Unit 4 Solutions PRECIPITATION COMMON ION EFFECT 4









- Slides: 11

Unit 4 Solutions PRECIPITATION COMMON ION EFFECT

4. 3. 4 Precipitation Reactions �Sometimes when two aqueous solutions are mixed together a solid is produced. This solid is called a precipitate, and the reaction is known as a precipitation reaction. �We can use the Solubility Table to predict whether a precipitate will form.

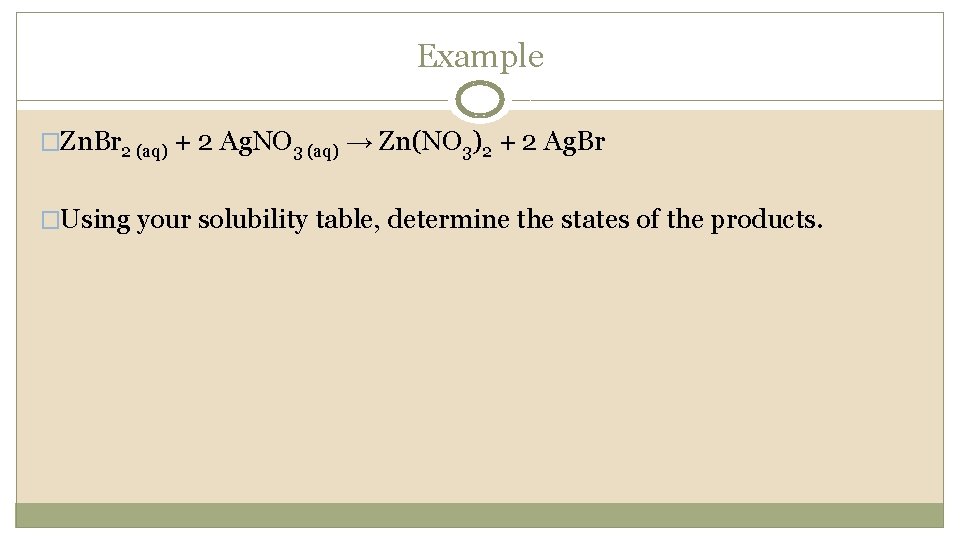
Example �Zn. Br 2 (aq) + 2 Ag. NO 3 (aq) → Zn(NO 3)2 + 2 Ag. Br �Using your solubility table, determine the states of the products.

�Let’s write our solutions out in long form, remember - anything that is (aq) is really present as separate ions. This, however, ONLY applies to aqueous states. �Thus we can rewrite our equation as: �Notice that some ions are the same on both sides of the equation, indicating they did not change during the reaction. �Ions that are present in a reaction but do not participate are called spectator ions.

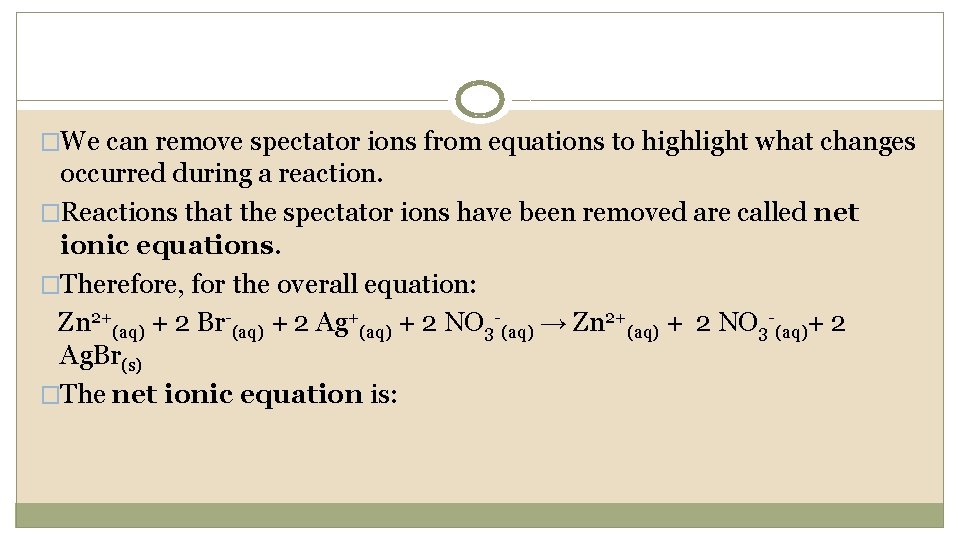
�We can remove spectator ions from equations to highlight what changes occurred during a reaction. �Reactions that the spectator ions have been removed are called net ionic equations. �Therefore, for the overall equation: Zn 2+(aq) + 2 Br-(aq) + 2 Ag+(aq) + 2 NO 3 -(aq) → Zn 2+(aq) + 2 NO 3 -(aq)+ 2 Ag. Br(s) �The net ionic equation is:

�Keep in mind that a reaction does not always take place. �Let's look at what happens when we mix solutions of Mg(CH 3 COO)2 and (NH 4)2 SO 4. �Write a balanced equation and predict if the products. Then predict the physical states of the products (reactants are aq. ) �Mg(CH 3 COO)2 (aq) + (NH 4)2 SO 4 (aq)→

Selective Precipitation �Knowledge of precipitation reactions also allows us to use selective precipitation to separate mixtures of ions. �For example. . . �We have a solution that contains the following cations: �Ag+, Cu 2+, and Mg 2+ ; each of these cations is associated with nitrate ions, NO 3 -. �Compounds containing nitrate are ALWAYS soluble. So the nitrate ions will remain spectator ions, so we will not need to consider them

�We wish to isolate each ion by causing each one to precipitate out of solution. Once a solid forms, we can filter the solid precipitate out, leaving the other ions in solution. �We are given the following solutions to use: �Na 2 S, Na. Cl, and Na. OH. �Sodium ions, Na+, always form soluble compounds. So Na+ will remain as a spectator ion. So we are given solutions of the following anions: �S 2 -, Cl-, and OH-

�We add these one at a time to our cation mixture. We need to determine the order so that we have ONE AND ONLY ONE precipitate occur each addition, removing one of the cations from the solution. Then continue until all cations are isolated. �Prepare a chart, with the cations we need to separate along one axis and anions along the other axis:


�Practice problems 4. 3. 4 �Assignment