The Mole Concept The Mole n Measures the




















- Slides: 32

The Mole Concept

The Mole n Measures the amount of a substance n Relates to the number of particles, mass, and volume of a substance

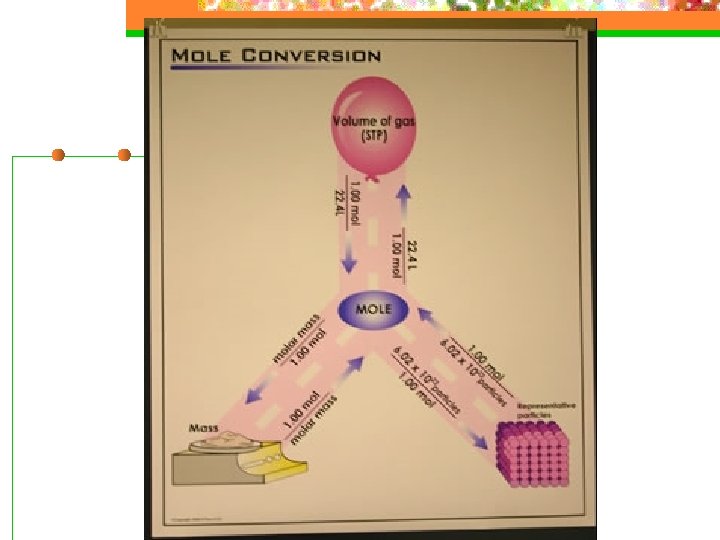
The Mole n Avogadro’s Number n 6. 02 x 1023 particles = 1 mole n Particles = atoms, molecules, or formula units

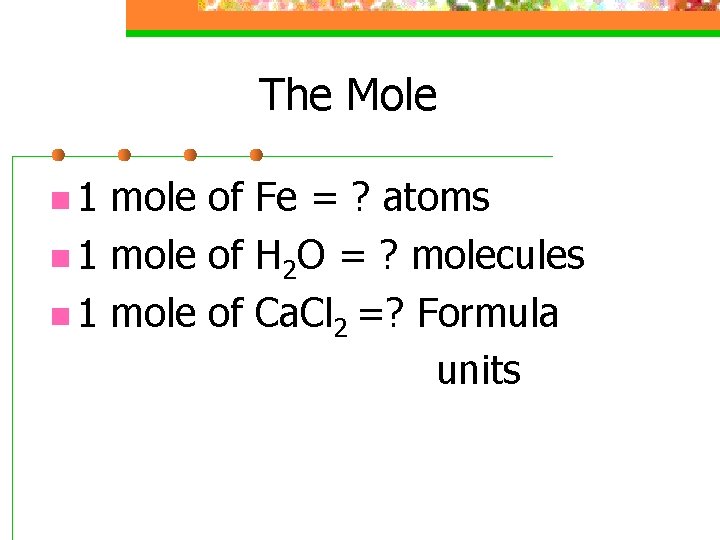
The Mole n 1 mole of Fe = ? atoms n 1 mole of H 2 O = ? molecules n 1 mole of Ca. Cl 2 =? Formula units

The Mole n How many moles is 1. 25 x 1023 atoms of Mg?

The Mole n How many C atoms are in 2. 12 mole of propane C 3 H 8?

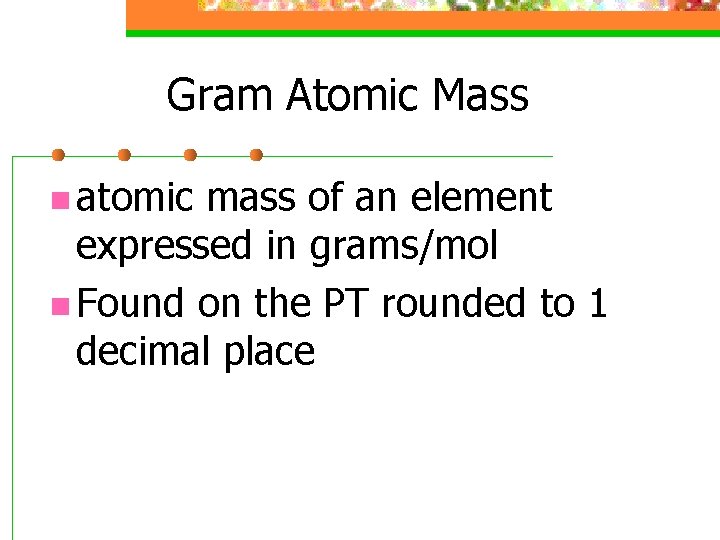
Gram Atomic Mass n atomic mass of an element expressed in grams/mol n Found on the PT rounded to 1 decimal place

Gram Atomic Mass

Gram Atomic Mass n What Cl? is the gam of N, O, H, Na,

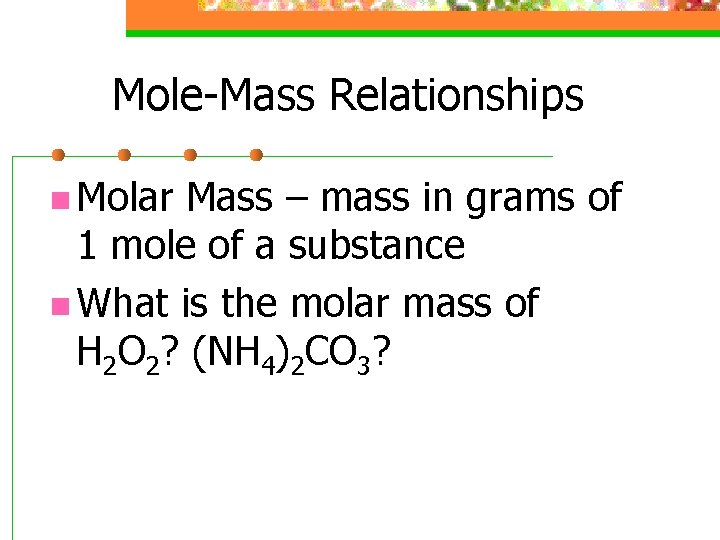
Mole-Mass Relationships n Molar Mass – mass in grams of 1 mole of a substance n What is the molar mass of H 2 O 2? (NH 4)2 CO 3?

Summary/Warmup n What conversion is used between moles and particles? n How is Molar Mass calculated?
Mole-Mass Relationships n What is the molar mass of oxygen? n How many grams are in 3. 0 mol of oxygen? n How many moles are in 2. 30 g of oxygen?

Mole-Volume Relationships n Molar Volume – At STP, the volume of 1 mole of any gas is 22. 4 L n STP – Standard Temperature (0 o. C) and Standard Pressure (1 atm)

Mole-Volume Relationships n Determine the volume in Liters of 0. 60 mol of SO 2 at STP.

Complex Mole Conversions n How many grams are in 3. 48 x 1023 formula units of iron(III)oxide?

Complex Mole Conversions n How many atoms are in 10. 0 L of Argon gas at STP?

Complex Mole Conversions n How many atoms of Zinc are in 10. 0 g of Zn?

Complex Mole Conversions n How many molecules are in 718 g dinitrogen trioxide?

Complex Mole Conversions n What is the volume of 2. 00 g of water at STP?
n Why was the mole of oxygen molecules excited when he left the singles bar? n He got Avogadro's number!

Mole Relationships

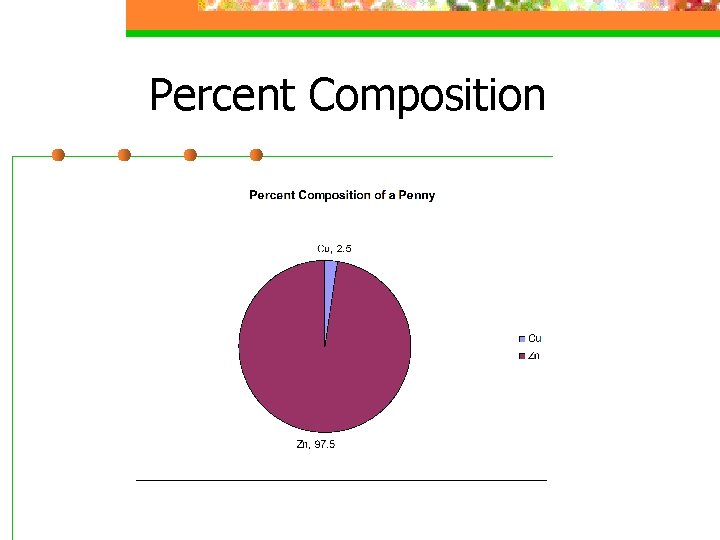
Percent Composition

Percent Composition %mass of an element =g element/ g compound x 100

Percent Composition n An 8. 20 g piece of Mg combines completely with 5. 40 g of O to form a compound. What is the percent composition?

Percent Composition n If only the chemical formula of the compound is known divide the mass of each element in a mole of a compound by the molar mass and multiply by 100

Percent Composition n Calculate the percent composition of C 3 H 8

Emperical Formula n Gives the lowest whole number ratio of atoms of elements in a compound n May or may not be the same as the molecular formula

Emperical Formula n What is the ef of a compound that is 25. 9% N and 74. 1% O

Molecular Formulas n Is either the same as the ef or a whole number multiple of it n Determined from ef and molar mass of the mf n MM of mf/ MM of ef = whole number multiple

Molecular Formulas n Calculate the molecular formula of the compound whose molar mass is 60. 0 g/mol and emperical formula is CH 4 N.

Putting it all together n Determine the ef and mf of Caffeine which contains 49. 5%C, 5. 15%H, 28. 9%N, and 16. 5%O; and the molar mass is 195 g/mol.

Putting it all together n Determine the ef and mf of Ibuprofen which contains 75. 69%C, 8. 80%H, and 15. 51%O ; and the molar mass is 206 g/mol.