Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) NO CHANGE Predict in which direction the equilibrium moves as a result of the following stress: Removing C 6 H 12 O 6(s) K does not depend on concentration of solid C 6 H 12 O 6

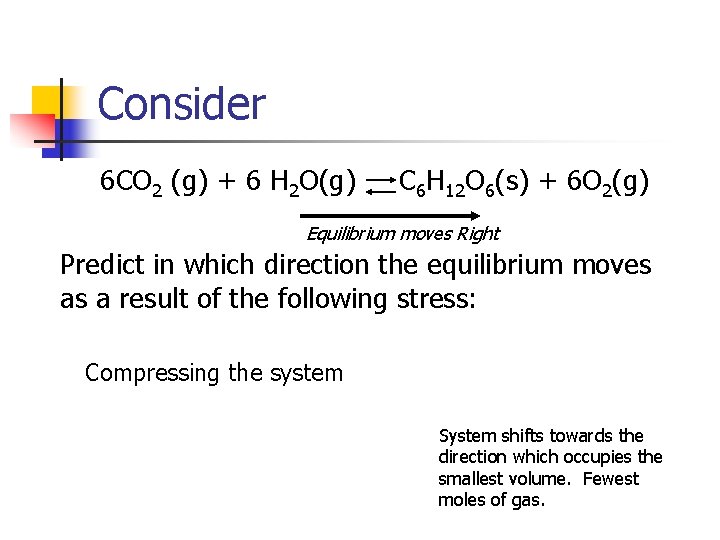
Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) Equilibrium moves Right Predict in which direction the equilibrium moves as a result of the following stress: Compressing the system System shifts towards the direction which occupies the smallest volume. Fewest moles of gas.

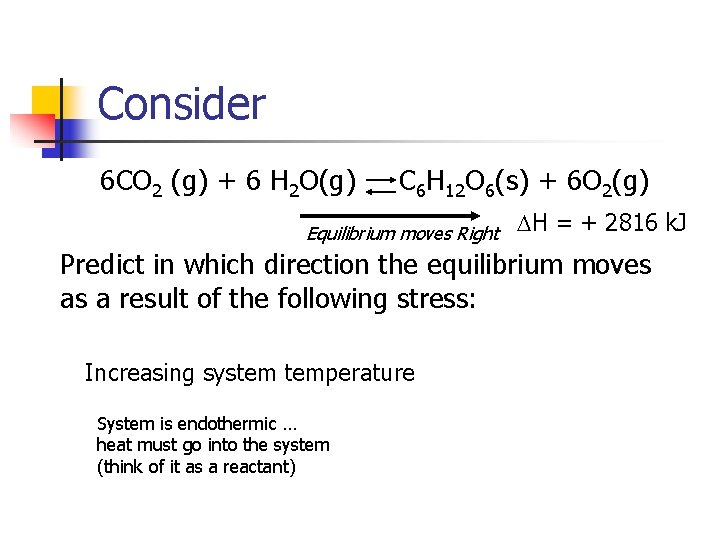
Consider 6 CO 2 (g) + 6 H 2 O(g) C 6 H 12 O 6(s) + 6 O 2(g) Equilibrium moves Right DH = + 2816 k. J Predict in which direction the equilibrium moves as a result of the following stress: Increasing system temperature System is endothermic … heat must go into the system (think of it as a reactant)

Consider this Co. Cl 2 (g) Co (g) + Cl 2(g) K=2. 19 x 10 -10 When [COCl 2] is 3. 5 x 10 -3 M, [CO] is 1. 1 x 10 -5 M, and [Cl 2] is 3. 25 x 10 -6 M is the system at equilibrium? Q= Reaction quotient

Compare Q and K Q = 1. 02 x 10 -8 K = 2. 19 x 10 -10 System is not at equilibrium, if it were the ratio would be 2. 19 x 10 -10 When Q>K TOO MUCH PRODUCT TO BE AT EQUILIRBIUM Equilibrium moves to the left Q<K TOO MUCH REACTANT TO BE AT EQUILIRBIUM Equilibrium moves to the Right Q=K System is at Equilibrium

Solubility Product Introduction to Ksp

Solubility Product solubility-product the product of the solubilities solubility-product constant => Ksp constant that is equal to the solubilities of the ions produced when a substance dissolves

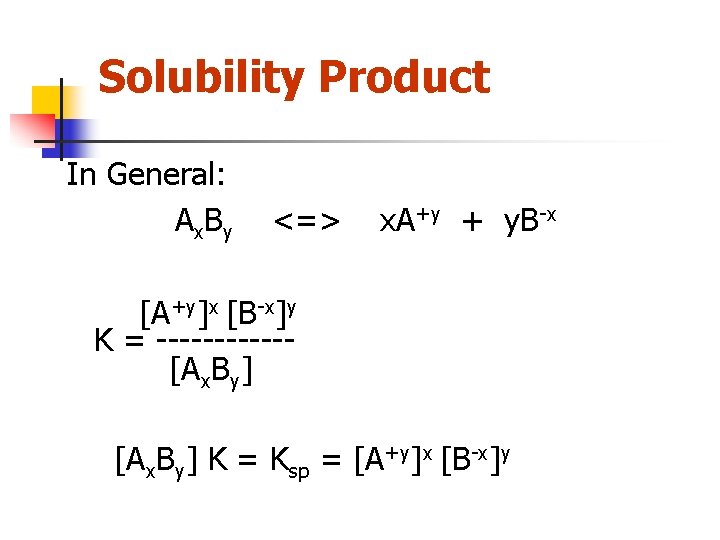
Solubility Product In General: A x. B y <=> x. A+y + y. B-x [A+y]x [B-x]y K = ------[Ax. By] K = Ksp = [A+y]x [B-x]y

Solubility Product For silver sulfate Ag 2 SO 4 (s) <=> 2 Ag+(aq) + SO 4 -2(aq) Ksp = [Ag+]2[SO 4 -2]

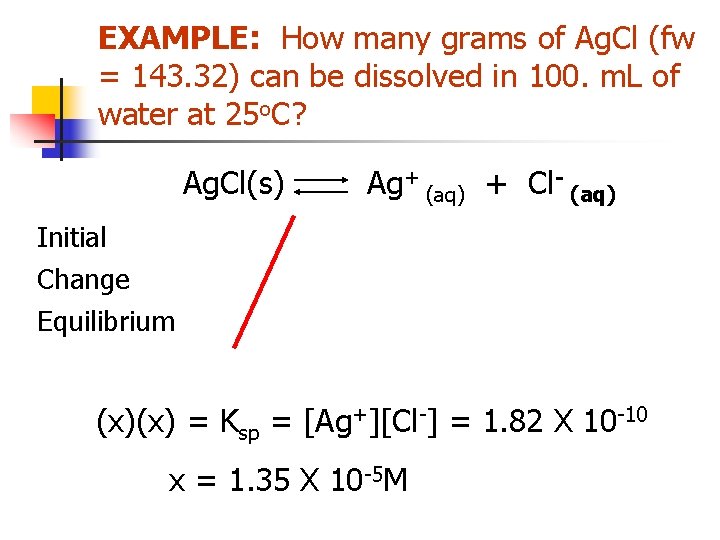
Solubility of a Precipitate in Pure Water EXAMPLE: How many grams of Ag. Cl (fw = 143. 32) can be dissolved in 100. m. L of water at 25 o. C? Ag. Cl <=> Ag+ + Cl. Ksp = [Ag+][Cl-] = 1. 82 X 10 -10 (Appen. F) let x = molar solubility = [Ag+] = [Cl-]

EXAMPLE: How many grams of Ag. Cl (fw = 143. 32) can be dissolved in 100. m. L of water at 25 o. C? Ag. Cl(s) Initial Change Equilibrium Ag+ (aq) + Cl- (aq) Some - - -x -x +x +x (x)(x) = Ksp = [Ag+][Cl-] = 1. 82 X 10 -10 x = 1. 35 X 10 -5 M

EXAMPLE: How many grams of Ag. Cl (fw = 143. 32) can be dissolved in 100. m. L of water at 25 o. C? x = 1. 35 X 10 -5 M n How many grams is that in 100 ml? # grams = (M. W. ) (Volume) (Molarity) = 143. 32 g mol-1 (. 100 L) (1. 35 x 10 -5 mol L-1) = 1. 93 X 10 -4 g = 0. 193 mg

The Common Ion Effect

The Common Ion Effect common ion effect n a salt will be less soluble if one of its constituent ions is already present in the solution
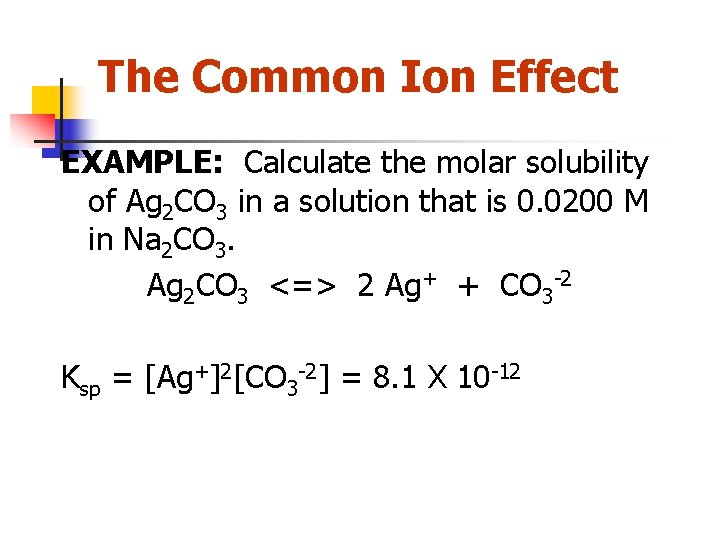
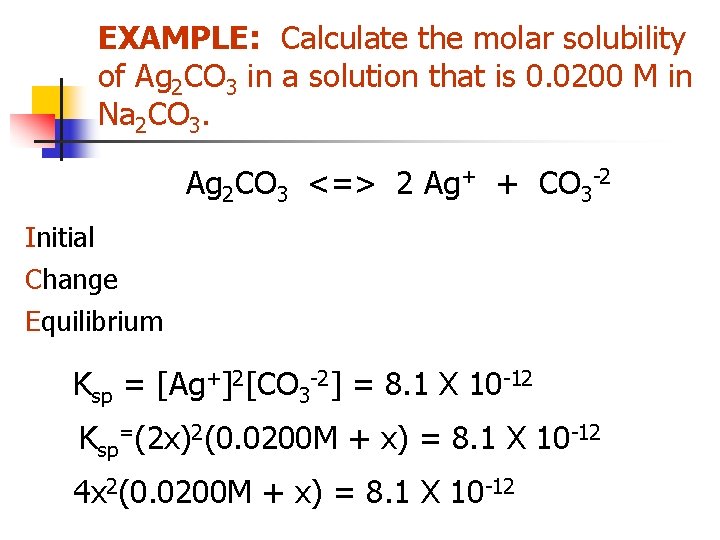
The Common Ion Effect EXAMPLE: Calculate the molar solubility of Ag 2 CO 3 in a solution that is 0. 0200 M in Na 2 CO 3. Ag 2 CO 3 <=> 2 Ag+ + CO 3 -2 Ksp = [Ag+]2[CO 3 -2] = 8. 1 X 10 -12

EXAMPLE: Calculate the molar solubility of Ag 2 CO 3 in a solution that is 0. 0200 M in Na 2 CO 3. Ag 2 CO 3 <=> 2 Ag+ + CO 3 -2 Initial Change Equilibrium Solid - 0. 0200 M -x Solid +2 x +x 0. 0200+x Ksp = [Ag+]2[CO 3 -2] = 8. 1 X 10 -12 Ksp=(2 x)2(0. 0200 M + x) = 8. 1 X 10 -12 4 x 2(0. 0200 M + x) = 8. 1 X 10 -12

EXAMPLE: Calculate the molar solubility of Ag 2 CO 3 in a solution that is 0. 0200 M in Na 2 CO 3. 4 x 2(0. 0200 M + x) = 8. 1 X 10 -12 no exact solution to a 3 rd order equation, need to make some approximation first, assume the X is very small compared to 0. 0200 M 4 X 2(0. 0200 M) = 8. 1 X 10 -12 X= 1. 0 X 10 -5 M

EXAMPLE: Calculate the molar solubility of Ag 2 CO 3 in a solution that is 0. 0200 M in Na 2 CO 3. X = 1. 0 X 10 -5 M (1. 3 X 10 -4 M in pure water) Second check assumption [CO 3 -2] = 0. 0200 M + X ~ 0. 0200 M + 0. 00001 M ~ 0. 0200 M Assumption is ok!

Separation by Precipitation

Separation by Precipitation Complete separation can mean a lot … we should define complete. Complete means that the concentration of the less soluble material has decreased to 1 X 10 -6 M or lower before the more soluble material begins to precipitate

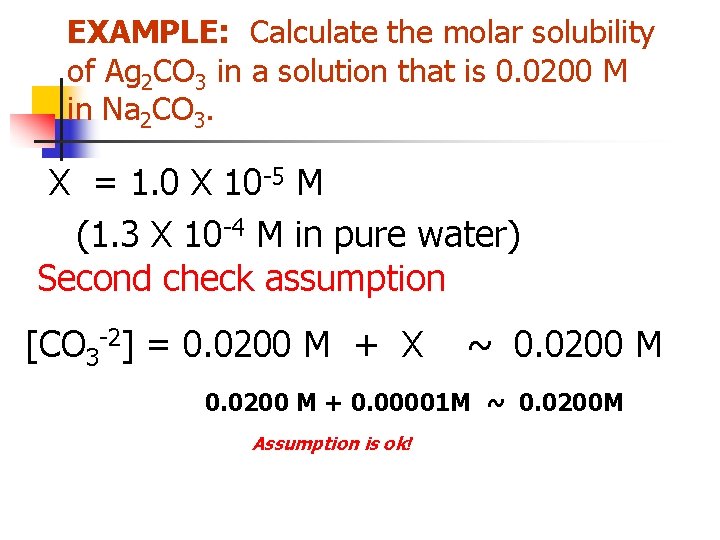
Separation by Precipitation EXAMPLE: Can Fe+3 and Mg+2 be separated quantitatively as hydroxides from a solution that is 0. 10 M in each cation? If the separation is possible, what range of OH- concentrations is permissible. Two competing reactions Fe(OH)3(s) Fe 3+ + 3 OH- Mg(OH)2(s) Mg 2+ + 2 OH-
![EXAMPLE Separate Iron and Magnesium Ksp Fe3OH3 2 X 10 39 Ksp EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-46.jpg)
EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp = [Mg+2][OH-]2 = 7. 1 X 10 -12 Assume quantitative separation requires that the concentration of the less soluble material to have decreased to < 1 X 10 -6 M before the more soluble material begins to precipitate.
![EXAMPLE Separate Iron and Magnesium Ksp Fe3OH3 2 X 10 39 Ksp EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-47.jpg)
EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 Ksp = [Mg+2][OH-]2 = 7. 1 X 10 -12 Assume [Fe+3] = 1. 0 X 10 -6 M What will be the [OH-] required to reduce the [Fe+3] to [Fe+3] = 1. 0 X 10 -6 M ? Ksp = [Fe+3][OH-]3 = 2 X 10 -39
![EXAMPLE Separate Iron and Magnesium Ksp Fe3OH3 2 X 10 39 1 EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1.](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-48.jpg)
EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1. 0 X 10 -6 M)*[OH-]3 = 2 X 10 -39

Add OH- Mg 2+ Fe 3+ 3+ Fe 2+ Mg Mg 2+ Fe 3+ 3+ Fe 3+ Mg 2+ 2+ Fe Mg Fe 3+ Mg 2+ 2+ Mg Fe 3+

Mg 2+ Mg 2+ Mg 2+ 2+ Mg 2+ Fe 3+ @ equilibrium What is the [OH-] when ^ this happens Is this [OH-] (that is in solution) great enough to start precipitating Mg 2+? Fe(OH)3(s)
![EXAMPLE Separate Iron and Magnesium Ksp Fe3OH3 2 X 10 39 1 EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1.](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-51.jpg)
EXAMPLE: Separate Iron and Magnesium? Ksp = [Fe+3][OH-]3 = 2 X 10 -39 (1. 0 X 10 -6 M)*[OH-]3 = 2 X 10 -39
![EXAMPLE Separate Iron and Magnesium What OH is required to begin the precipitation of EXAMPLE: Separate Iron and Magnesium? What [OH-] is required to begin the precipitation of](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-52.jpg)
EXAMPLE: Separate Iron and Magnesium? What [OH-] is required to begin the precipitation of Mg(OH)2? [Mg+2] = 0. 10 M Ksp = (0. 10 M)[OH-]2 = 7. 1 X 10 -12 [OH-] = 8. 4 X 10 -6 M
![EXAMPLE Separate Iron and Magnesium equilibrium OH to completely remove Fe 3 EXAMPLE: Separate Iron and Magnesium? @ equilibrium [OH-] to ‘completely’ remove Fe 3+ ^](https://slidetodoc.com/presentation_image_h/b170bbedf36d1025ac7f07f103951a6d/image-53.jpg)
EXAMPLE: Separate Iron and Magnesium? @ equilibrium [OH-] to ‘completely’ remove Fe 3+ ^ -11 = 1. 3 X 10 M [OH-] to start removing Mg 2+ = 8. 4 X 10 -6 M “All” of the Iron will be precipitated b/f any of the magnesium starts to precipitate!!