The optimal choice of gonadotrophin in Gn RH


















- Slides: 37

The optimal choice of gonadotrophin in Gn. RH antagonist protocols Prof Dr P Devroey

Recent trends in ART practice • Increasing use of Gn. RH antagonists with lower doses of gonadotrophin • Increasing use of ICSI over the last decade • Increasing use of single embryo transfer • Increasing use of embryo culture to blastocyst stage • Increasing use of vitrification instead of slow-freezing

Why the MEGASET trial? • MEGASET compares HP-h. MG (MENOPUR®) with r. FSH (PUREGON®) in a setting that addresses these recent trends in ART practice • Randomised, assessor-blind, parallel groups, multicentre trial to demonstrate non-inferiority of HPh. MG compared to r. FSH with respect to ongoing pregnancy rates

Participating clinics 25 clinics in 7 countries

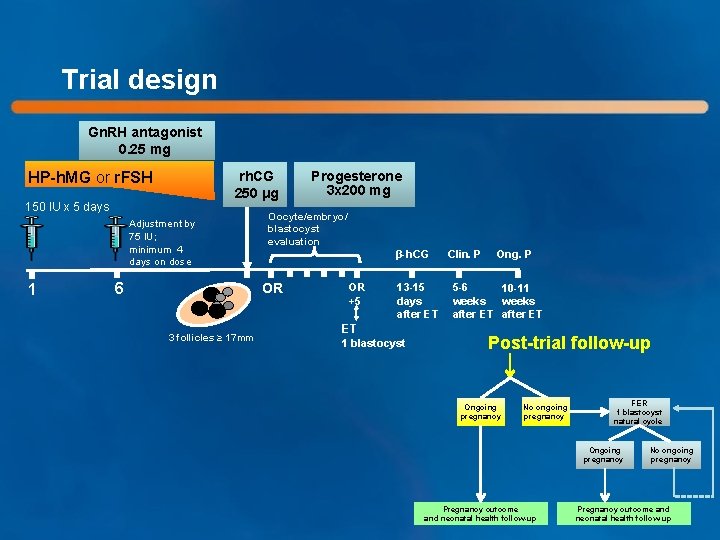
Key design features • • • Women 18– 34 years BMI 18– 24. 9 kg/m 2 Gn. RH antagonist No programming 150 IU starting dose ICSI Blastocyst culture Single blastocyst transfer on Day 5 2 weeks luteal support Vitrification Replacement of a single warmed blastocyst in a natural cycle

Trial design Gn. RH antagonist 0. 25 mg HP-h. MG or r. FSH rh. CG 250 μg 150 IU x 5 days Adjustment by 75 IU; minimum 4 days on dose 1 6 Oocyte/embryo/ blastocyst evaluation β-h. CG OR 3 follicles ≥ 17 mm Progesterone 3 x 200 mg OR +5 13 -15 days after ET ET 1 blastocyst Clin. P Ong. P 5 -6 10 -11 weeks after ET Post-trial follow-up Ongoing pregnancy No ongoing pregnancy FER 1 blastocyst natural cycle Ongoing pregnancy Pregnancy outcome and neonatal health follow-up No ongoing pregnancy Pregnancy outcome and neonatal health follow-up

Investigations: All patients • Endocrine profile • Follicular development • Ovarian response • Endometrial profile • Pregnancy rates • Cumulus mass appearance • Oocyte maturation, fertilisation • Embryo quality • Blastocyst quality

Additional investigations: Subgroups of patients • Early-mid follicular phase endocrine profile • Intrafollicular endocrine profile • Uterine contractility • Modelling of follicles • Modelling of endometrium • Gene expression in cumulus cells (mechanical dissection and enzymatic denudation)

METHODOLOGY

Primary endpoint of the study • Ongoing pregnancy rates beyond 10– 11 weeks after ET in a fresh cycle

Power calculation • Estimated ongoing pregnancy rate of 30% was derived from previous studies on single blastocyst transfer • Non-inferiority margin was set at – 10% (absolute) • At least 660 cycles was required to achieve a study power of 80%

Analysis of data • Modified Intention-to-treat (ITT) analysis – All subjects who have been randomised and exposed to at least one dose of investigational medicinal product were analysed according to the actual treatment • Per protocol analysis – All subjects from the modified ITT, except those who are excluded because of a major protocol deviation were analysed

EMBRYO ASSESSMENT

Embryo morphology assessment and grading • Local embryologists only; no central evaluation • Interobserver agreement and intraobserver reproducibility were validated in the MERi. T trial showing good–excellent agreement on overall embryo morphology assessment and grading 1 • Embryos were graded according to the Gardner and Schoolcraft classification system 2 1. Arce et al. Hum Reprod 2006; 21: 2141– 2148 2. Gardner and Schoolcraft. In: Towards reproductive certainty (Eds Jansen & Mortimer). The plenary proceedings of the 11 th world congress on in vitro fertilization and human reproductive genetics. The Parthenon Publishing Group. 1999. Pp 378– 388

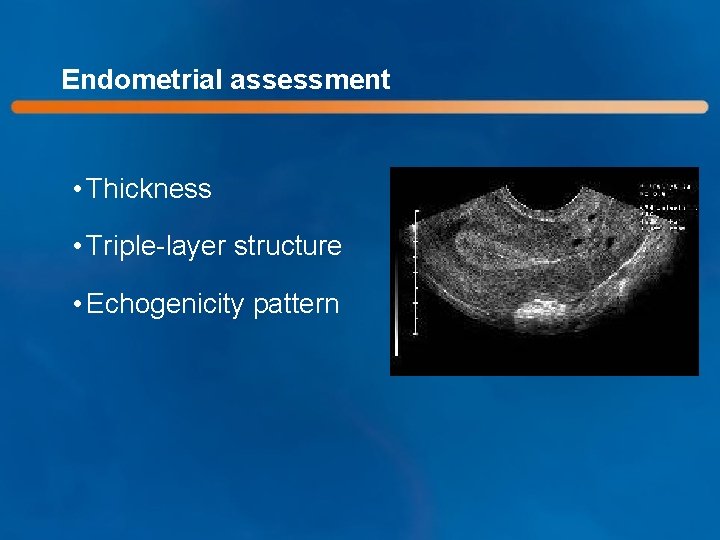
Endometrial assessment • Thickness • Triple-layer structure • Echogenicity pattern

SUBJECT DISPOSITION

Consort diagram Screened (N=810) Randomised and exposed (n=749) HP-h. MG (ITT; N=374) r. FSH (ITT; N=375) Oocyte retrieval N=362 Embryo transfer N=305 Embryo transfer N=316 β-h. CG visit N=305 β-h. CG visit N=316 Ongoing pregnancy visit N=107

BASELINE PARAMETERS

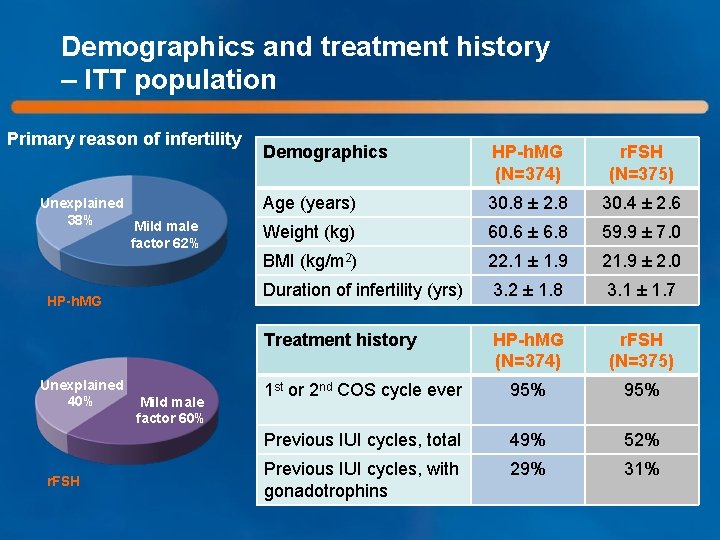
Demographics and treatment history – ITT population Primary reason of infertility Unexplained 38% Mild male factor 62% HP-h. MG Unexplained 40% r. FSH Mild male factor 60% Demographics HP-h. MG (N=374) r. FSH (N=375) Age (years) 30. 8 ± 2. 8 30. 4 ± 2. 6 Weight (kg) 60. 6 ± 6. 8 59. 9 ± 7. 0 BMI (kg/m 2) 22. 1 ± 1. 9 21. 9 ± 2. 0 Duration of infertility (yrs) 3. 2 ± 1. 8 3. 1 ± 1. 7 Treatment history HP-h. MG (N=374) r. FSH (N=375) 1 st or 2 nd COS cycle ever 95% Previous IUI cycles, total 49% 52% Previous IUI cycles, with gonadotrophins 29% 31%

ENDOCRINE PROFILE

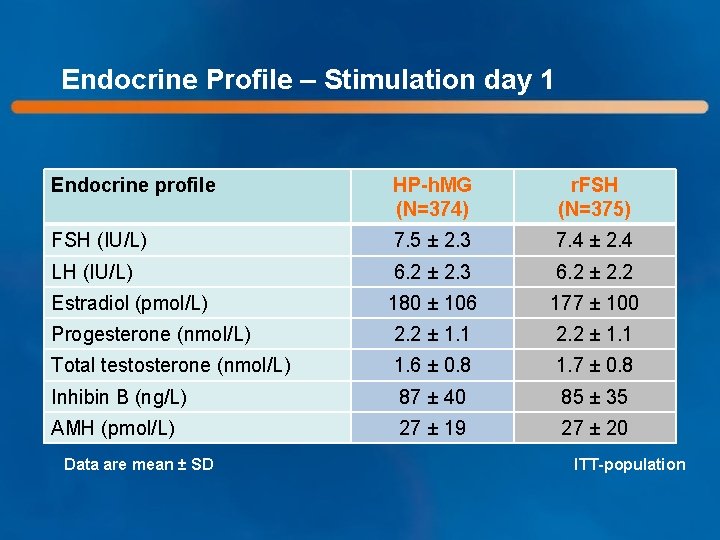
Endocrine Profile – Stimulation day 1 Endocrine profile HP-h. MG (N=374) r. FSH (N=375) FSH (IU/L) 7. 5 ± 2. 3 7. 4 ± 2. 4 LH (IU/L) 6. 2 ± 2. 3 6. 2 ± 2. 2 Estradiol (pmol/L) 180 ± 106 177 ± 100 Progesterone (nmol/L) 2. 2 ± 1. 1 Total testosterone (nmol/L) 1. 6 ± 0. 8 1. 7 ± 0. 8 Inhibin B (ng/L) 87 ± 40 85 ± 35 AMH (pmol/L) 27 ± 19 27 ± 20 Data are mean ± SD ITT-population

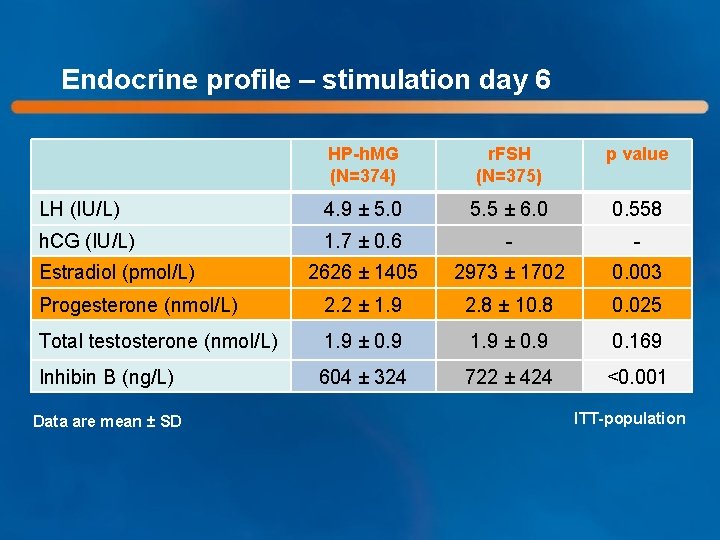
Endocrine profile – stimulation day 6 HP-h. MG (N=374) r. FSH (N=375) p value LH (IU/L) 4. 9 ± 5. 0 5. 5 ± 6. 0 0. 558 h. CG (IU/L) 1. 7 ± 0. 6 - - 2626 ± 1405 2973 ± 1702 0. 003 Progesterone (nmol/L) 2. 2 ± 1. 9 2. 8 ± 10. 8 0. 025 Total testosterone (nmol/L) 1. 9 ± 0. 9 0. 169 Inhibin B (ng/L) 604 ± 324 722 ± 424 <0. 001 Estradiol (pmol/L) Data are mean ± SD ITT-population

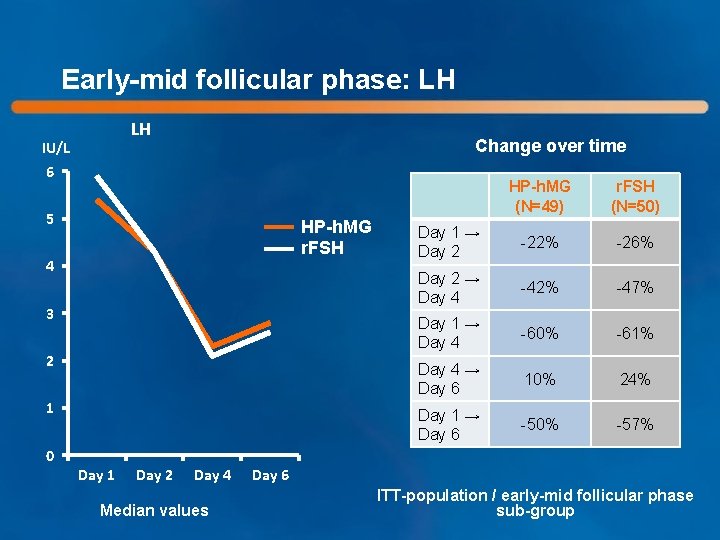
Early-mid follicular phase: LH LH IU/L Change over time 6 5 HP-h. MG r. FSH 4 3 2 1 HP-h. MG (N=49) r. FSH (N=50) Day 1 → Day 2 -22% -26% Day 2 → Day 4 -42% -47% Day 1 → Day 4 -60% -61% Day 4 → Day 6 10% 24% Day 1 → Day 6 -50% -57% 0 Day 1 Day 2 Day 4 Median values Day 6 ITT-population / early-mid follicular phase sub-group

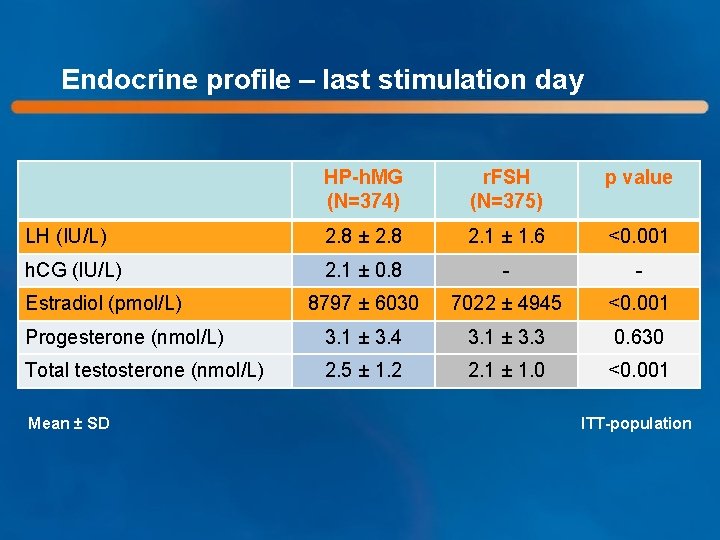
Endocrine profile – last stimulation day HP-h. MG (N=374) r. FSH (N=375) p value LH (IU/L) 2. 8 ± 2. 8 2. 1 ± 1. 6 <0. 001 h. CG (IU/L) 2. 1 ± 0. 8 - - 8797 ± 6030 7022 ± 4945 <0. 001 Progesterone (nmol/L) 3. 1 ± 3. 4 3. 1 ± 3. 3 0. 630 Total testosterone (nmol/L) 2. 5 ± 1. 2 2. 1 ± 1. 0 <0. 001 Estradiol (pmol/L) Mean ± SD ITT-population

Premature luteinization* - LH ≥ 10 IU/L - Progesterone ≥ 1 ng/m. L (3. 18 nmol/L) HP-h. MG (N=374) r. FSH (N=375) 5. 9% 6. 1% ITT-population *Both LH and progesterone criteria to be met at the same visit (ie. Stimulation Day 6 or Last Stimulation Day)

TREATMENT EFFICIENCY

Follicular development Stimulation Day 6 HP-h. MG r. FSH Last Stimulation Day p<0. 05 HP-h. MG r. FSH p<0. 05 Mean data ITT-population

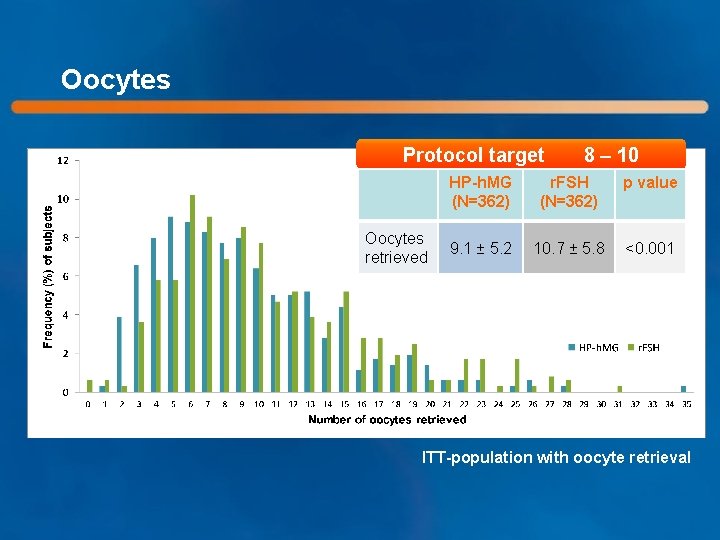
Oocytes Protocol target Oocytes retrieved 8 – 10 HP-h. MG (N=362) r. FSH (N=362) p value 9. 1 ± 5. 2 10. 7 ± 5. 8 <0. 001 ITT-population with oocyte retrieval

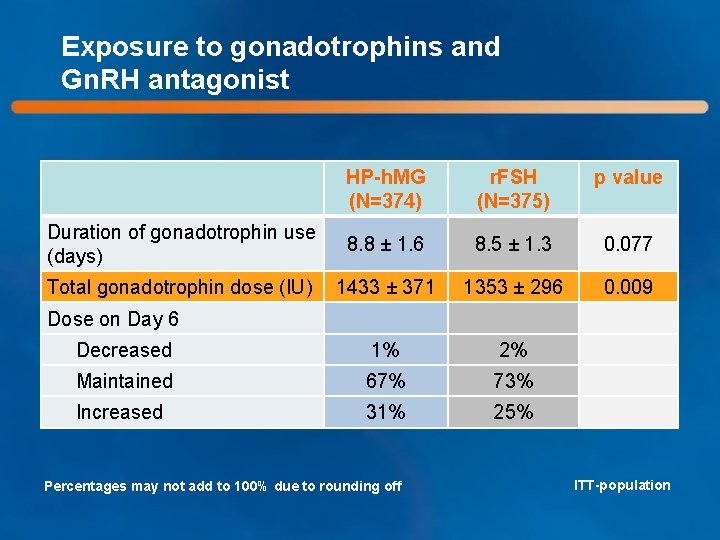
Exposure to gonadotrophins and Gn. RH antagonist HP-h. MG (N=374) r. FSH (N=375) p value Duration of gonadotrophin use (days) 8. 8 ± 1. 6 8. 5 ± 1. 3 0. 077 Total gonadotrophin dose (IU) 1433 ± 371 1353 ± 296 0. 009 Decreased 1% 2% Maintained 67% 73% Increased 31% 25% Dose on Day 6 Percentages may not add to 100% due to rounding off ITT-population

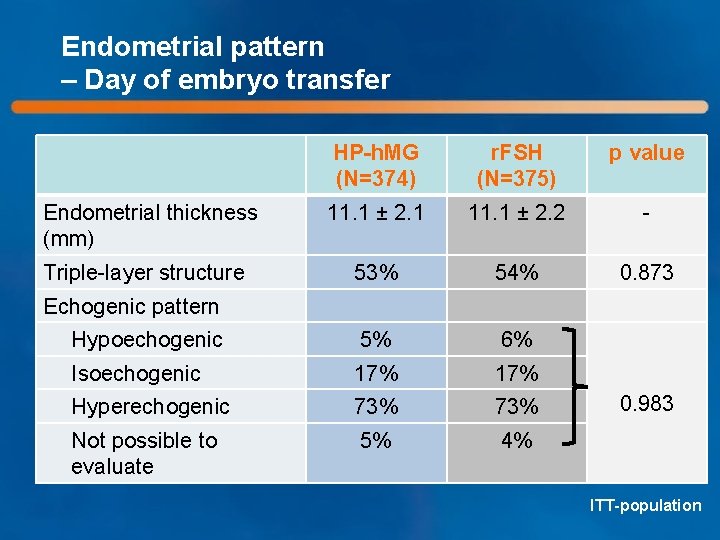
Endometrial pattern – Day of embryo transfer HP-h. MG (N=374) r. FSH (N=375) p value 11. 1 ± 2. 1 11. 1 ± 2. 2 - 53% 54% 0. 873 Hypoechogenic 5% 6% Isoechogenic 17% Hyperechogenic 73% Not possible to evaluate 5% 4% Endometrial thickness (mm) Triple-layer structure Echogenic pattern 0. 983 ITT-population

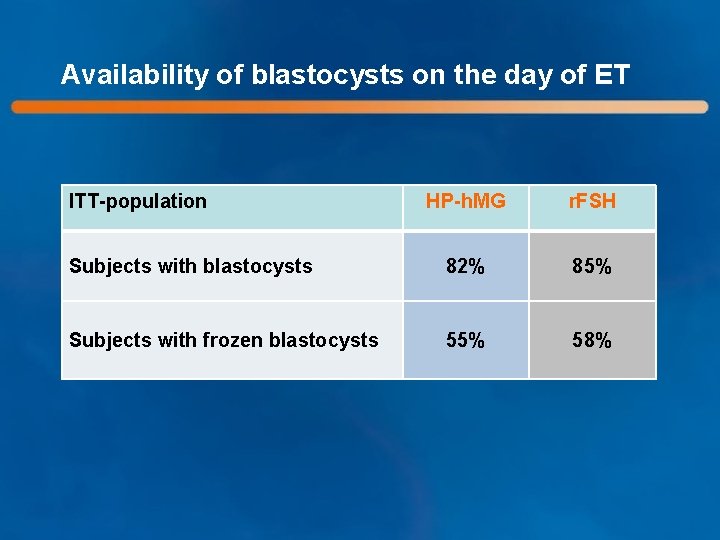
Availability of blastocysts on the day of ET ITT-population HP-h. MG r. FSH Subjects with blastocysts 82% 85% Subjects with frozen blastocysts 55% 58%

Ongoing pregnancy rate per started cycle: Primary endpoint HP-h. MG – r. FSH HP-h. MG r. FSH PP 30. 0% 27. 0% 3. 0% (-3. 8; 9. 8) ITT 28. 9% 26. 7% 2. 2% (-4. 2; 8. 6) Difference (95% CI) Non-inferiority was demonstrated for both PP- and ITT-populations, as the lower limit of the 95% confidence interval was above the preestablished non-inferiority margin of -10%

Pregnancy rates per started cycle ITT-population PP-population

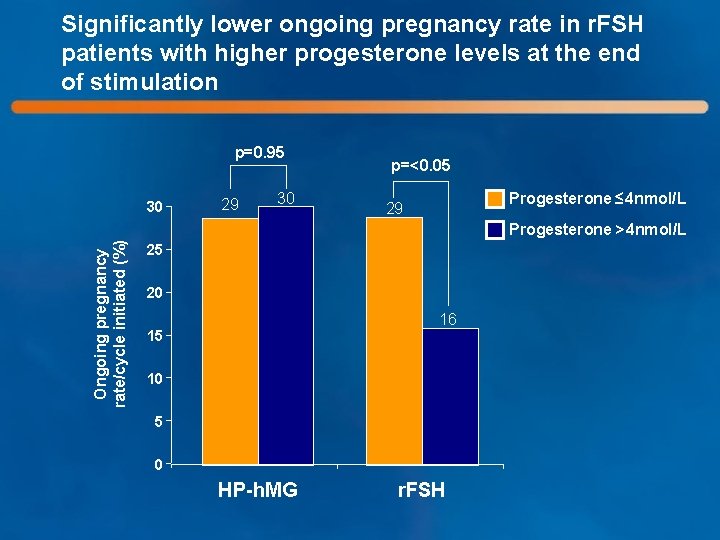
Significantly lower ongoing pregnancy rate in r. FSH patients with higher progesterone levels at the end of stimulation p=0. 95 30 29 30 p=<0. 05 Progesterone ≤ 4 nmol/L 29 Ongoing pregnancy rate/cycle initiated (%) Progesterone >4 nmol/L 25 20 16 15 10 5 0 HP-h. MG r. FSH

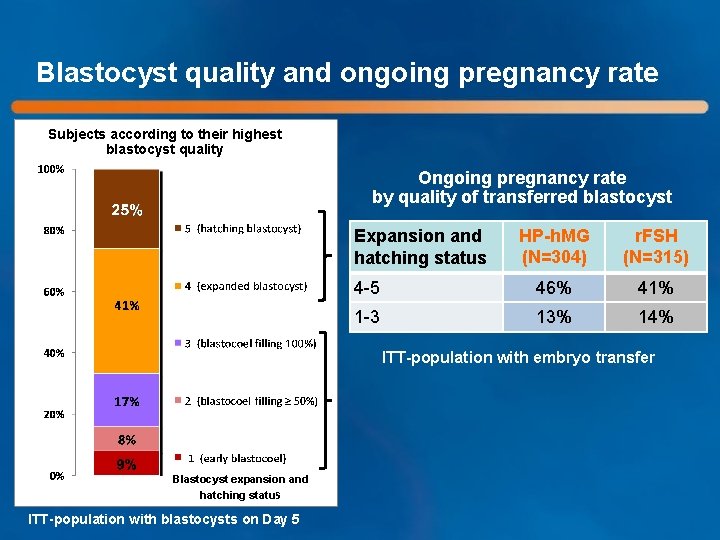
Blastocyst quality and ongoing pregnancy rate Subjects according to their highest blastocyst quality Ongoing pregnancy rate by quality of transferred blastocyst HP-h. MG (N=304) r. FSH (N=315) 4 -5 46% 41% 1 -3 13% 14% Expansion and hatching status ITT-population with embryo transfer Blastocyst expansion and hatching status ITT-population with blastocysts on Day 5

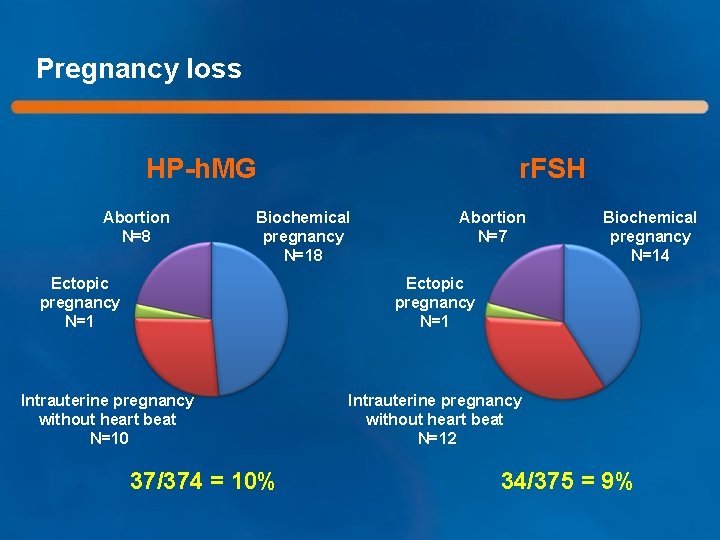
Pregnancy loss HP-h. MG Abortion N=8 r. FSH Biochemical pregnancy N=18 Ectopic pregnancy N=1 Abortion N=7 Biochemical pregnancy N=14 Ectopic pregnancy N=1 Intrauterine pregnancy without heart beat N=10 37/374 = 10% Intrauterine pregnancy without heart beat N=12 34/375 = 9%

Conclusions • Primary endpoint of MEGASET study was achieved • Largest multicentre, multinational RCT of HP-h. MG vs r. FSH addressing new trends in ART in a robust, high quality innovative trial with ICSI • Demonstrates single blastocyst transfer is effective with mild stimulation and lower number of oocytes • Reinforces the importance of progesterone during the late follicular phase – Higher pregnancy rate with HP-h. MG than r. FSH when progesterone >4 nmol/L
 Optimal choice
Optimal choice Good choice or bad choice
Good choice or bad choice đại từ thay thế
đại từ thay thế Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc 101012 bằng
101012 bằng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worm breton là gì
Tư thế worm breton là gì Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Chúa yêu trần thế
Chúa yêu trần thế điện thế nghỉ
điện thế nghỉ Fecboak
Fecboak Một số thể thơ truyền thống
Một số thể thơ truyền thống Sơ đồ cơ thể người
Sơ đồ cơ thể người Công thức tính thế năng
Công thức tính thế năng Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu