MOLE CONCEPT STOICHIOMETRY CHEMISTRYX CHAPTER 4 Mole Concept


- Slides: 14

MOLE CONCEPT & STOICHIOMETRY CHEMISTRY-X CHAPTER 4

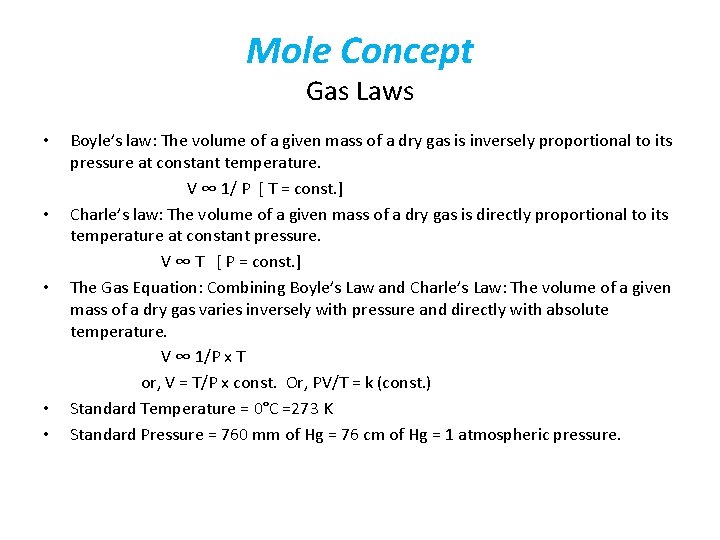
Mole Concept Gas Laws • • • Boyle’s law: The volume of a given mass of a dry gas is inversely proportional to its pressure at constant temperature. V ∞ 1/ P [ T = const. ] Charle’s law: The volume of a given mass of a dry gas is directly proportional to its temperature at constant pressure. V ∞ T [ P = const. ] The Gas Equation: Combining Boyle’s Law and Charle’s Law: The volume of a given mass of a dry gas varies inversely with pressure and directly with absolute temperature. V ∞ 1/P x T or, V = T/P x const. Or, PV/T = k (const. ) Standard Temperature = 0°C =273 K Standard Pressure = 760 mm of Hg = 76 cm of Hg = 1 atmospheric pressure.

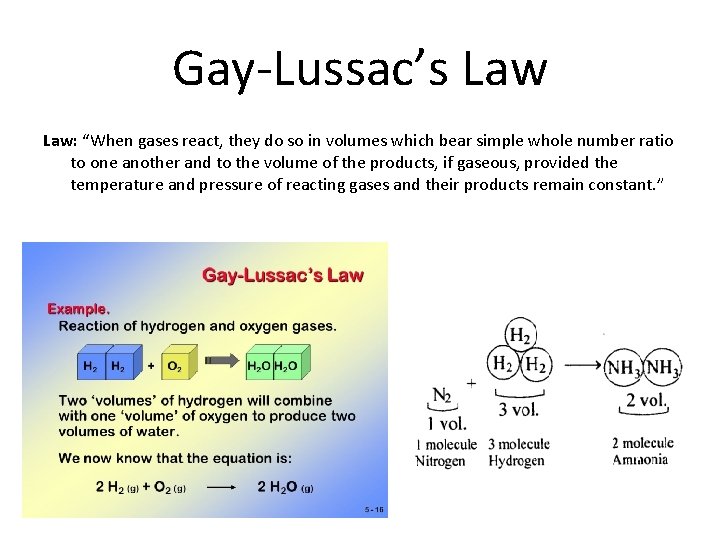
Gay-Lussac’s Law: “When gases react, they do so in volumes which bear simple whole number ratio to one another and to the volume of the products, if gaseous, provided the temperature and pressure of reacting gases and their products remain constant. ”

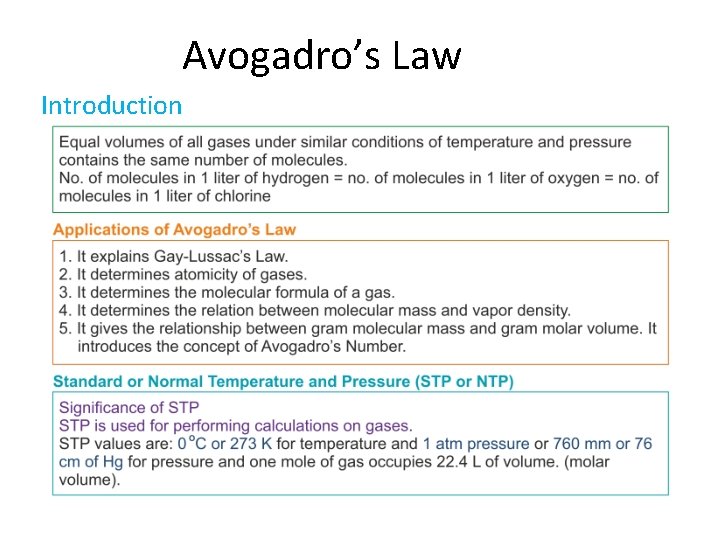
Avogadro’s Law Introduction

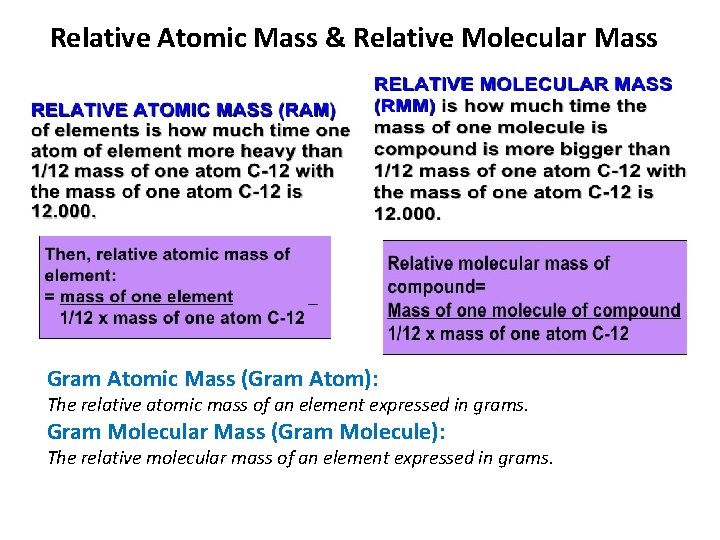
Relative Atomic Mass & Relative Molecular Mass Gram Atomic Mass (Gram Atom): The relative atomic mass of an element expressed in grams. Gram Molecular Mass (Gram Molecule): The relative molecular mass of an element expressed in grams.

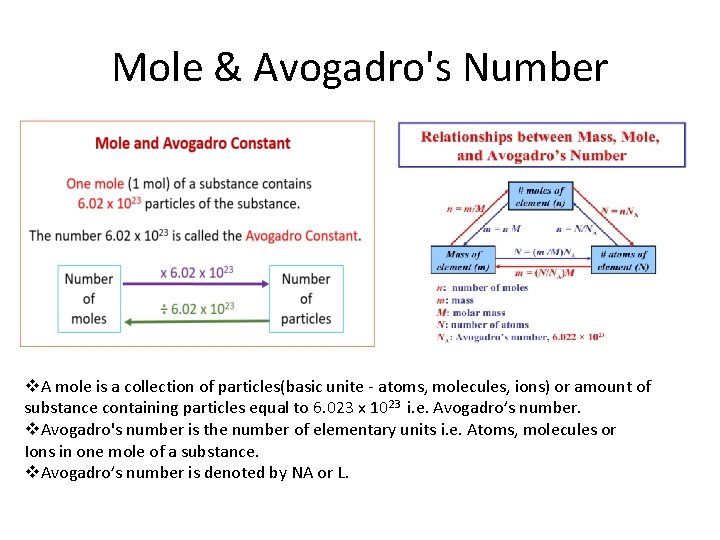
Mole & Avogadro's Number v. A mole is a collection of particles(basic unite - atoms, molecules, ions) or amount of substance containing particles equal to 6. 023 x 1023 i. e. Avogadro’s number. v. Avogadro's number is the number of elementary units i. e. Atoms, molecules or Ions in one mole of a substance. v. Avogadro’s number is denoted by NA or L.

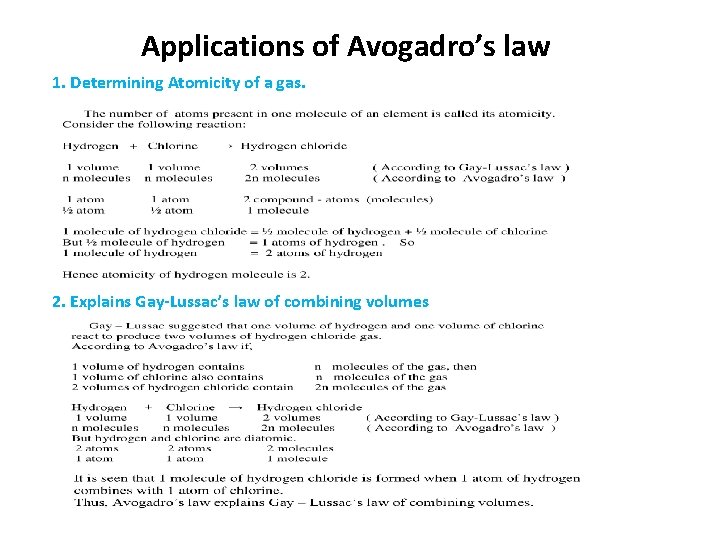
Applications of Avogadro’s law 1. Determining Atomicity of a gas. 2. Explains Gay-Lussac’s law of combining volumes

Applications of Avogadro’s law 3. Determining the relation between molecular weight and vapour density

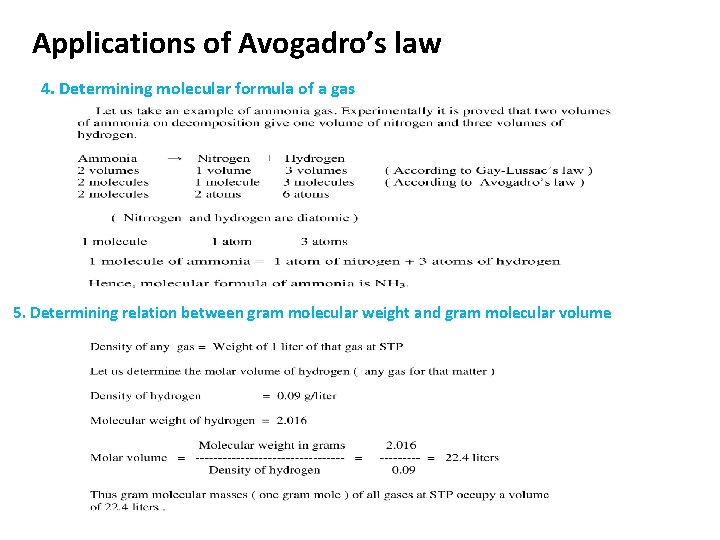
Applications of Avogadro’s law 4. Determining molecular formula of a gas 5. Determining relation between gram molecular weight and gram molecular volume

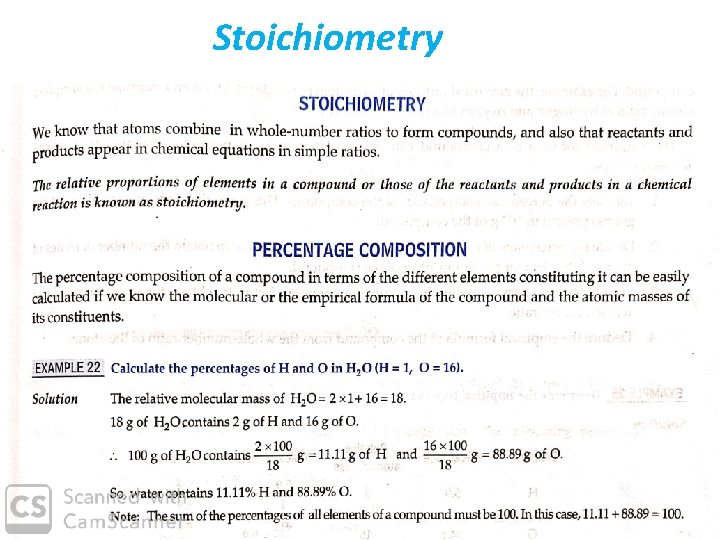
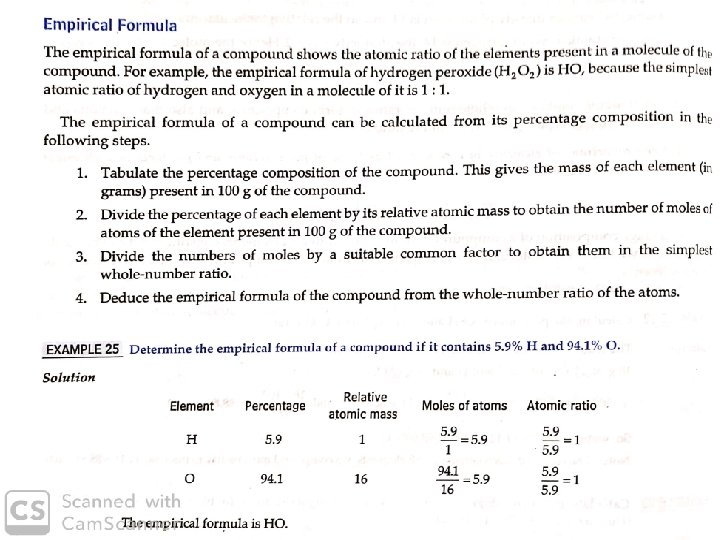
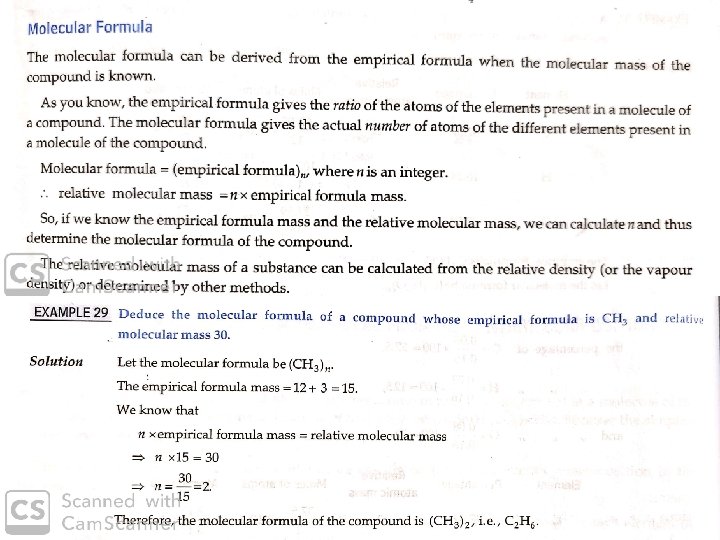
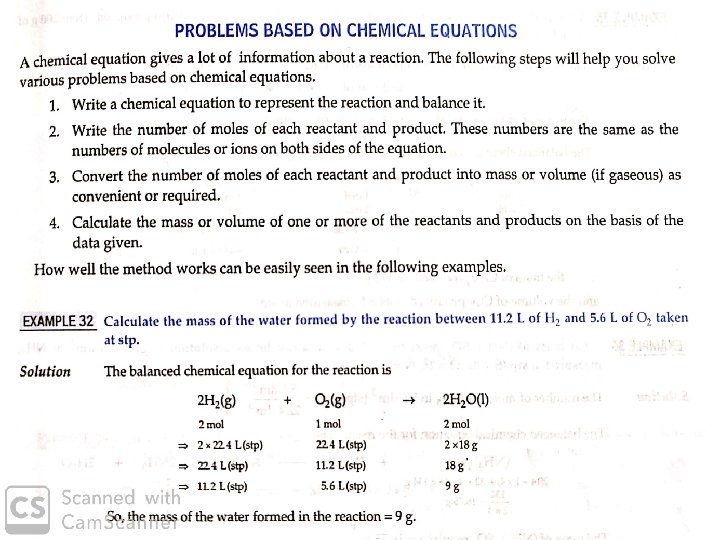
Stoichiometry




THE END ~ Rajshree Ranjana