Unit 3 The Quantum Model The Quantum Mechanical




- Slides: 35

Unit 3 The Quantum Model

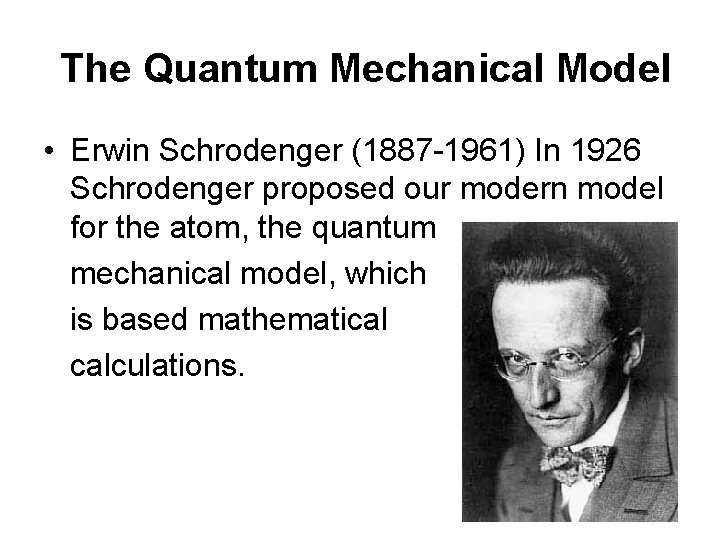
The Quantum Mechanical Model • Erwin Schrodenger (1887 -1961) In 1926 Schrodenger proposed our modern model for the atom, the quantum mechanical model, which is based mathematical calculations.

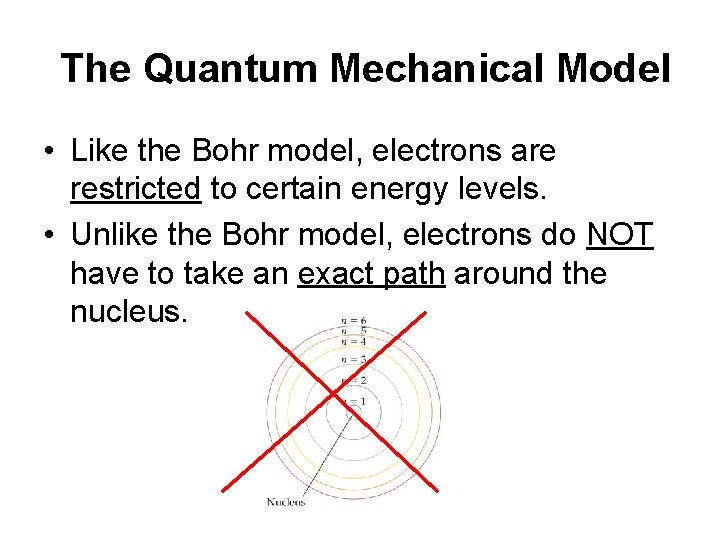
The Quantum Mechanical Model • Like the Bohr model, electrons are restricted to certain energy levels. • Unlike the Bohr model, electrons do NOT have to take an exact path around the nucleus.

The Quantum Mechanical Model The importance of the quantum mechanical model is that it determines: 1. allowed energies an electron can have 2. how likely it is to find the electron in a particular location

The Quantum Mechanical Model -We cannot know both the location and velocity of an electron (Heisenberg’s uncertainty principle), thus Schrodinger’s equation does not tell us the exact location of the electron, rather it describes the probability that an electron will be at a certain location in the atom.

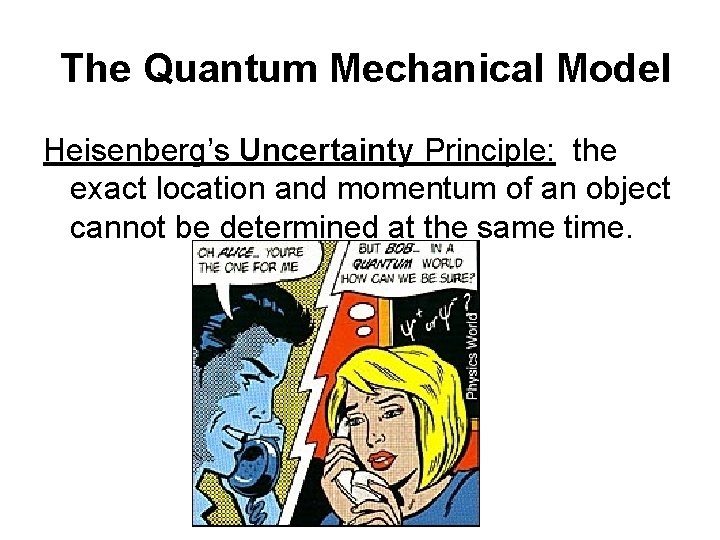
The Quantum Mechanical Model Heisenberg’s Uncertainty Principle: the exact location and momentum of an object cannot be determined at the same time.

• Because it behaves as a particle, a baseball follows a well-defined path as it travels from the pitcher to the catcher. Because of their wave nature, an electron's path cannot be precisely known. The best we can do is to calculate the probability of the electron following a specific path.

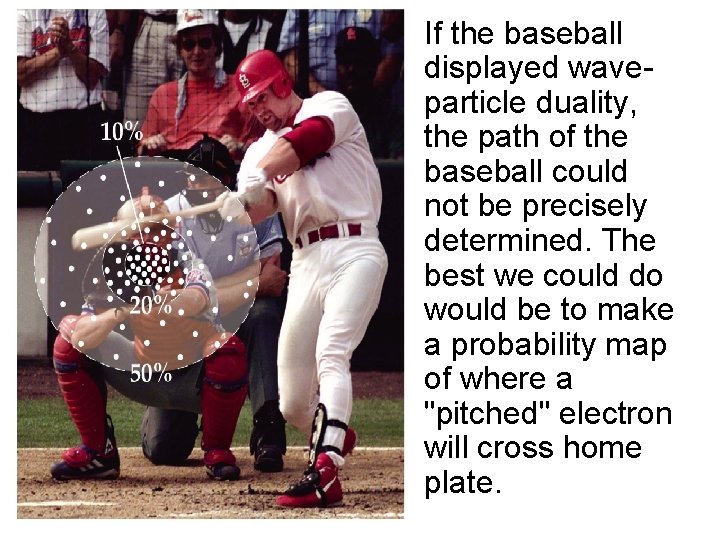
• If the baseball displayed waveparticle duality, the path of the baseball could not be precisely determined. The best we could do would be to make a probability map of where a "pitched" electron will cross home plate.

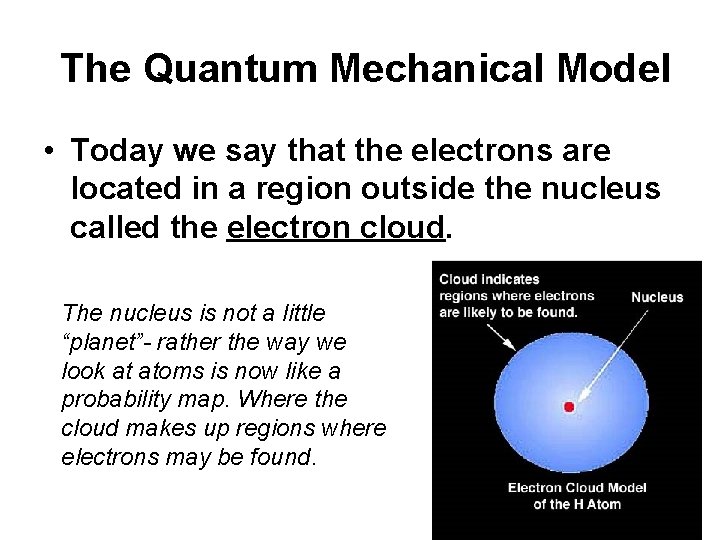
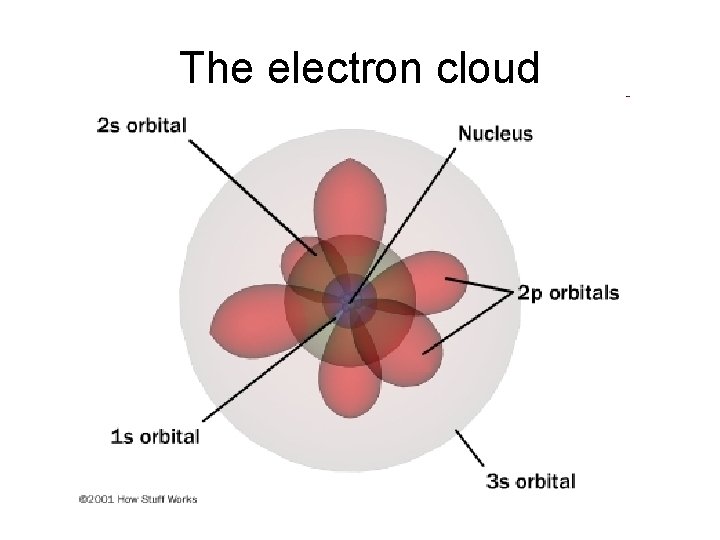
The Quantum Mechanical Model • Today we say that the electrons are located in a region outside the nucleus called the electron cloud. The nucleus is not a little “planet”- rather the way we look at atoms is now like a probability map. Where the cloud makes up regions where electrons may be found.

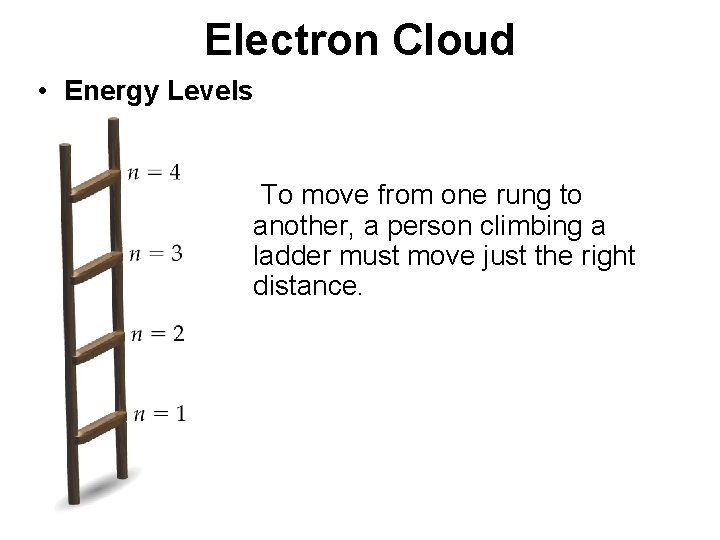
Electron Cloud • Energy Levels To move from one rung to another, a person climbing a ladder must move just the right distance.

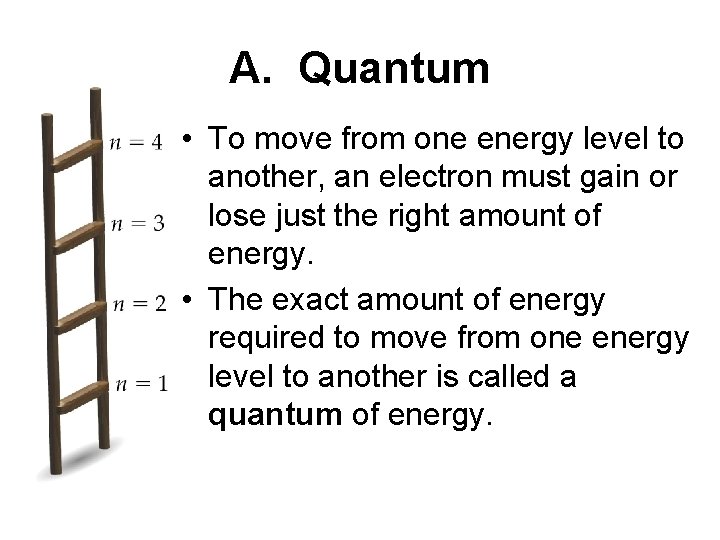
A. Quantum • To move from one energy level to another, an electron must gain or lose just the right amount of energy. • The exact amount of energy required to move from one energy level to another is called a quantum of energy.

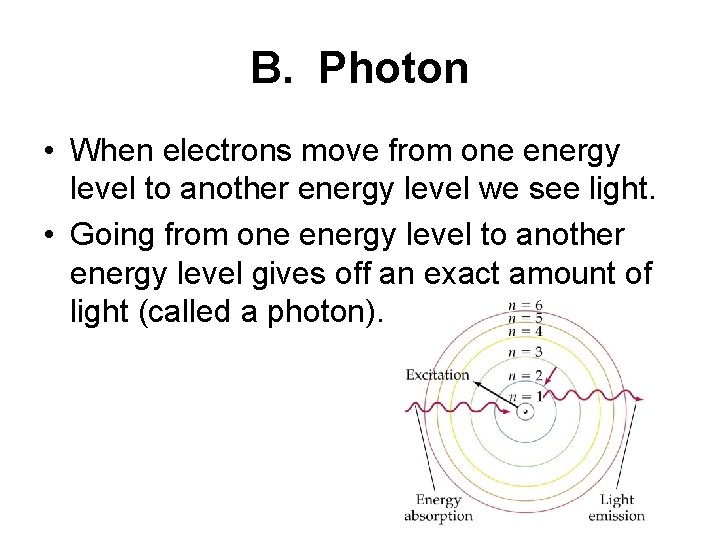
B. Photon • When electrons move from one energy level to another energy level we see light. • Going from one energy level to another energy level gives off an exact amount of light (called a photon).

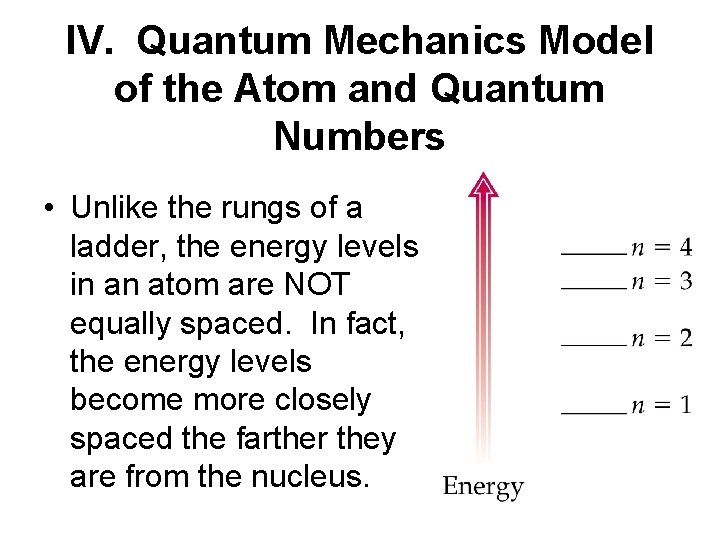
IV. Quantum Mechanics Model of the Atom and Quantum Numbers • Unlike the rungs of a ladder, the energy levels in an atom are NOT equally spaced. In fact, the energy levels become more closely spaced the farther they are from the nucleus.

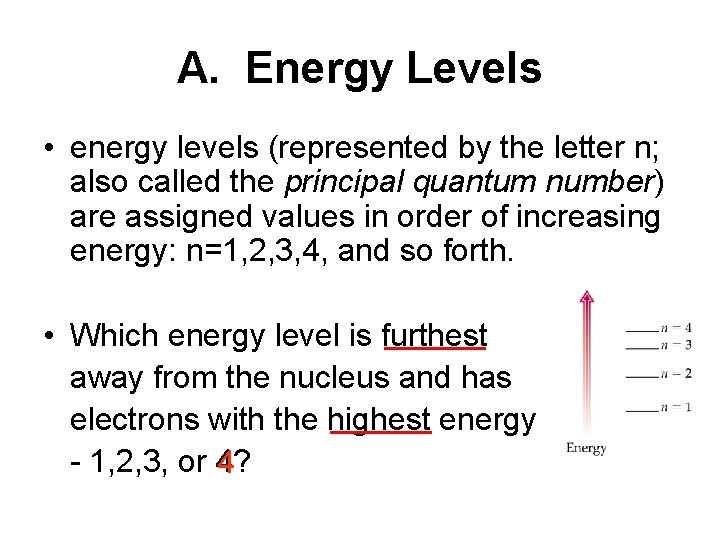
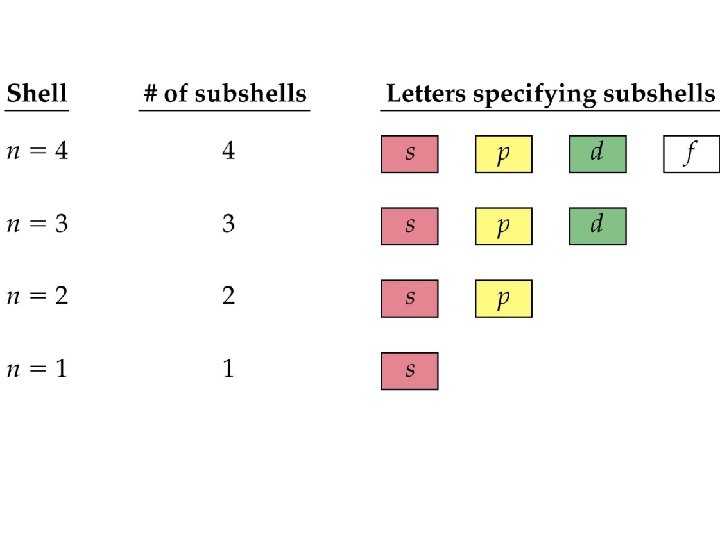
A. Energy Levels • energy levels (represented by the letter n; also called the principal quantum number) are assigned values in order of increasing energy: n=1, 2, 3, 4, and so forth. • Which energy level is furthest away from the nucleus and has electrons with the highest energy - 1, 2, 3, or 4? 4

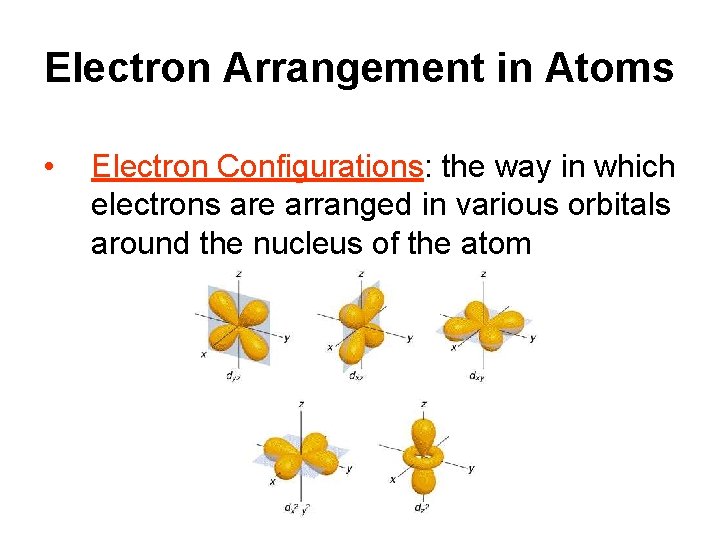
B. Sublevels • Within each energy level, the electrons are located in various sublevels – there are 4 different sublevels s, p, d, and f. • s, p, d, and f describe the shapes of the orbitals. ) Remember we’re talking about what the probability map looks like!


C. Orbitals Where are the electrons in the various sublevels located in relation to the nucleus? • Electrons are NOT confined to a fixed circular path, they are, however, found in definite regions of the atoms – these regions are called atomic orbitals! • Each orbital can only hold 2 electrons at a time

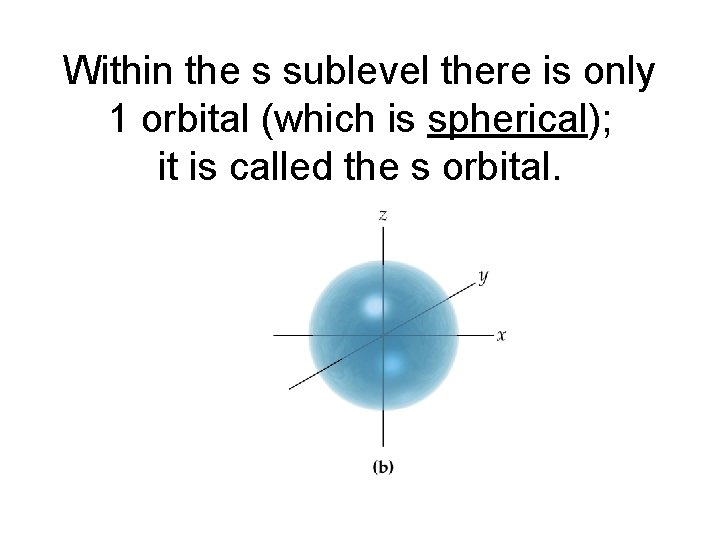
Within the s sublevel there is only 1 orbital (which is spherical); it is called the s orbital.

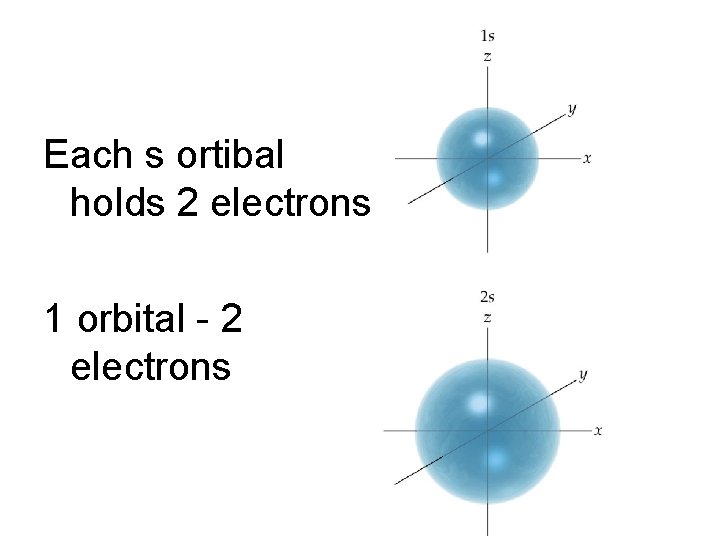
Each s ortibal holds 2 electrons 1 orbital - 2 electrons

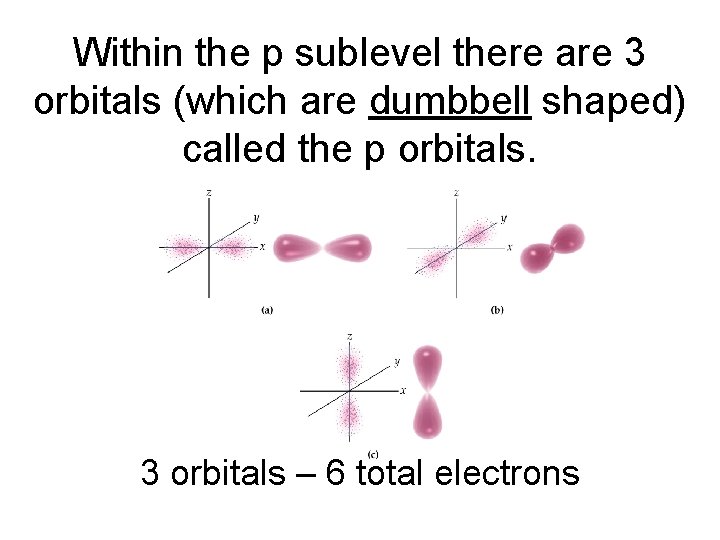
Within the p sublevel there are 3 orbitals (which are dumbbell shaped) called the p orbitals. 3 orbitals – 6 total electrons

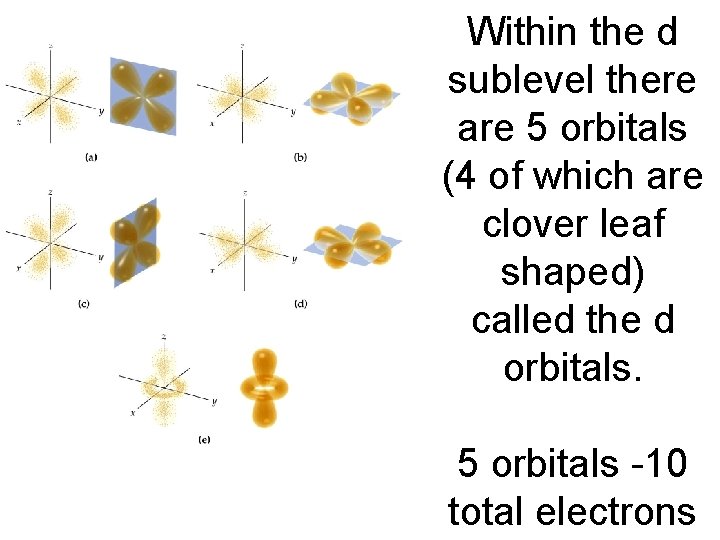
Within the d sublevel there are 5 orbitals (4 of which are clover leaf shaped) called the d orbitals. 5 orbitals -10 total electrons

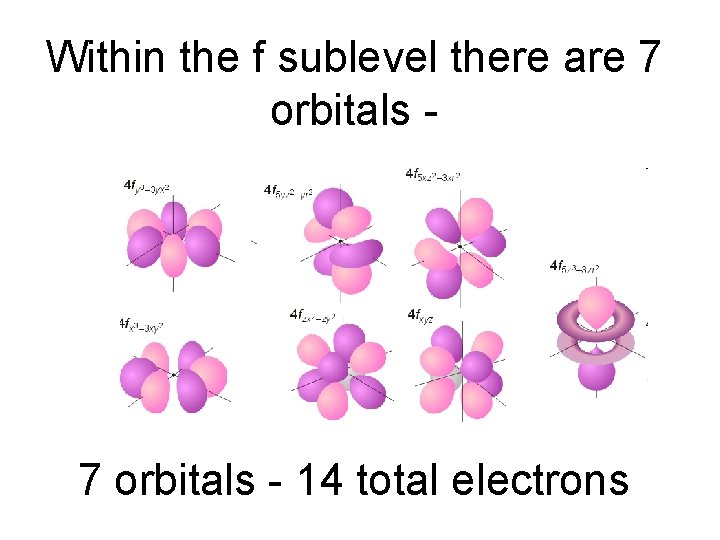
Within the f sublevel there are 7 orbitals - 14 total electrons

The electron cloud

Electron Arrangement in Atoms • Electron Configurations: the way in which electrons are arranged in various orbitals around the nucleus of the atom

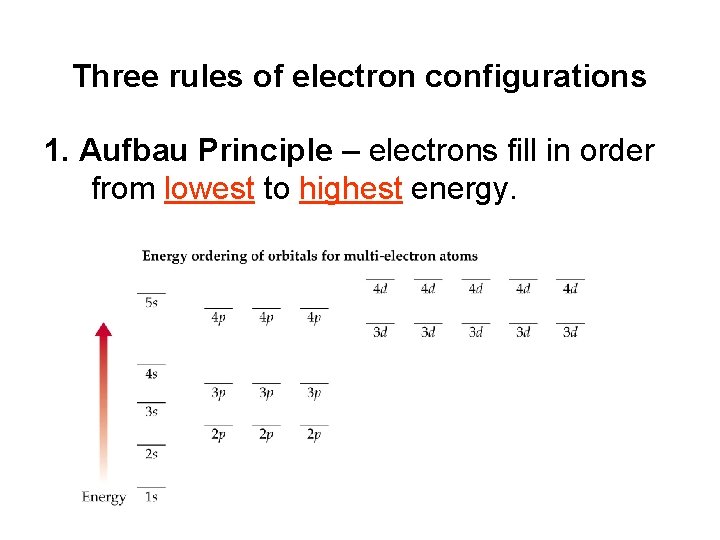
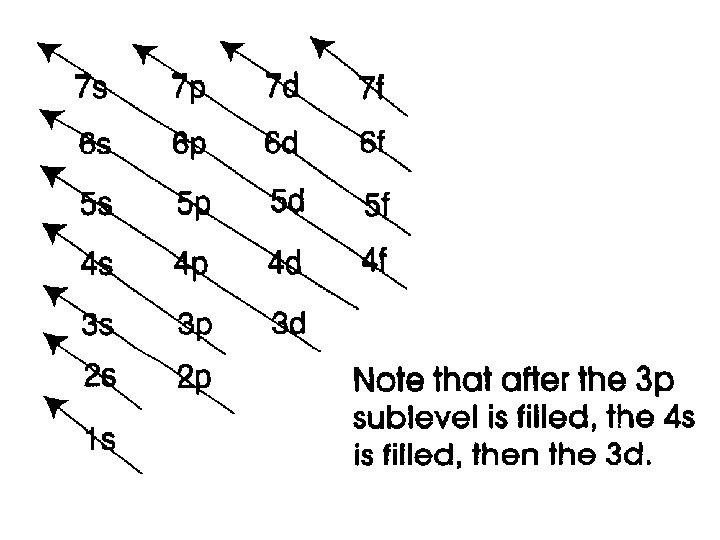
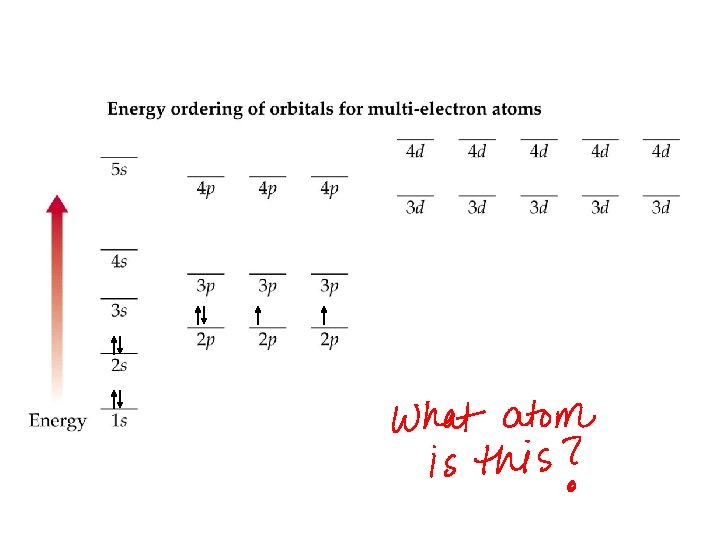
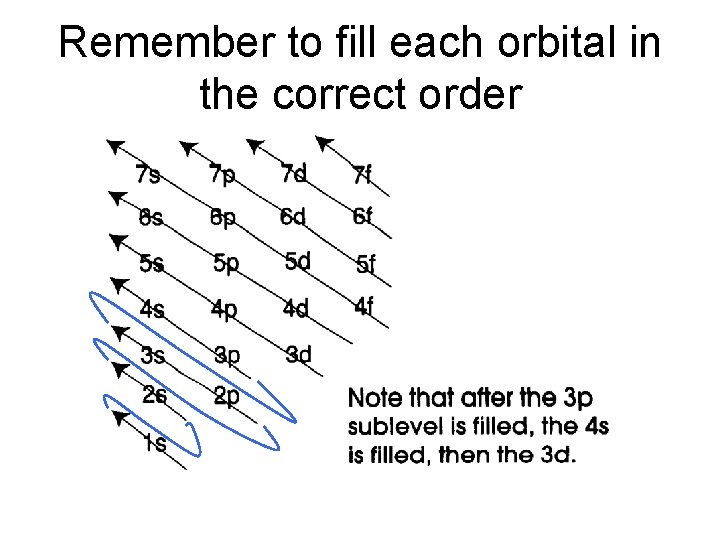
Three rules of electron configurations 1. Aufbau Principle – electrons fill in order from lowest to highest energy.


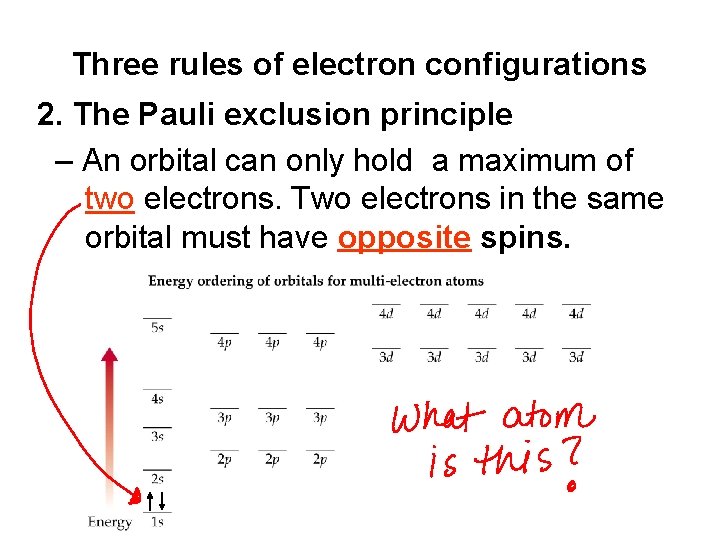
Three rules of electron configurations 2. The Pauli exclusion principle – An orbital can only hold a maximum of two electrons. Two electrons in the same orbital must have opposite spins.


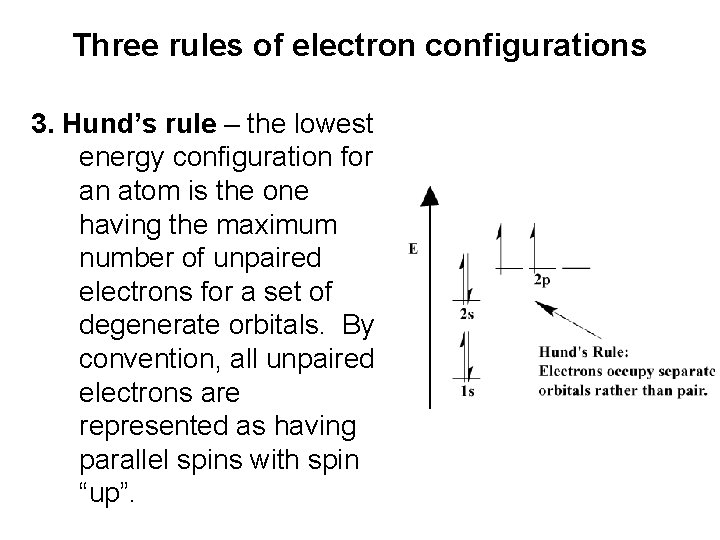
Three rules of electron configurations 3. Hund’s rule – the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons for a set of degenerate orbitals. By convention, all unpaired electrons are represented as having parallel spins with spin “up”.

Writing Electron Configurations (3 ways)

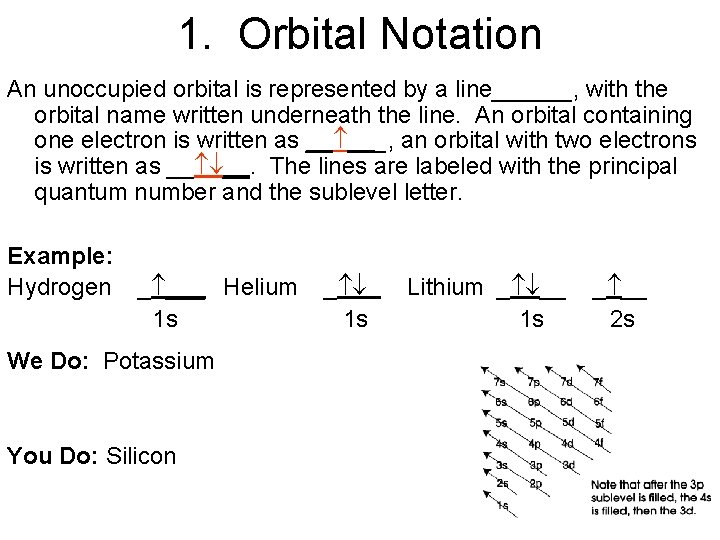
1. Orbital Notation An unoccupied orbital is represented by a line______, with the orbital name written underneath the line. An orbital containing one electron is written as __ ___, an orbital with two electrons is written as __ __. The lines are labeled with the principal quantum number and the sublevel letter. Example: Hydrogen _ ___ Helium 1 s We Do: Potassium You Do: Silicon _ 1 s Lithium _ __ 1 s _ __ 2 s

Remember to fill each orbital in the correct order

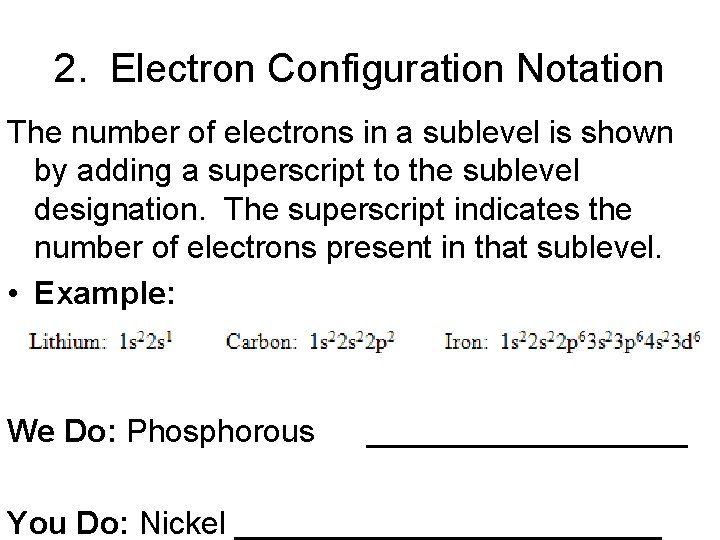
2. Electron Configuration Notation The number of electrons in a sublevel is shown by adding a superscript to the sublevel designation. The superscript indicates the number of electrons present in that sublevel. • Example: We Do: Phosphorous _________ You Do: Nickel ____________

3. Noble Gas Notation (Shorthand Notation) In order to write less, a noble gas can stand for that element’s number of electrons Example: Sodium [Ne] 3 s 1 The [Ne] stands for the first 10 electrons because neon has 10 electrons 10 + 1 = 11 electrons We Do: Phosphorous You Do: Nickel

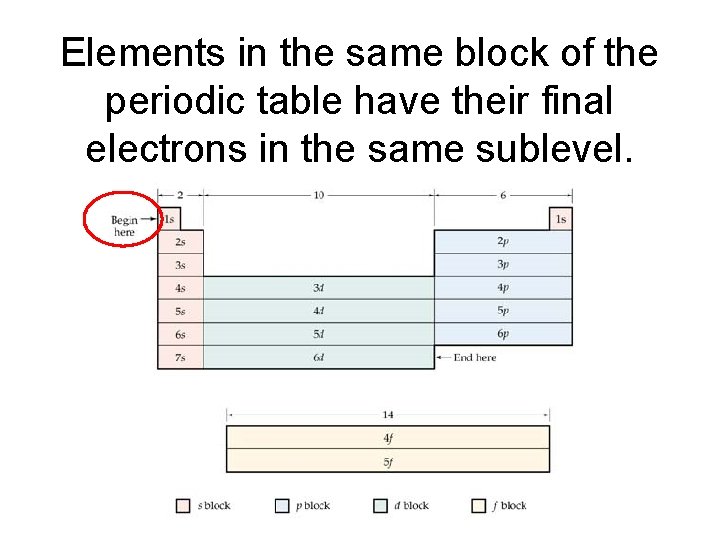
Elements in the same block of the periodic table have their final electrons in the same sublevel.