THE QUANTUM MECHANICAL MODEL OF THE ATOM WAS









- Slides: 24

THE QUANTUM MECHANICAL MODEL OF THE ATOM WAS DEVELOPED THROUGH THE STUDY OF LIGHT. ISAAC NEWTON(1642 -1727)-LIGHT CONSISTS OF PARTICLES 1900 -LIGHT TRAVELS IN WAVES

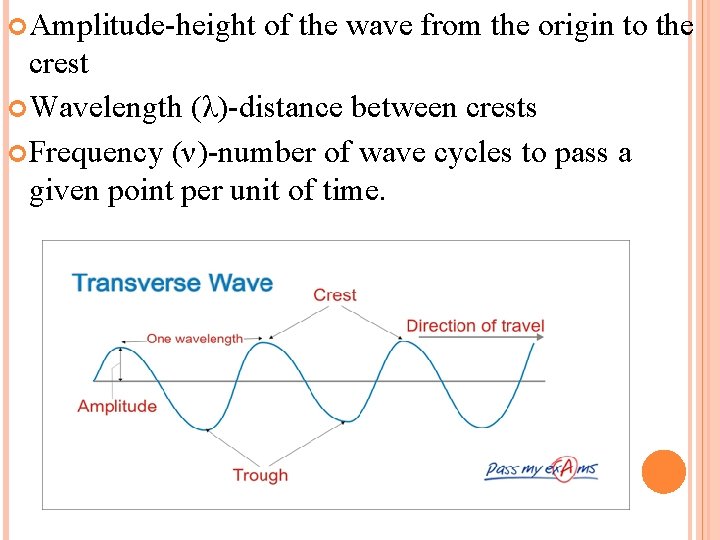
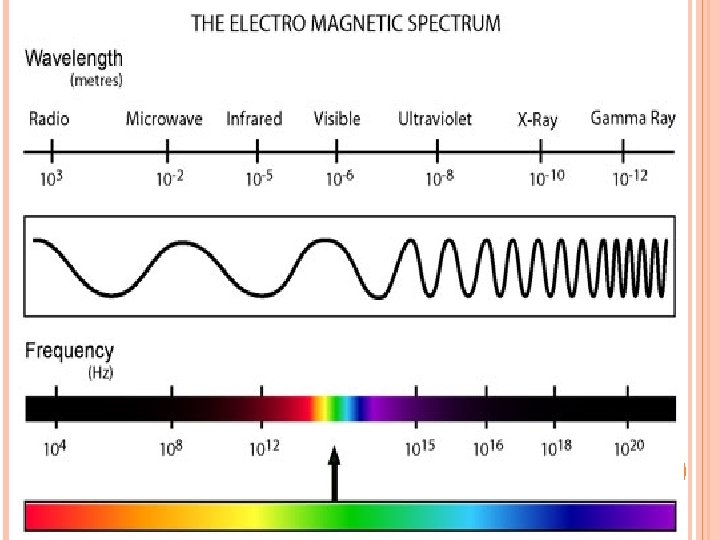
Amplitude-height of the wave from the origin to the crest Wavelength (λ)-distance between crests Frequency (ν)-number of wave cycles to pass a given point per unit of time.


ν = c/λ 8 c = speed of light 3. 0 x 10 m/s λ = wavelength (m) -1 ν = frequency (Hz or s )

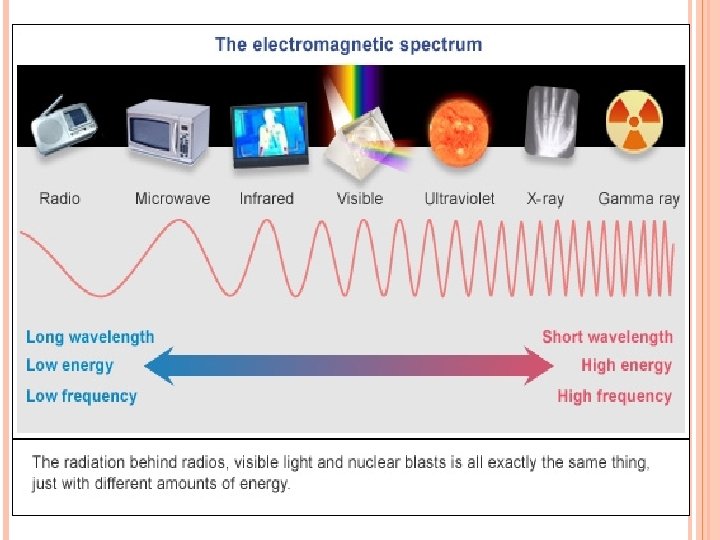
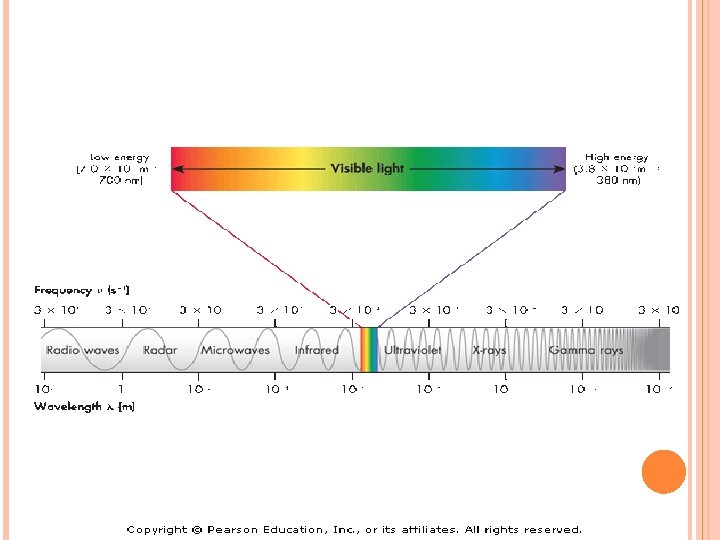
Electromagnetic Radiation-consists of radiation over a broadband of wavelengths (Radio waves to gamma waves) Light-electromagnetic waves that travel at 3. 0 x 108 m/s




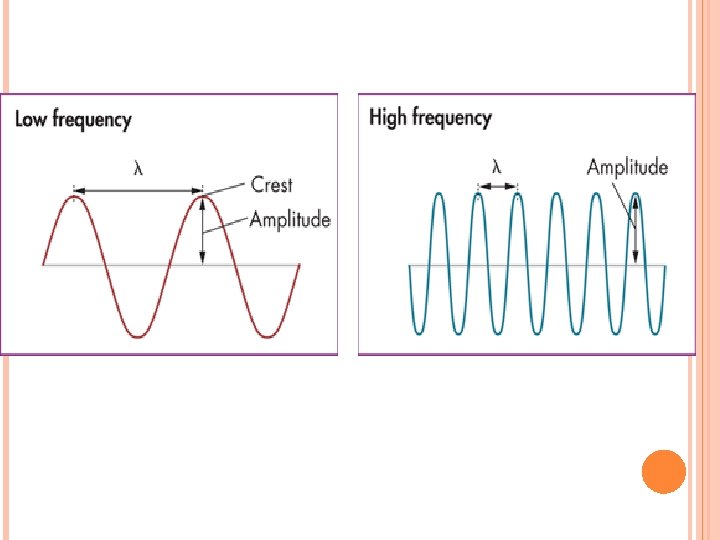
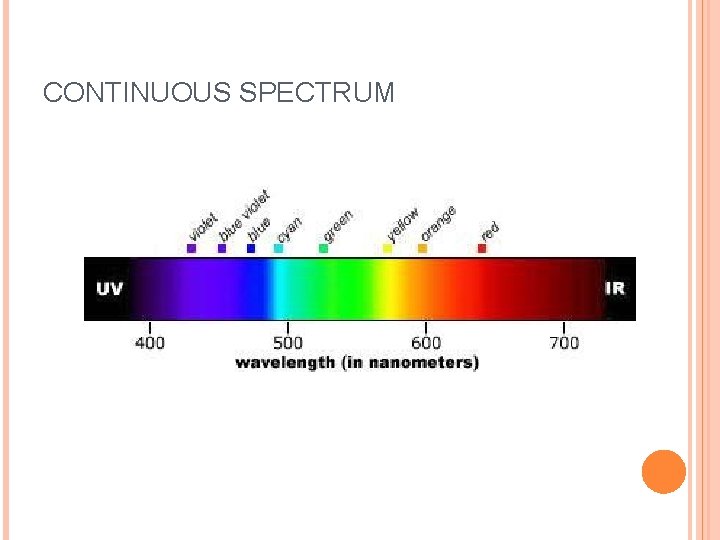
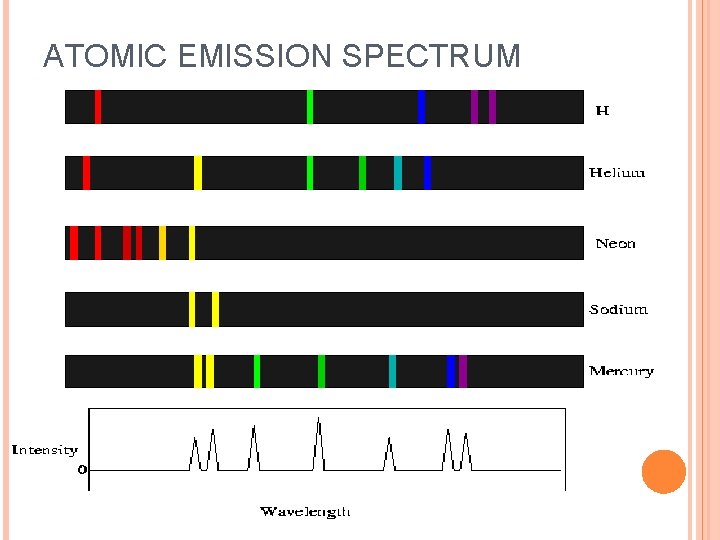
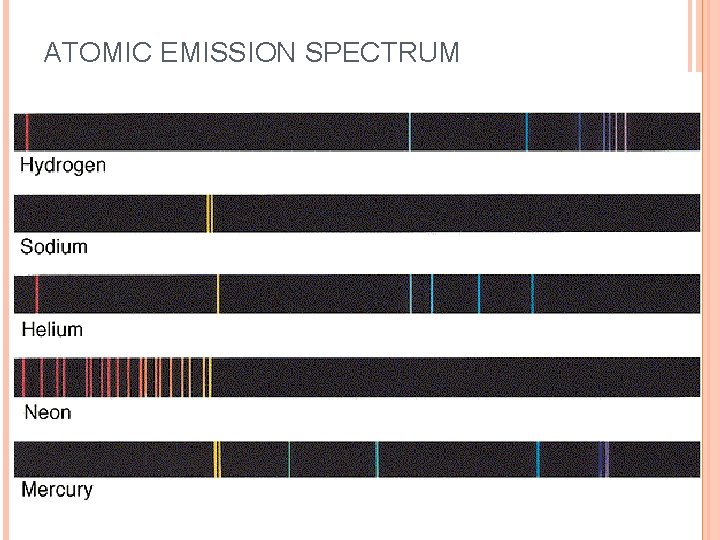
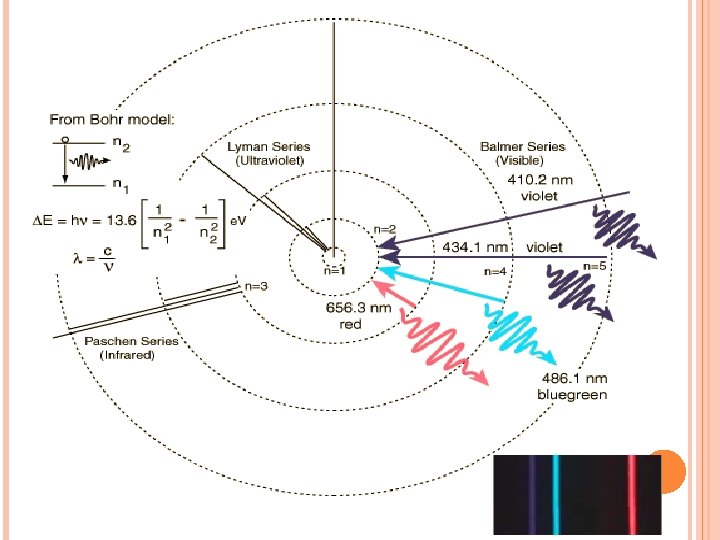
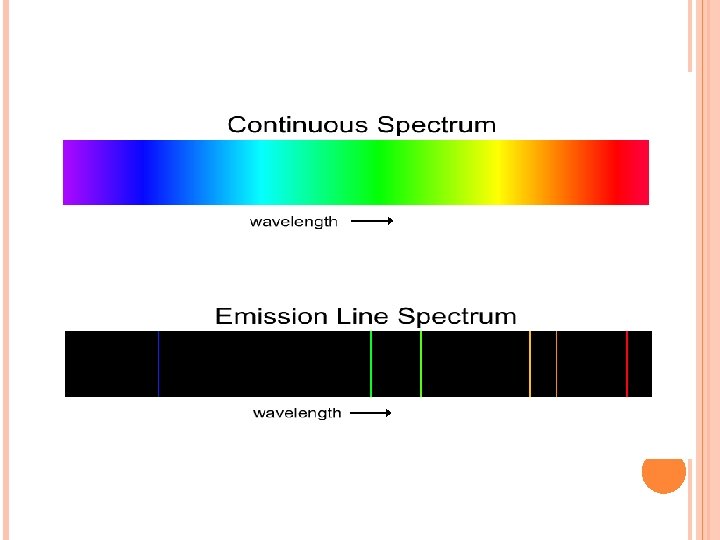
When sunlight passes through a prism light separates into a spectrum of color ROYGBIV Red-longest wavelength-lowest frequency Violet-shortest wavelength-highest frequency Atomic Emission Spectrum-passing light emitted by an element through a prism Consist of relatively few lines Unique to each element White light gives a continuous spectrum.

CONTINUOUS SPECTRUM

ATOMIC EMISSION SPECTRUM

ATOMIC EMISSION SPECTRUM

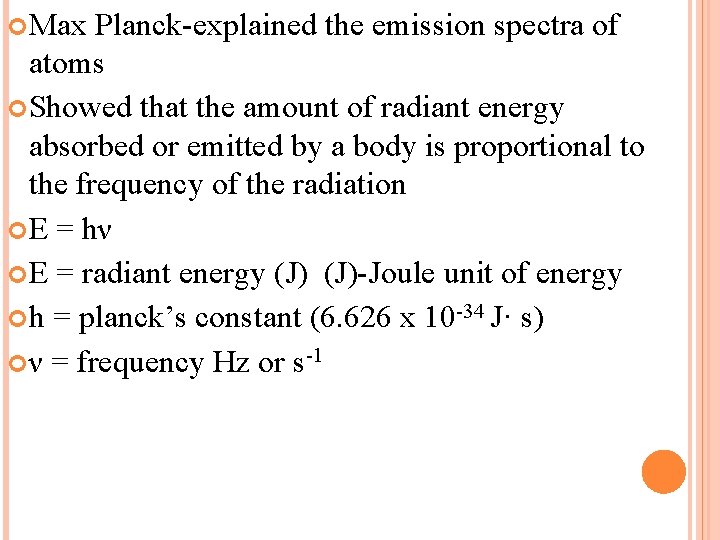
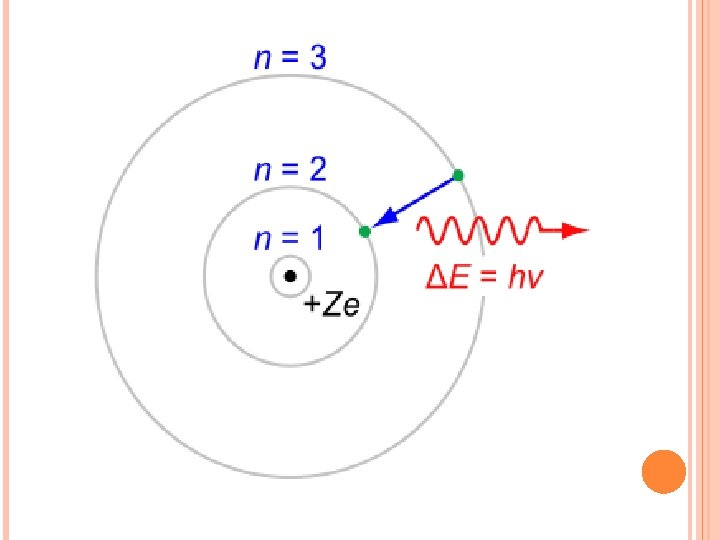
Max Planck-explained the emission spectra of atoms Showed that the amount of radiant energy absorbed or emitted by a body is proportional to the frequency of the radiation E = hν E = radiant energy (J)-Joule unit of energy h = planck’s constant (6. 626 x 10 -34 J· s) ν = frequency Hz or s-1



If the frequency of a violet spectrum line is at 2. 50 x 1014 Hz, how much energy does each photon of this light have? What is the frequency of radiation whose wavelength is 2. 40 10– 5 cm? What is the wavelength of the radiation whose frequency is 5. 00 × 1015 s– 1? In what region of the electromagnetic spectrum is this radiation?

1. 2. What are the two equations we discussed yesterday? What do the symbols represent in each equation and what are the units for each of those symbols?

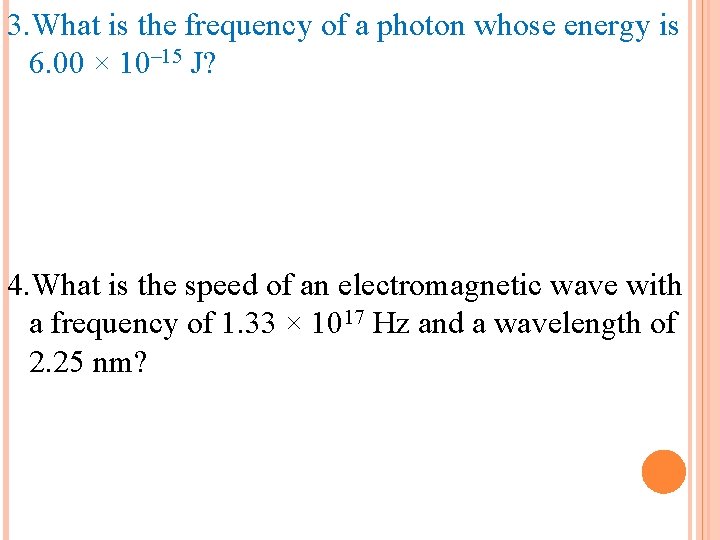
1. Calculate the wavelength of a yellow light emitted by a sodium lamp if the frequency of the radiation is 5. 0 x 1014 s-1. 2. What is the energy of a photon whose frequency is 2. 22 × 1014 s– 1? 3. What is the frequency of a photon whose energy is 6. 00 × 10– 15 J? 4. What is the speed of an electromagnetic wave with a frequency of 1. 33 × 1017 Hz and a wavelength of 2. 25 nm?

Calculate the wavelength of a yellow light emitted by a sodium lamp if the frequency of the radiation is 5. 0 x 1014 s-1. 1. 2. What is the energy of a photon whose frequency is 2. 22 × 1014 s– 1?

3. What is the frequency of a photon whose energy is 6. 00 × 10– 15 J? 4. What is the speed of an electromagnetic wave with a frequency of 1. 33 × 1017 Hz and a wavelength of 2. 25 nm?

1. J. J. Thomson discovered what? What did he use to discover this? What was his model called? 2. Rutherford discovered what? What experiment did he use? 3. What are the four types of sublevels? How many total electrons can each sublevel hold? 4. How many electrons total can occupy the 3 rd energy level? 5. The 4 th energy level has how many orbitals total? 6. Write the electron configuration for Kr and Bi.

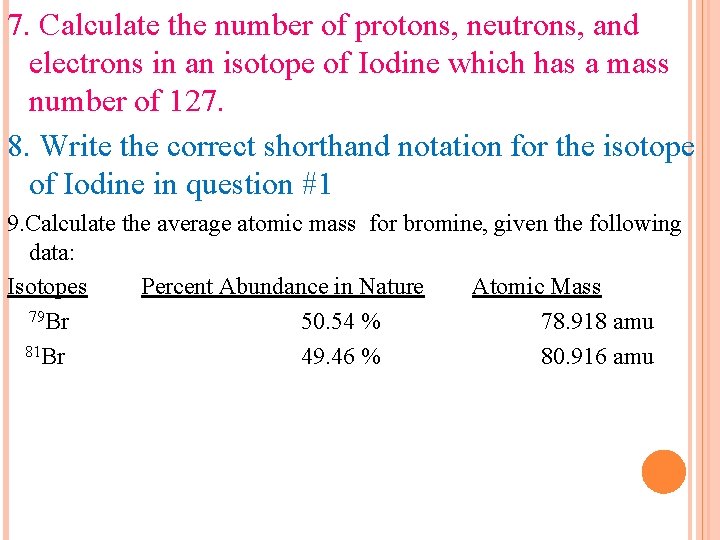
7. Calculate the number of protons, neutrons, and electrons in an isotope of Iodine which has a mass number of 127. 8. Write the correct shorthand notation for the isotope of Iodine in question #1 9. Calculate the average atomic mass for bromine, given the following data: Isotopes Percent Abundance in Nature Atomic Mass 79 Br 50. 54 % 78. 918 amu 81 Br 49. 46 % 80. 916 amu

1. Put the visible colors in order of increasing frequency. 2. The electromagnetic spectrum consists of radiation over a broad band of wavelengths. What type of radiation has the lowest frequency? The highest frequency? 3. What are three subatomic particles and their charges? 4. An inexpensive laser that is available to the public emits light that has a wavelength of 670 nm. What are the color and frequency of the radiation? 5. X-rays are used to diagnose diseases of internal body organs. What is the frequency of an X-ray with a wavelength of 1. 15 × 10− 10 m?
