The Quantum Mechanical Model of the Atom Matter


























- Slides: 75

The Quantum. Mechanical Model of the Atom

Matter and Energy By 1900, physicists thought that the nature of energy and matter was well understood and distinct. Matter, a collection of particles, have mass and a defined position in space. Radiant energy, as waves, is massless and delocalized. It was also believed that particles of matter could absorb or emit any energy, without restriction.

The Failure of Classical Physics Observations of the behavior of subatomic particles in the early 1900 s could not be predicted or explained using classical physics. Very small particles such as electrons appear to interact with electromagnetic radiation (light) differently than object we can see and handle.

Electromagnetic Radiation Early atomic scientists studied the interaction of matter with electromagnetic radiation. Electromagnetic radiation, or radiant energy, includes visible light, infrared, micro and radio waves, and X-rays and ultraviolet light.

Electromagnetic Radiation Electromagnetic radiation travels in waves. The waves of radiant energy have three important characteristics: 1. Wavelength - λ - (lambda) 2. Frequency – ν – (nu) 3. Speed – c – the speed of light

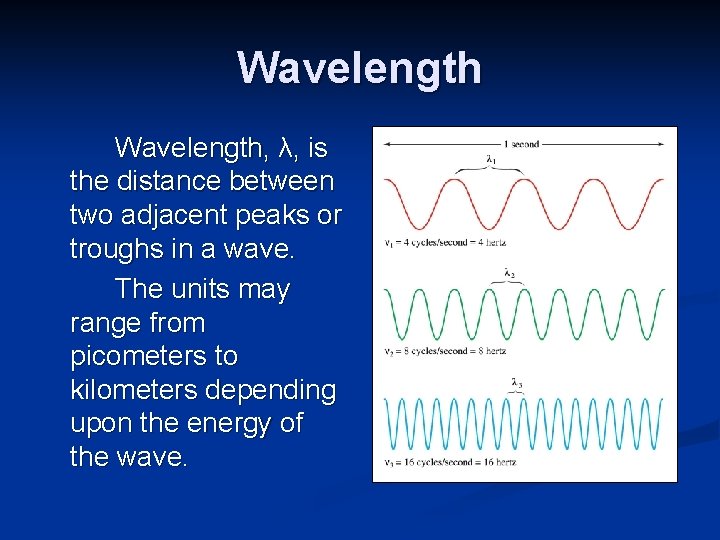
Wavelength, λ, is the distance between two adjacent peaks or troughs in a wave. The units may range from picometers to kilometers depending upon the energy of the wave.

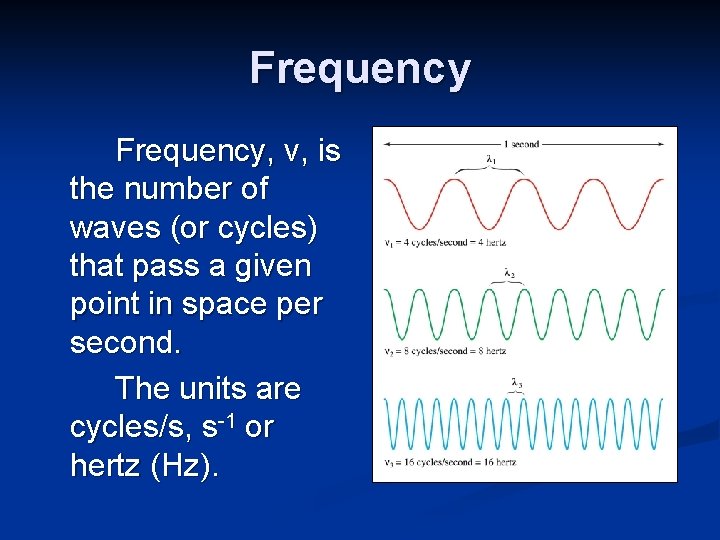
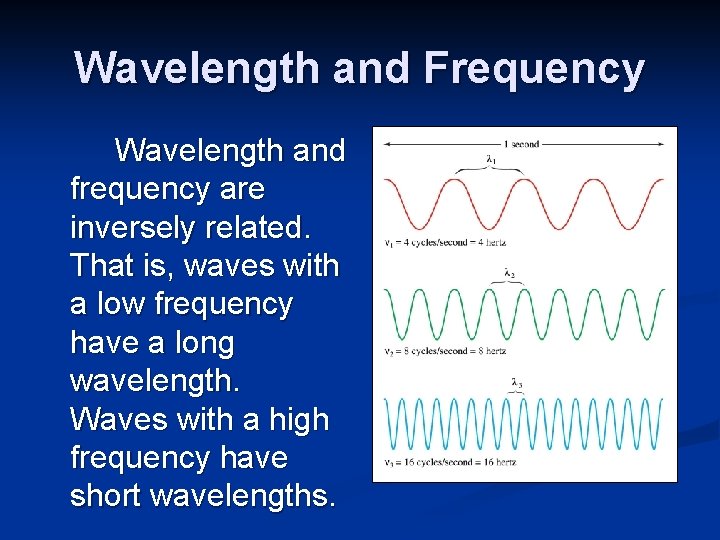
Frequency, ν, is the number of waves (or cycles) that pass a given point in space per second. The units are cycles/s, s-1 or hertz (Hz).

The Speed of Light All electromagnetic radiation travels at the same speed. The speed of light ( c ) is: c = 2. 9979 x 108 m/s

Wavelength and Frequency Wavelength and frequency are inversely related. That is, waves with a low frequency have a long wavelength. Waves with a high frequency have short wavelengths.

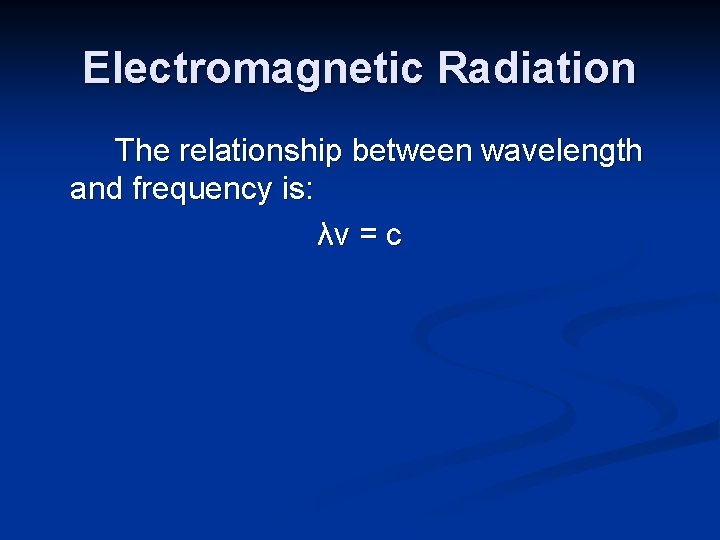
Electromagnetic Radiation The relationship between wavelength and frequency is: λν = c

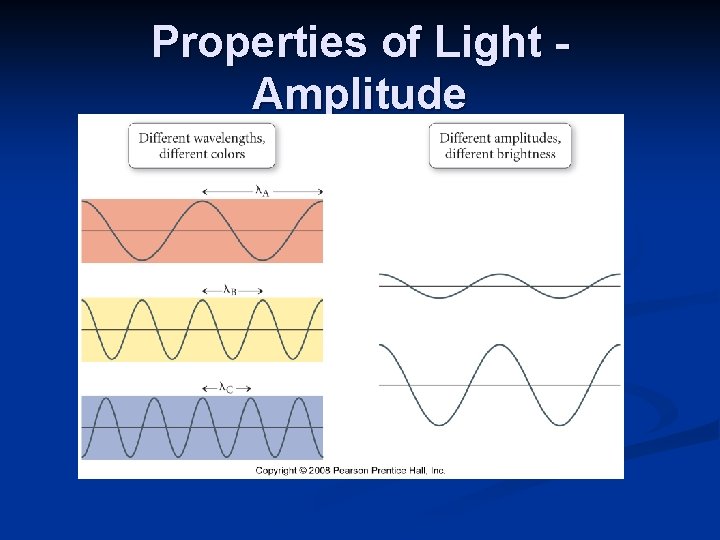
Properties of Light Amplitude

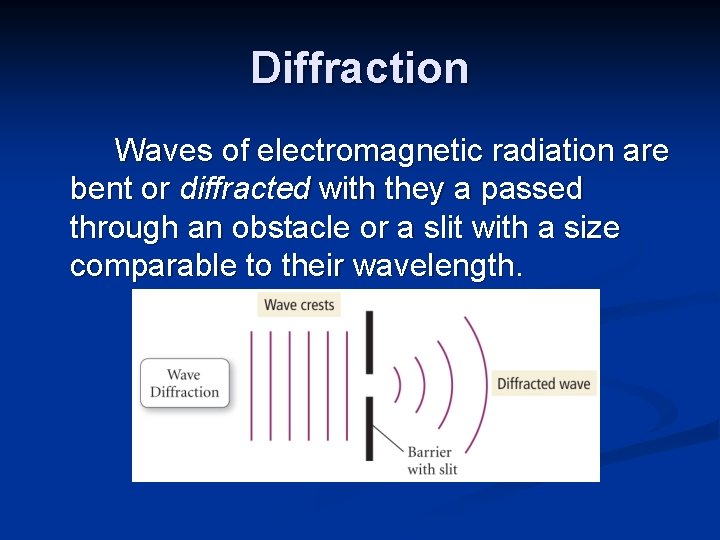
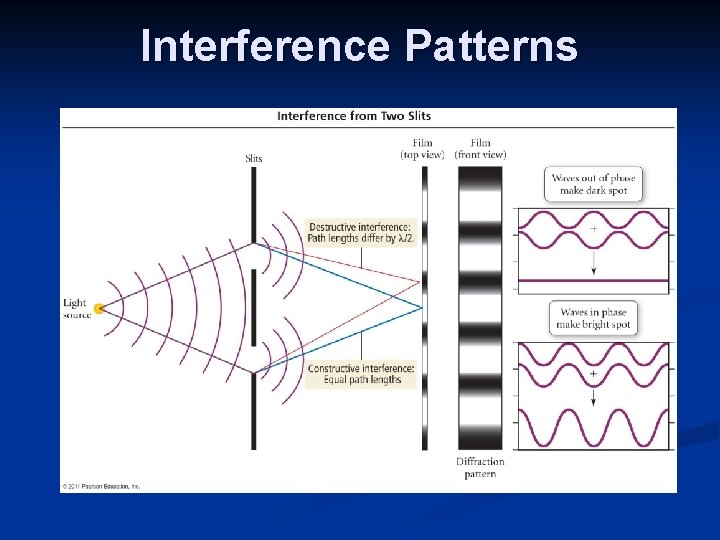
Diffraction Waves of electromagnetic radiation are bent or diffracted with they a passed through an obstacle or a slit with a size comparable to their wavelength.


Interference Patterns

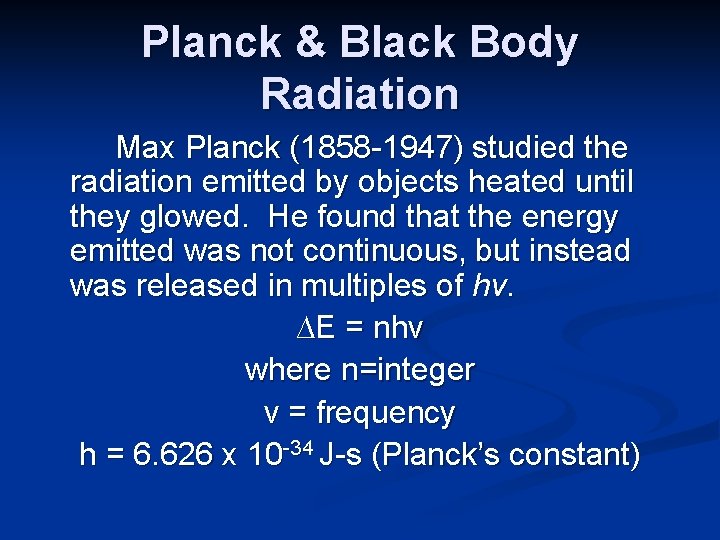
Planck & Black Body Radiation Max Planck (1858 -1947) studied the radiation emitted by objects heated until they glowed. He found that the energy emitted was not continuous, but instead was released in multiples of hν. ∆E = nhν where n=integer ν = frequency h = 6. 626 x 10 -34 J-s (Planck’s constant)

Planck & Black Body Radiation ∆E = nhν Planck’s work showed that when matter and energy interact, the energy is quantized, and can occur only in discrete units or bundles with energy of hν. Each packet or bundle of energy is called a quantum. A fraction of a quantum is never emitted.

Einstein – Photoelectric Effect Albert Einstein (1879 -1955) won a Nobel Prize for his explanation of the photoelectric effect. When light of sufficient energy strikes the surface of a metal, electrons are emitted from the metal surface. Each metal has a characteristic minimum frequency, νo , called the threshold frequency, needed for electrons to be emitted.


The Photoelectric Effect

Observations 1. No electrons are emitted if the frequency of light used is less than νo, regardless of the intensity of the light. 2. For light with a frequency≥ νo , electrons are emitted. The number of electrons increases with the intensity of the light. 3. For light with a frequency > νo , the electrons are emitted with greater kinetic energy.

Explanation Einstein proposed that light is quantized, consisting of a stream of “particles” called photons. If the photon has sufficient energy, it can “knock off” an electron from the metal surface. If the energy of the photon is greater than that needed to eject an electron, the excess energy is transferred to the electron as kinetic energy.

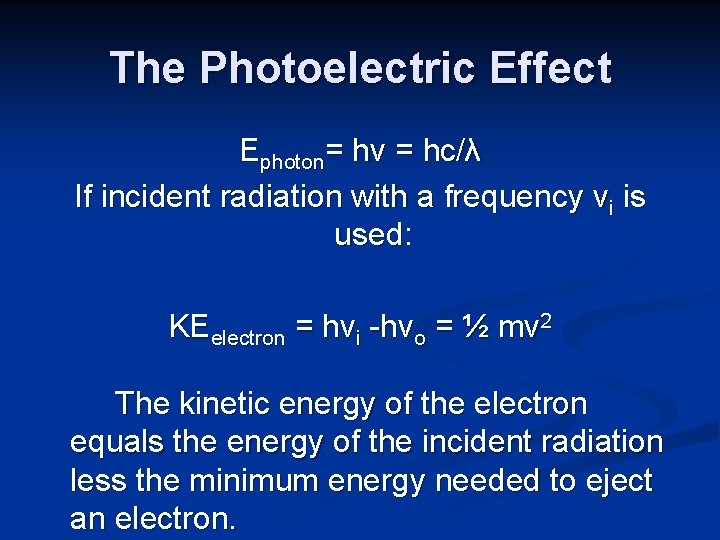
The Photoelectric Effect Ephoton= hν = hc/λ If incident radiation with a frequency νi is used: KEelectron = hνi -hνo = ½ mv 2 The kinetic energy of the electron equals the energy of the incident radiation less the minimum energy needed to eject an electron.

The Photoelectric Effect The frequency hνo is the minimum energy needed to eject an electron from a specific metal. This energy is called the binding energy of the emitted electron.

Particle-Wave Duality Einstein’s work suggested that the incident photon behaved like a particle. If it “hits” the metal surface with sufficient energy (hνi), the excess energy of the photon is transferred to the ejected electron. In the atomic scale, waves of radiant energy have particle-like properties.

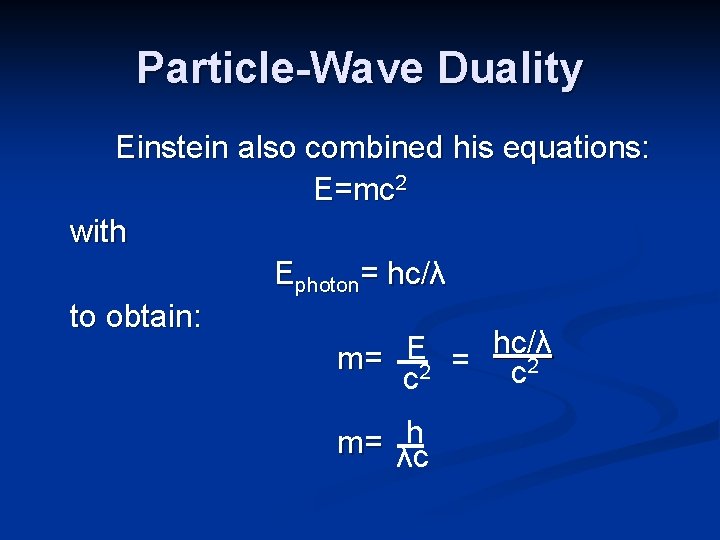
Particle-Wave Duality Einstein also combined his equations: E=mc 2 with Ephoton= hc/λ to obtain: hc/λ E m= 2 = c 2 c h m= λc

Particle-Wave Duality The apparent mass of radiant energy can be calculated. Although a wave lacks any mass at rest, at times, it behaves as if it has mass. Einstein’s equation was confirmed by experiments done by Arthur Compton in 1922. Collisions between X-rays and electrons confirmed the “mass” of the radiation.

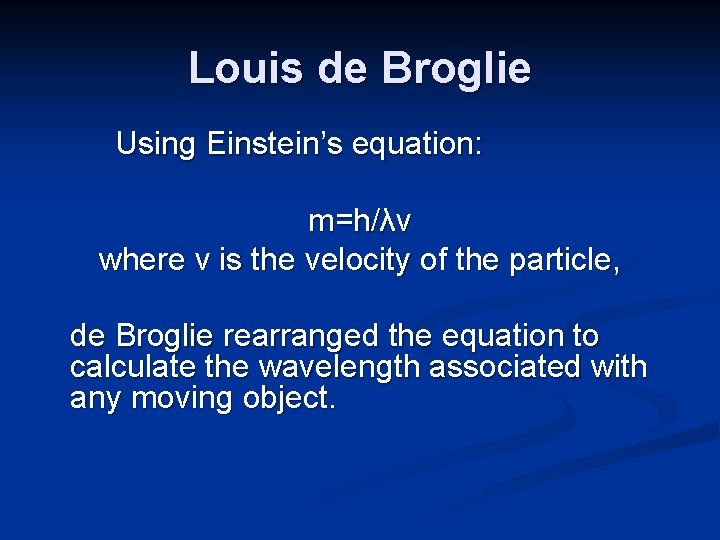
Louis de Broglie Einstein showed that waves can behave like particles. In 1923, Louis de Broglie (1892 -1987) proposed that moving electrons have wave-like properties.

Louis de Broglie Using Einstein’s equation: m=h/λv where v is the velocity of the particle, de Broglie rearranged the equation to calculate the wavelength associated with any moving object.

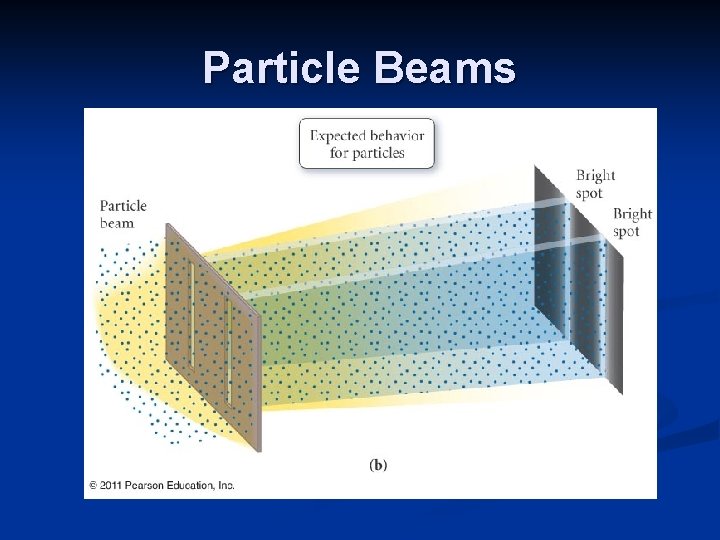
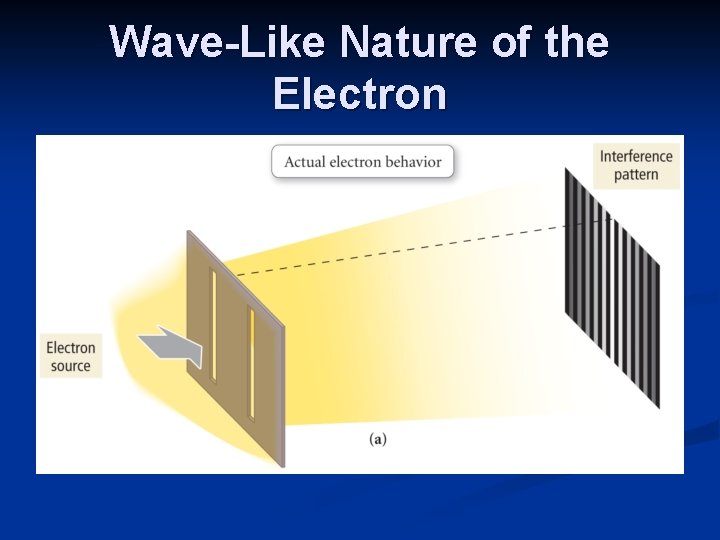
Louis de Broglie λ=h/mv de Broglie’s equation was tested using a stream of electrons directed at a crystal. A diffraction pattern, due to the interaction of waves, resulted. The experiment showed that electrons have wave-like properties.

Particle Beams

Wave-Like Nature of the Electron

Particle-Wave Duality It is important to note that the wavelike properties of moving particles are insignificant in our everyday world. A moving object such as a car or a tennis ball has an incredibly small wavelength associated with it. It is on the atomic scale that the dual nature of particles and light become significant.

Emission Spectrum of Hydrogen When atoms are given extra energy, or excited, they give off the excess energy as light as they return to their original energy, or ground state. Hg H 2 H e

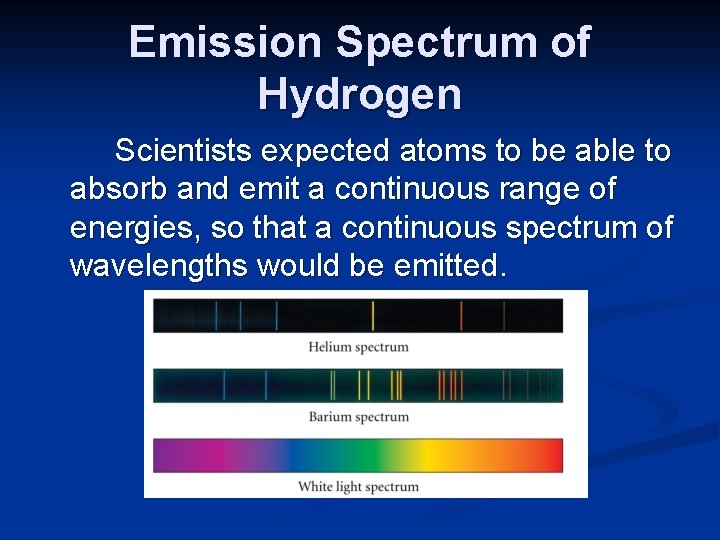
Emission Spectrum of Hydrogen Scientists expected atoms to be able to absorb and emit a continuous range of energies, so that a continuous spectrum of wavelengths would be emitted.

Emission Spectrum of Hydrogen A continuous spectrum in the visible range, would look like a rainbow, with all colors visible. Instead, hydrogen, and other excited atoms emit only specific wavelengths of light as they return to the ground state. A line spectrum results.

Emission Spectrum of Hydrogen

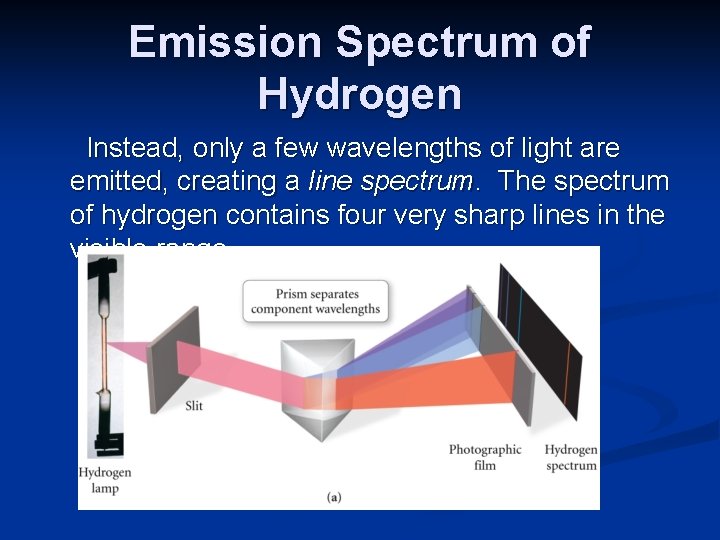
Emission Spectrum of Hydrogen Instead, only a few wavelengths of light are emitted, creating a line spectrum. The spectrum of hydrogen contains four very sharp lines in the visible range.

Emission Spectrum of Hydrogen The discrete lines in the spectrum indicate that the energy of the atom is quantized. Only specific energies exist in the excited atom, so only specific wavelengths of radiation are emitted.

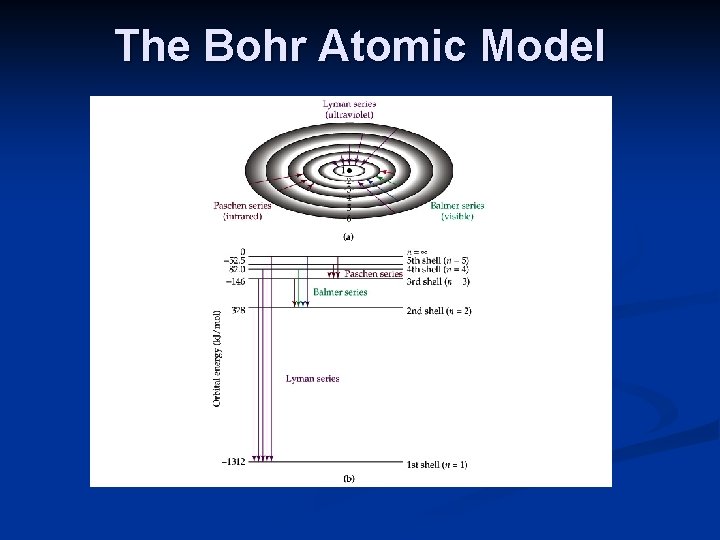
The Bohr Atomic Model In 1913, Niels Bohr (1885 -1962) proposed that the electron of hydrogen circles the nucleus in allowed orbits. That is, the electron is in its ground state in an orbit closest to the nucleus. As the atom becomes excited, the electron is promoted to an orbit further away from the nucleus.

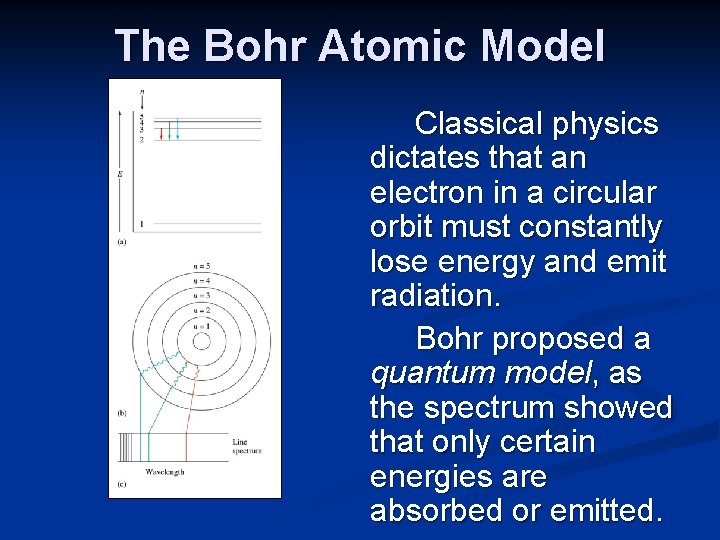
The Bohr Atomic Model Classical physics dictates that an electron in a circular orbit must constantly lose energy and emit radiation. Bohr proposed a quantum model, as the spectrum showed that only certain energies are absorbed or emitted.

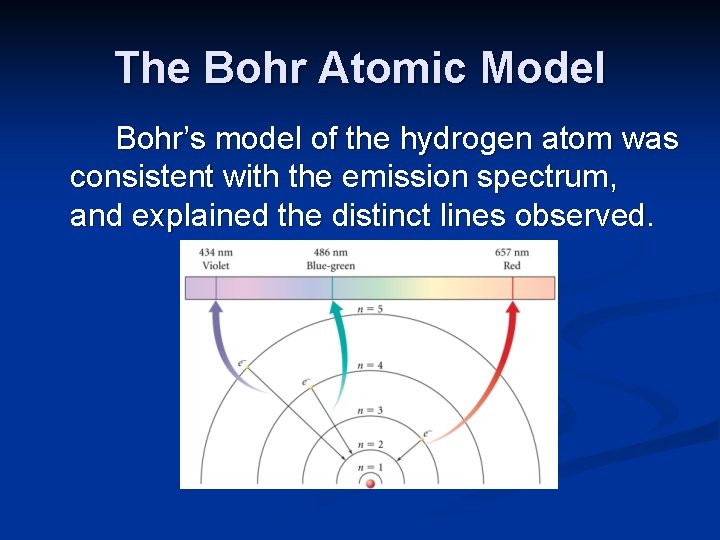
The Bohr Atomic Model Bohr’s model of the hydrogen atom was consistent with the emission spectrum, and explained the distinct lines observed.

The Bohr Atomic Model

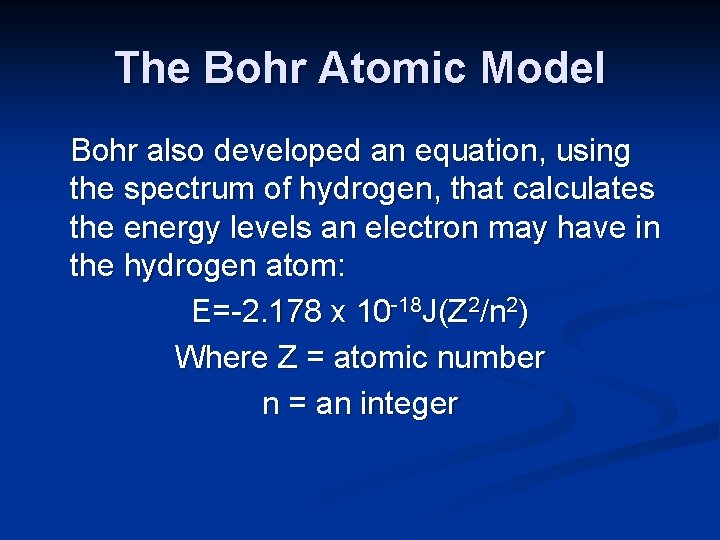
The Bohr Atomic Model Bohr also developed an equation, using the spectrum of hydrogen, that calculates the energy levels an electron may have in the hydrogen atom: E=-2. 178 x 10 -18 J(Z 2/n 2) Where Z = atomic number n = an integer

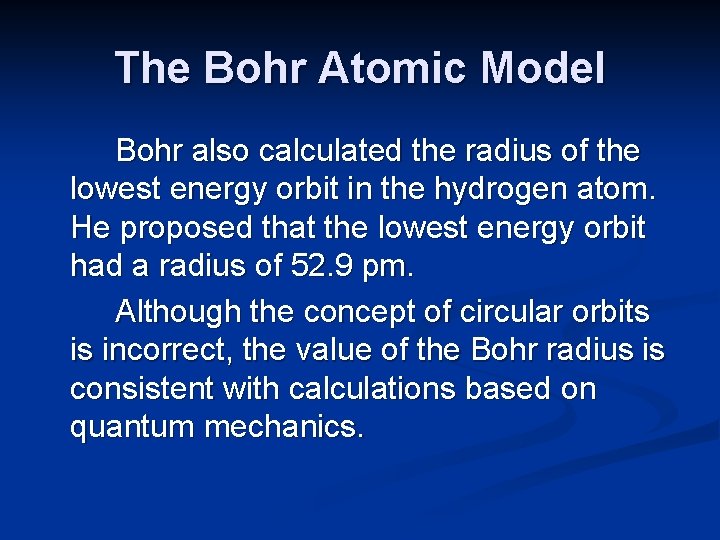
The Bohr Atomic Model Bohr also calculated the radius of the lowest energy orbit in the hydrogen atom. He proposed that the lowest energy orbit had a radius of 52. 9 pm. Although the concept of circular orbits is incorrect, the value of the Bohr radius is consistent with calculations based on quantum mechanics.

The Bohr Atomic Model The Bohr model didn’t work for atoms other than hydrogen. Though limited, Bohr’s approach did attempt to explain the quantized energy levels of electrons. Later developments showed that any attempt to define the path of the electron is incorrect.

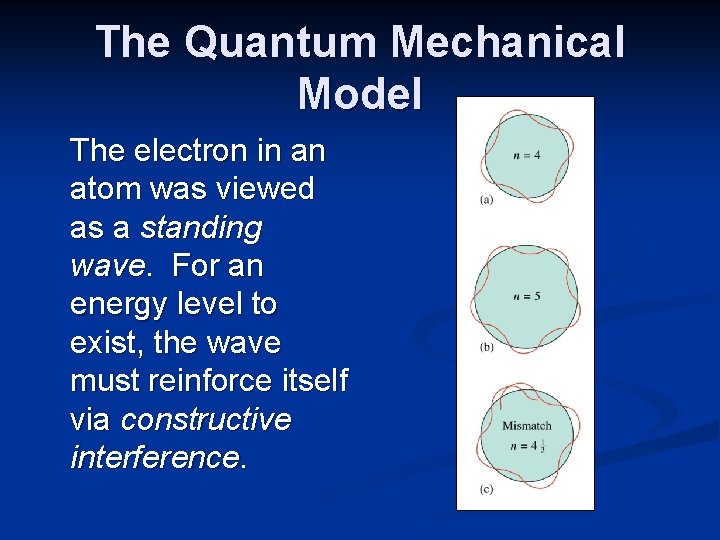
The Quantum Mechanical Model The quantum mechanical atomic model was developed based on theories of Werner Heisenberg (1901 -1976), Louis de Broglie (1892 -1987) and Erwin Schrödinger (1887 -1961). They focused on the wave-like nature of the moving electron.

The Quantum Mechanical Model The electron in an atom was viewed as a standing wave. For an energy level to exist, the wave must reinforce itself via constructive interference.

The Quantum Mechanical Model Schrödinger developed complex equations called wave functions ( Ψ). The wave functions can be used to calculate the energy of electrons, not only in hydrogen, but in other atoms.

The Quantum Mechanical Model The wave functions also describe various volumes or spaces where electrons of a specific energy are likely to be found. These spaces are called orbitals.

The Quantum Mechanical Model Orbitals are not orbits. The wave functions provide no information about the path of the electron. Instead, it provides the space in which there is a high probability (90%) of finding an electron with a specific energy.

The Heisenberg Uncertainty Principle Werner Heisenberg showed that, due to the wave nature of the electron, It is impossible to know both the precise position and the momentum of the electron at the same time. This is known as the Heisenberg Uncertainty Principle.

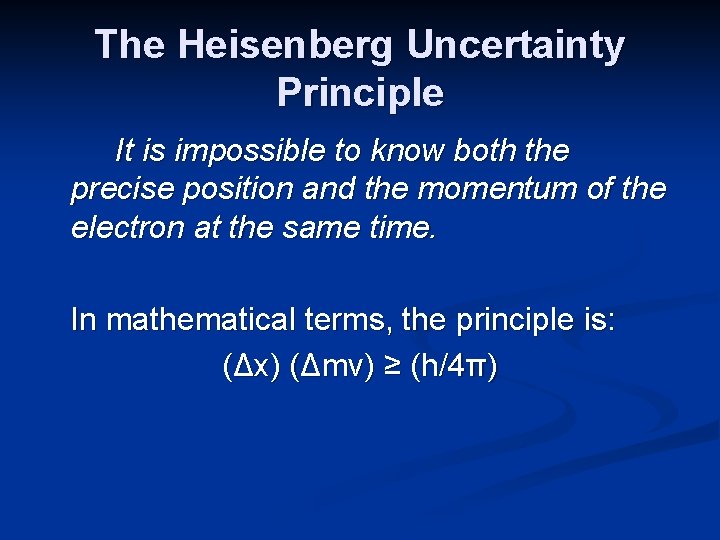
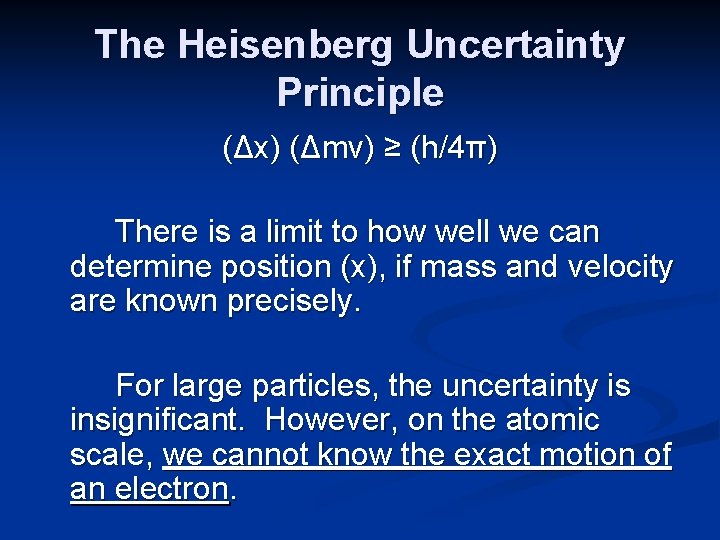
The Heisenberg Uncertainty Principle It is impossible to know both the precise position and the momentum of the electron at the same time. In mathematical terms, the principle is: (Δx) (Δmv) ≥ (h/4π)

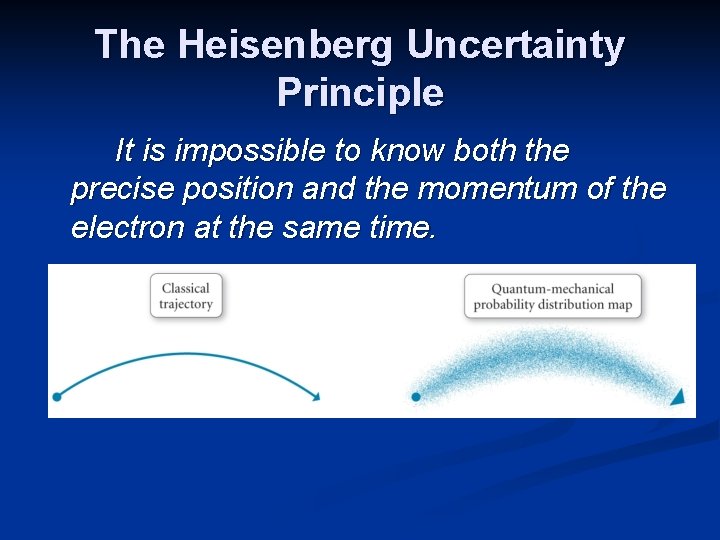
The Heisenberg Uncertainty Principle It is impossible to know both the precise position and the momentum of the electron at the same time.

The Heisenberg Uncertainty Principle (Δx) (Δmv) ≥ (h/4π) There is a limit to how well we can determine position (x), if mass and velocity are known precisely. For large particles, the uncertainty is insignificant. However, on the atomic scale, we cannot know the exact motion of an electron.

The Heisenberg Uncertainty Principle (Δx) (Δmv) ≥ (h/4π) For an electron in a hydrogen atom, the uncertainty in the position of the electron is similar in size to the entire hydrogen atom. Thus the location of the electron cannot be determined.

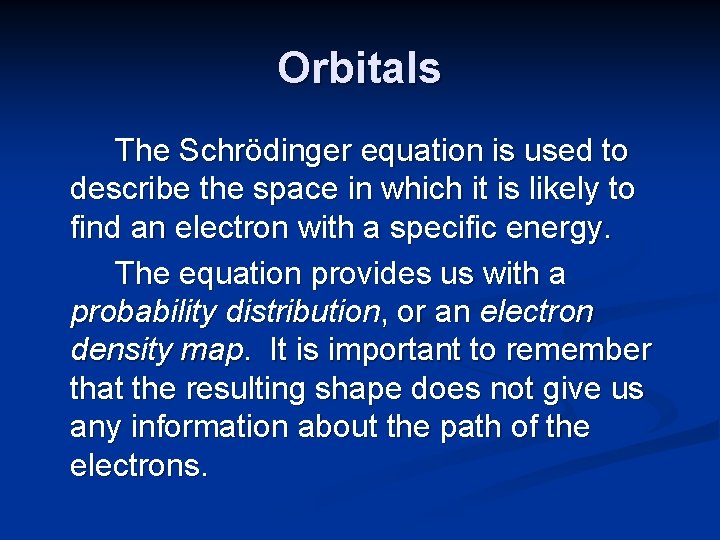
Orbitals The Schrödinger equation is used to describe the space in which it is likely to find an electron with a specific energy. The equation provides us with a probability distribution, or an electron density map. It is important to remember that the resulting shape does not give us any information about the path of the electrons.

Orbitals Each orbital described by the Schrodinger equations is associated with three interrelated quantum numbers which relate to the energy of electrons in the orbital and the probability of finding the electron within a particular volume.

Quantum Numbers The principal quantum number, n, determines the overall size and energy of an orbital. It is an integer with values of 1, 2, 3, etc. The angular momentum quantum number, l, determines the shape of the orbital. It is related to the more familiar designations of s, p, d and f.

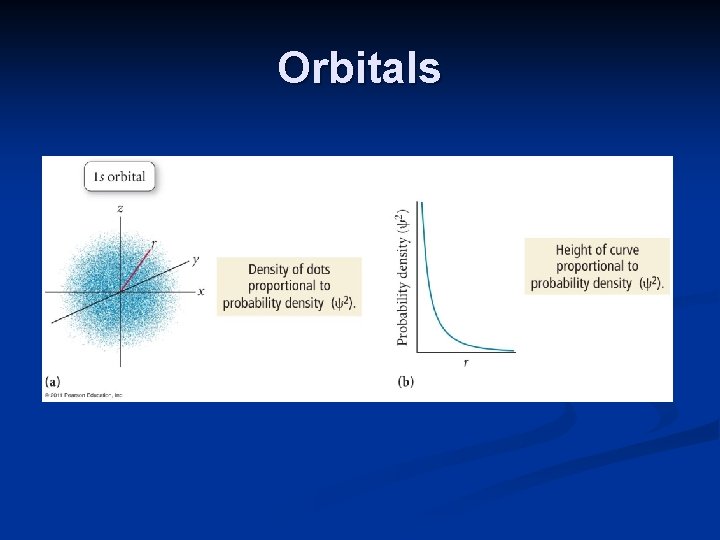
Orbitals The orbital of lowest energy is the 1 s orbital. The probability density, or probability of finding an electron per unit volume, shows electron density in all directions, creating a spherical shape. The probability density decreases with greater distance from the nucleus.

Orbitals

Orbitals

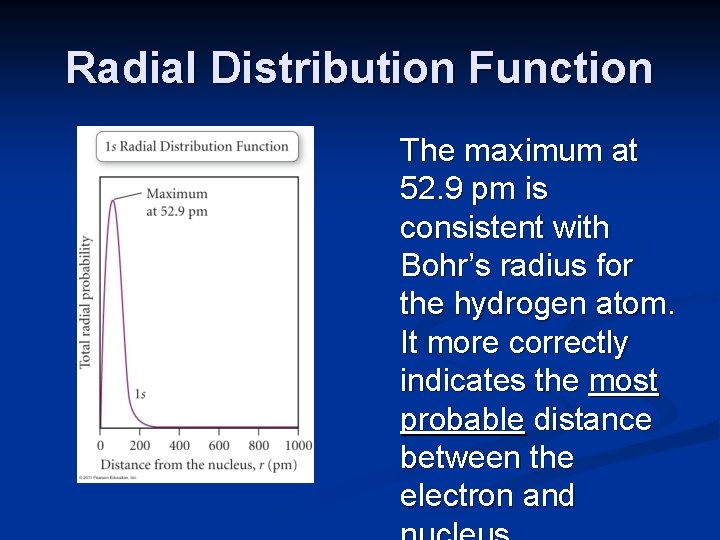
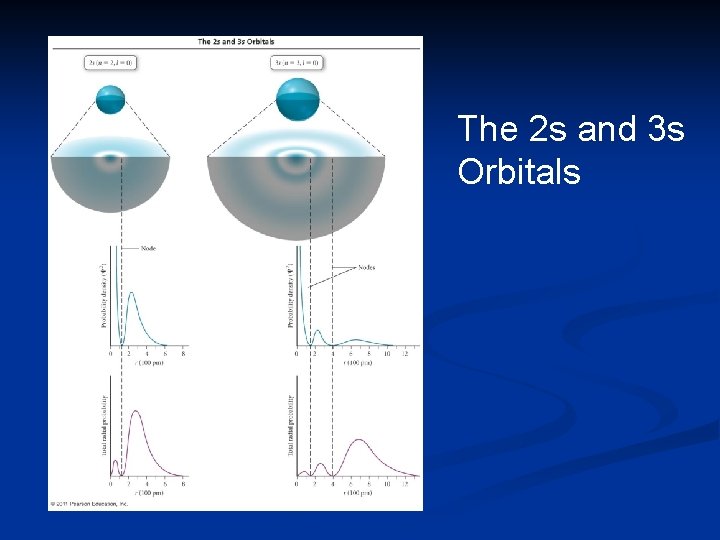
Radial Distribution Function The radial distribution function is a graphical representation of the probability of finding an electron in a thin spherical shell a specific distance from the nucleus. It shows that there is zero probability that the electron will be at the nucleus, and also indicates the most probable distance the electron will have from the nucleus.

Radial Distribution Function The maximum at 52. 9 pm is consistent with Bohr’s radius for the hydrogen atom. It more correctly indicates the most probable distance between the electron and

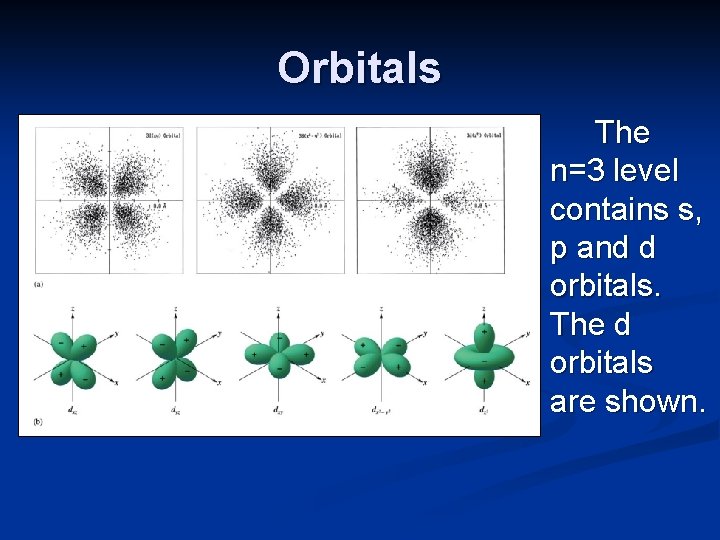
Orbitals The first energy level of hydrogen (n=1) consists of a 1 s orbital. The second energy level of hydrogen (n=2) consists of a 2 s orbital and 2 p orbitals. The third energy level of hydrogen (n=3) consists of a 3 s orbital, 3 p orbitals, and 3 d orbitals.

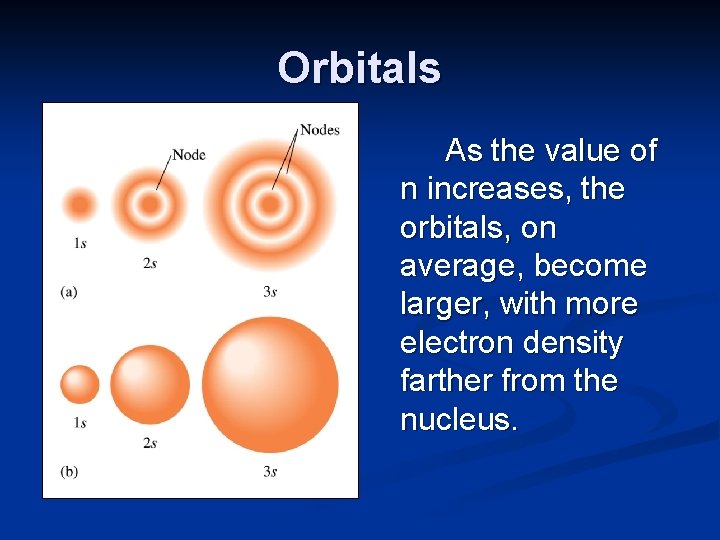
Orbitals As the value of n increases, the orbitals, on average, become larger, with more electron density farther from the nucleus.

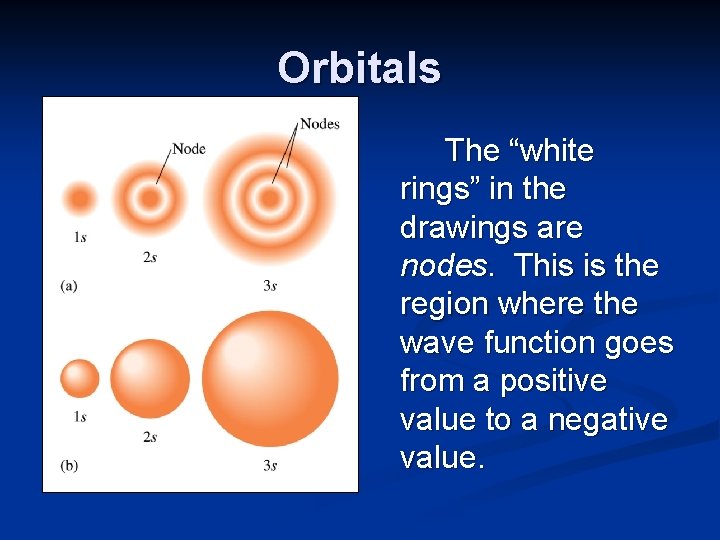
Orbitals The “white rings” in the drawings are nodes. This is the region where the wave function goes from a positive value to a negative value.

The 2 s and 3 s Orbitals

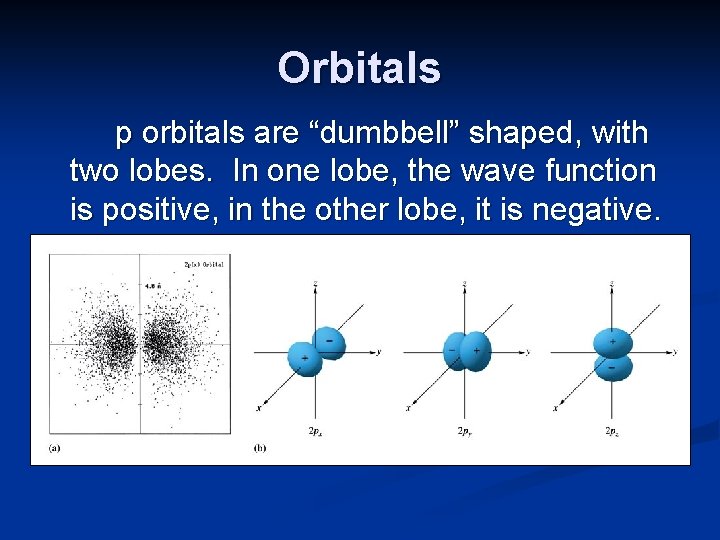
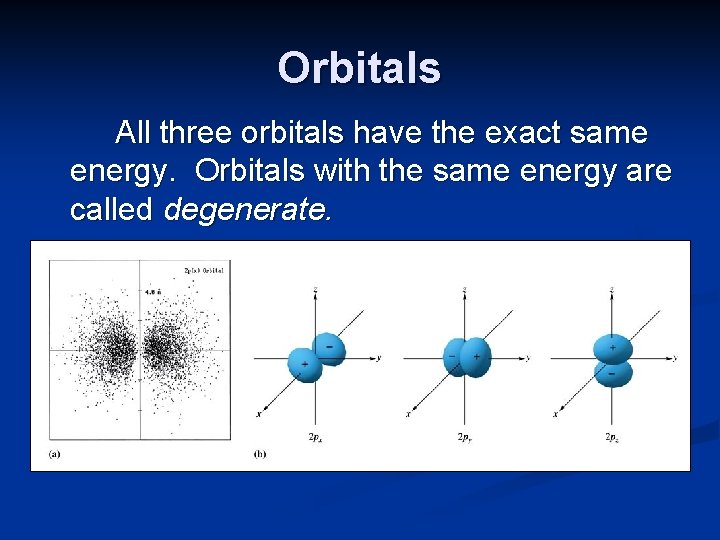
Orbitals p orbitals are “dumbbell” shaped, with two lobes. In one lobe, the wave function is positive, in the other lobe, it is negative.

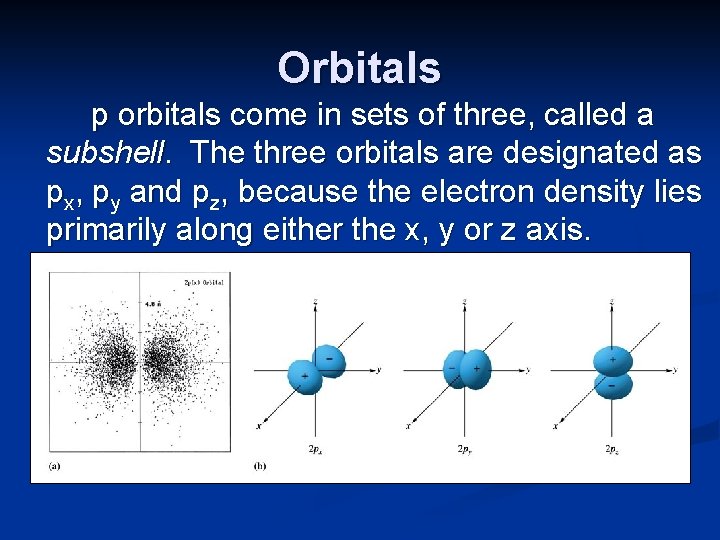
Orbitals p orbitals come in sets of three, called a subshell. The three orbitals are designated as px, py and pz, because the electron density lies primarily along either the x, y or z axis.

Orbitals All three orbitals have the exact same energy. Orbitals with the same energy are called degenerate.

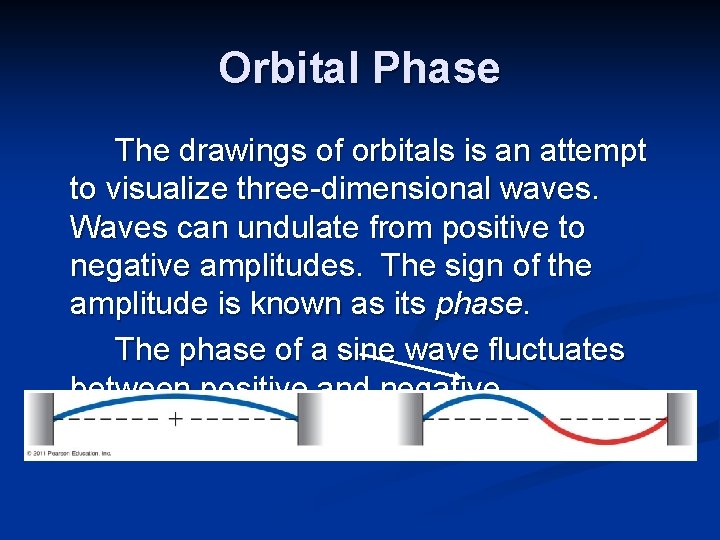
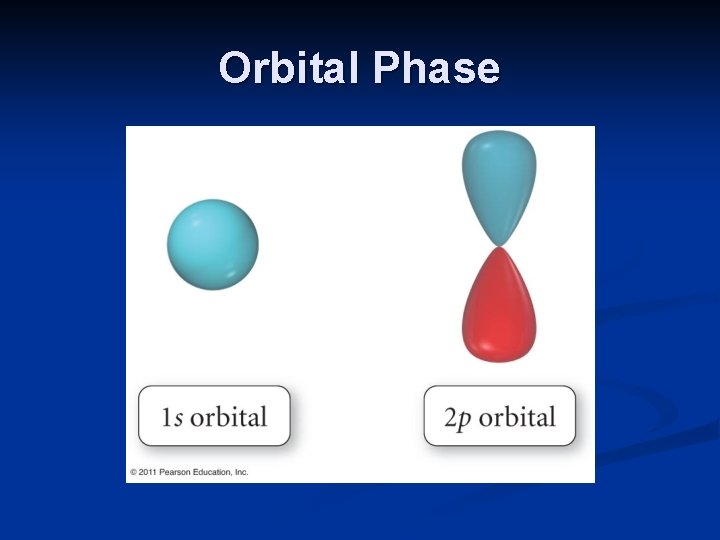
Orbital Phase The drawings of orbitals is an attempt to visualize three-dimensional waves. Waves can undulate from positive to negative amplitudes. The sign of the amplitude is known as its phase. The phase of a sine wave fluctuates between positive and negative.

Orbital Phase

Orbital Phase The phase of the wave functions or orbitals is quite important when atoms bond together. The orbitals must be of the same phase to overlap and form covalent bonds.

Orbitals The n=3 level contains s, p and d orbitals. The d orbitals are shown.

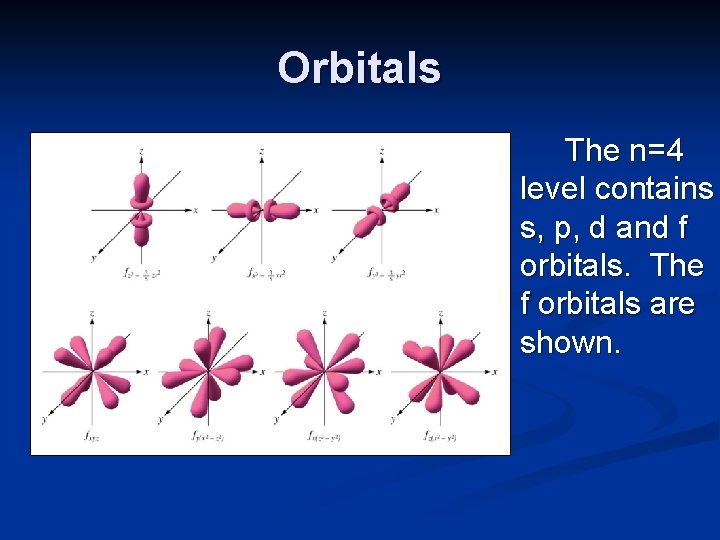
Orbitals The n=4 level contains s, p, d and f orbitals. The f orbitals are shown.