Quantum Model of the Atom The Atom is




















- Slides: 24

Quantum Model of the Atom

The Atom is like a Hotel, not a solar system Copy the diagram on the board if you have not done so.

Electrons do not circle a nucleus willy-nilly. They have assigned positions that only extra energy can cause them to jump into another position ( ex: photoelectric effect )

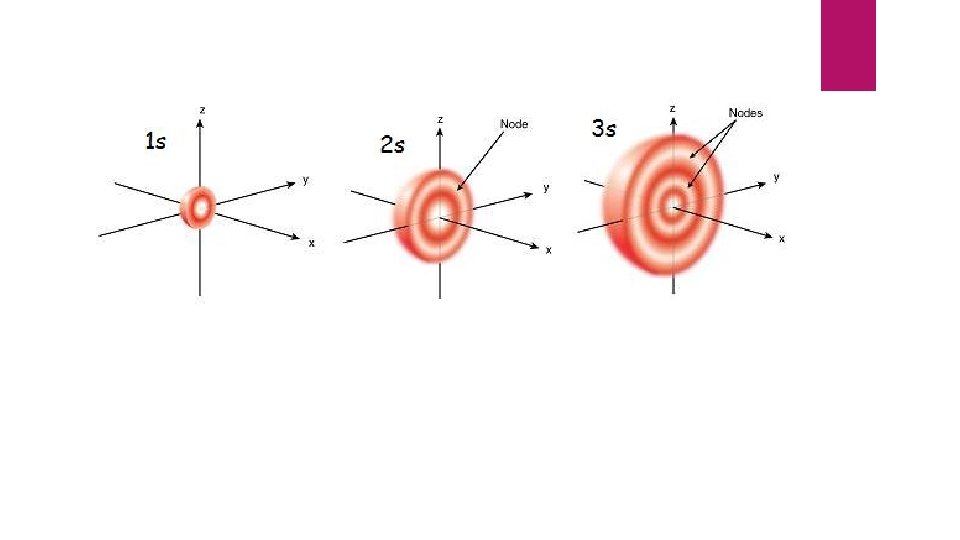
Unfortunately, we can not identify their EXACT position only the probable area we might find them. These are called orbitals.

Heisenberg Uncertainty Principle We can not know both the position and the momentum of a particle (electron )

Quantum Numbers These are “codes” for describing the area or “room” in which a certain electron might be located

Principal Quantum number “n” Describes the size and energy of the orbital , aka “hotel floor” Can be found on the periodic table is equal to 1 -∞

Practice: Looking at a periodic table… What is the principal quantum number for Titanium?

Practice: Looking at a periodic table… What is the principal quantum number for Titanium? n = 4

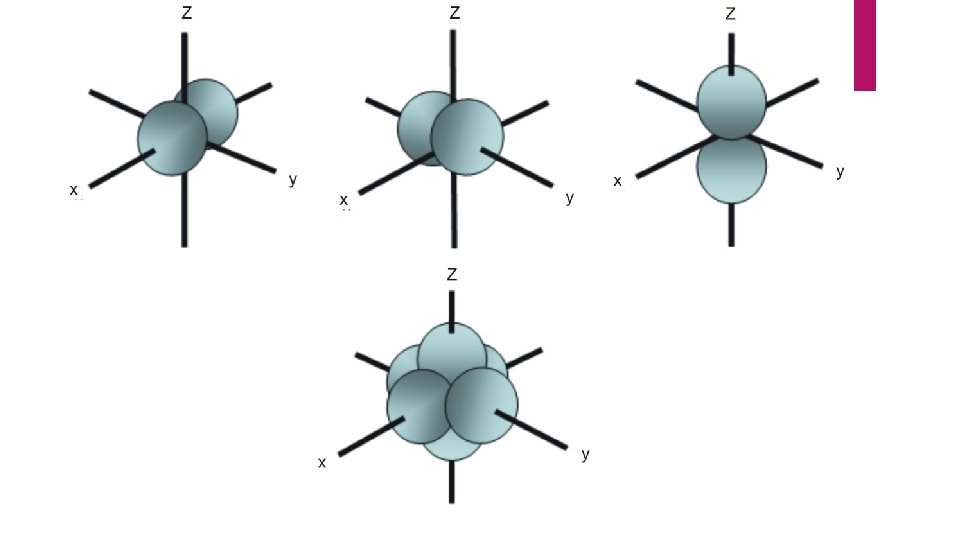
Angular momentum quantum number “ℓ “ Describes the shape of the space the electron is in. i. e. what kind of hotel room it is in. is equal to n -1 …… 0

Angular momentum quantum number “ℓ “ Describes the shape of the space the electron is in. i. e. what kind of hotel room it is in. is equal to n -1 …… 0 l = 3, 2, 1, 0

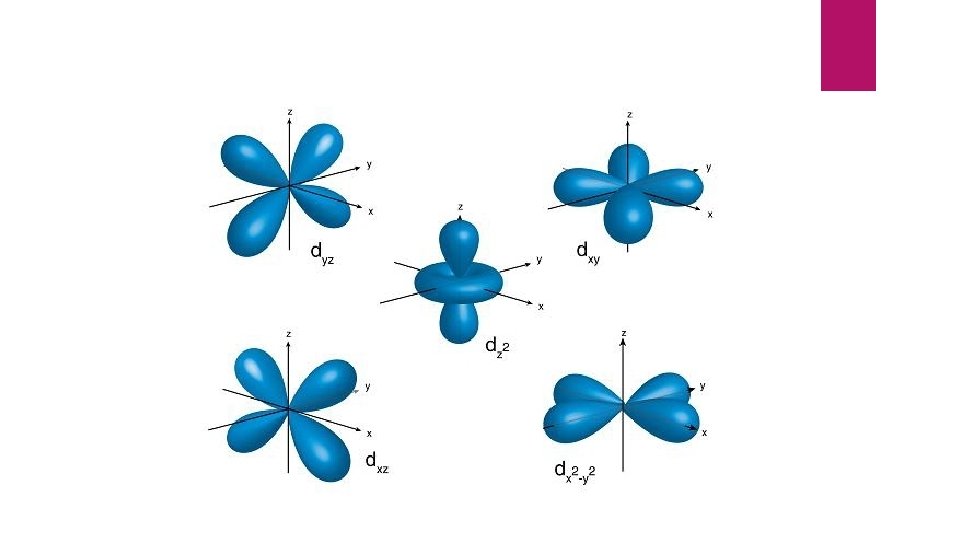
Let’s look at Titanium: n = 4 ℓ = 3 , 2, 1, 0 Each number is code for a different type of room the electron may be in.

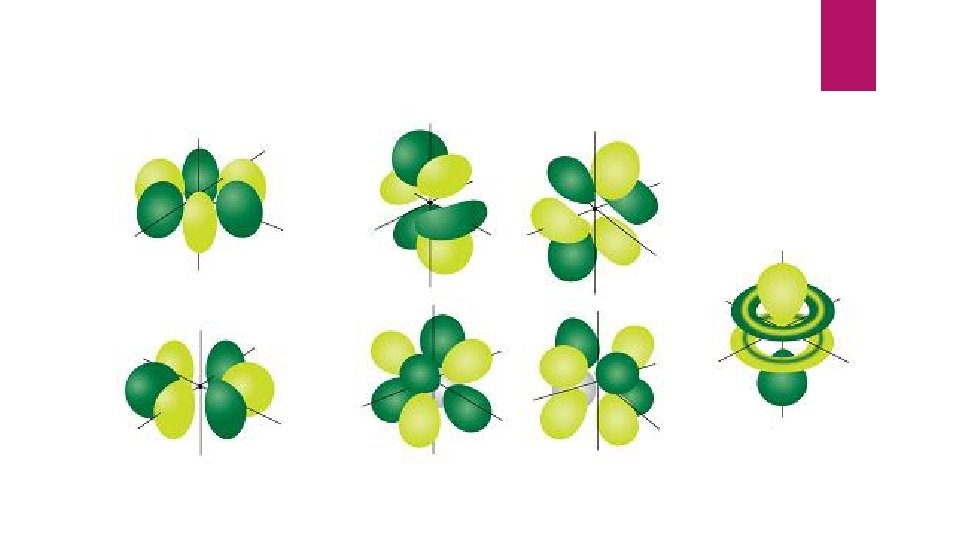
continued 3 = “f” orbital, a shape that I can’t describe, ( suite ) On the next slide I will show you pictures of these orbitals.





“ So Titanium is a 4 story hotel with electrons in all four types of orbitals Now let’s see why there are 3 ps, 5 ds and 7 fs ”

Magnetic Quantum number, mℓ Describes the position of the orbital in 3 -d space. It’s spatial orientation. i. e. in the center, by vending machines, at the end of the hall, etc

= +_ ℓ so titanium has +_3, +_2, +_ 1, 0 How many numbers is this? Take the largest orbital ( s, p, d or f) and that’s how many of them you have.

7 numbers , right? Let’s look at those f orbitals again…

Spin Quantum Number, ms Describes the spin of the electron, clockwise or counter = +1/2 or -1/2 ALWAYS

Practice writing the quantum numbers for: 1. Pd 2. U

Practice writing the quantum numbers for: 1. Pd n = 5, l = 4 3 2 1 0 ml = +- (4, 3, 2, 1), 0 Ms = +- ½ 2. U ?