Smallpox Vaccine Overview for Health Care Response Teams





































- Slides: 60

Smallpox Vaccine: Overview for Health Care Response Teams Thomas G. Franck, MD, MPH Regional Physician Consultant Office of Emergency Preparedness & Response Virginia Department of Health October, 2002 January 2003

Objectives To briefly review smallpox disease n To gain an in depth understanding of smallpox vaccine, including: n – history of smallpox vaccination – overview of vaccinia – indications – contraindications – normal response – complications

Taxonomy Ø Family: Poxviridae ØGenus: Orthopoxviruses § Smallpox § Cowpox (variola) § Monkeypox § Vaccinia 93% DNA Homology

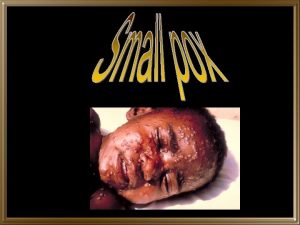
Smallpox Caused by Variola virus n Unique to humans n Person-to-person spread n – usually via close contact - droplets – contaminated materials (uncommon) – aerosolized droplet nuclei spread (rare) n 30% case-fatality rate on average

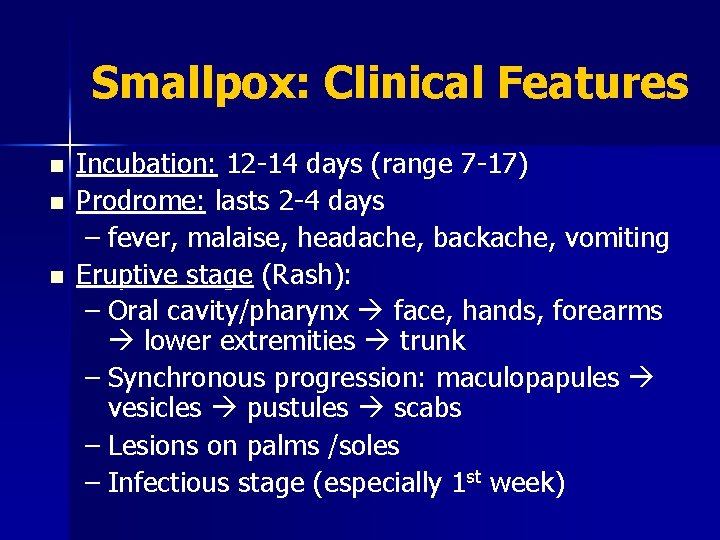
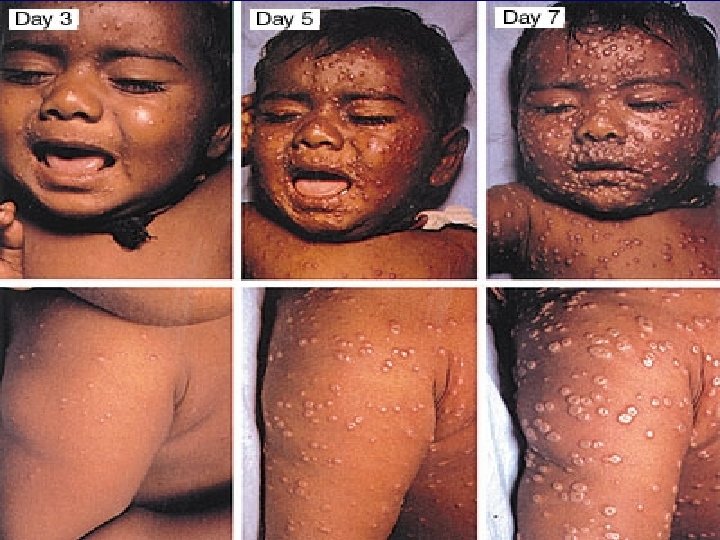
Smallpox: Clinical Features n n n Incubation: 12 -14 days (range 7 -17) Prodrome: lasts 2 -4 days – fever, malaise, headache, backache, vomiting Eruptive stage (Rash): – Oral cavity/pharynx face, hands, forearms lower extremities trunk – Synchronous progression: maculopapules vesicles pustules scabs – Lesions on palms /soles – Infectious stage (especially 1 st week)


11/25/2020

Smallpox - Treatment n Treatment – Supportive care – No treatment proven effective – Experimental treatment with antivirals, e. g. , Cidofovir n Prevention/Prophylaxis – Vaccination - protective if given within 3 days of exposure

Smallpox: Why the Concern Now? Last case in US in 1949 n Last naturally acquired case in 1977 n Disease declared eliminated by WHO in 1980 n Stocks of Variola virus held by U. S. & Russia n Bio Weapons programs in several countries n Recent Intelligence review: 4 countries may have covert stocks of smallpox virus – Russia, Iraq, North Korea, and France n

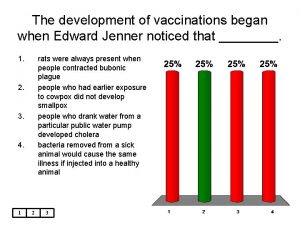
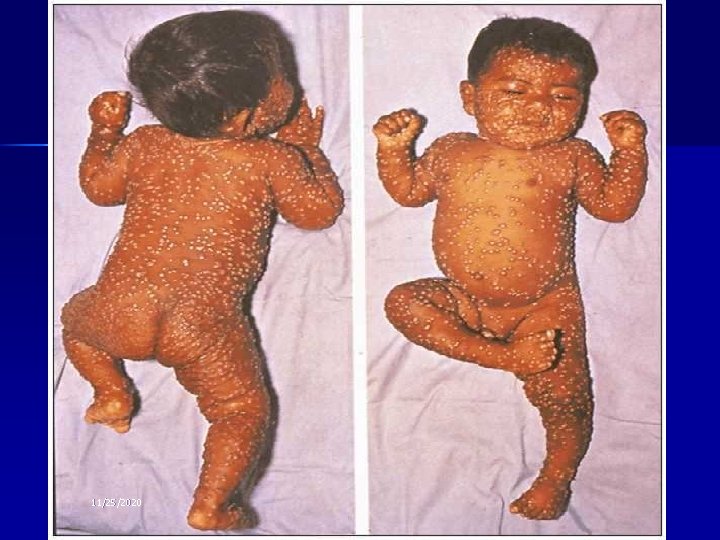
Smallpox Vaccine: History 1796: Edward Jenner develops vaccine (cowpox) 1805: Use of cows to produce vaccine 1940 s: Freeze-drying of Vaccinia 1965: Licensure of bifurcated needle 1972: Routine vaccination stopped in U. S. 1983: Vaccine removed from civilian market 1990: U. S. Military vaccination stops






Smallpox Vaccine n Live virus called “Vaccinia” n An orthopoxvirus, genetically distinct from other orthopoxviruses such as cowpox, monkeypox, and variola (cause of smallpox) n Origin unknown: May be a virus now extinct in nature

Vaccinia Vaccine n “Dryvax” (Wyeth Laboratories) n Contains NY City Board of Health strain n 2. 7 million doses licensed (phase 1)* n Enough vaccine “to vaccinate every single person in the country in an emergency”* *December 2002

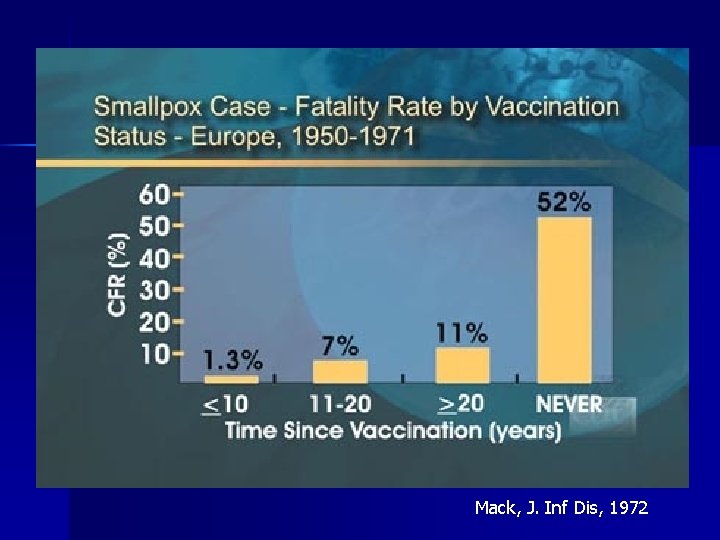
Vaccine Efficacy: Pre-Exposure § Reduces chance of getting infected (i. e. , decreases secondary attack rate) Ø 91%-97% reduction in cases among case contacts with vaccination scar § For those infected, reduces fatality rate and severity of disease

Mack, J. Inf Dis, 1972

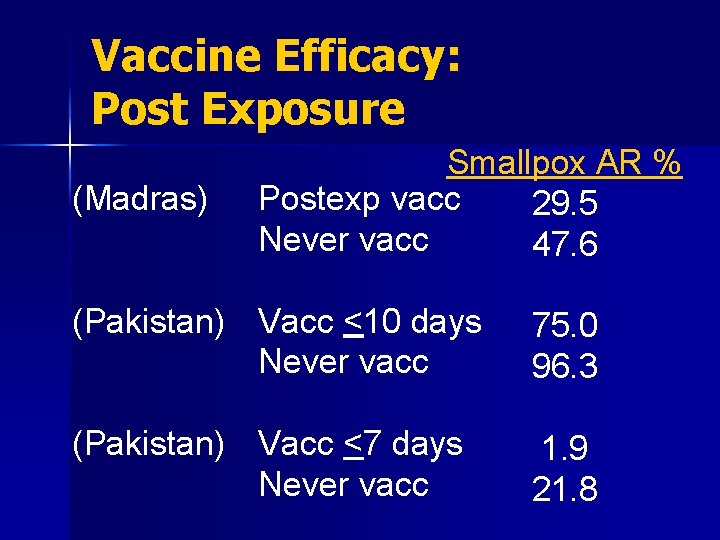
Vaccine Efficacy: Post Exposure n Generally prevents smallpox, or significantly decreases severity, if given within 3 days of exposure n Vaccination 4 to 7 days post-exposure still offered protection to many people, but significantly less than vaccination before 4 days

Vaccine Efficacy: Post Exposure (Madras) Smallpox AR % Postexp vacc 29. 5 Never vacc 47. 6 (Pakistan) Vacc <10 days Never vacc 75. 0 96. 3 (Pakistan) Vacc <7 days Never vacc 1. 9 21. 8

Duration of Immunity n n High level of protection (95 -100%) for 3 -5 years following vaccination Immunity wanes after 5 years, but some residual protection evident at 10 and even 20+ years Reduction in disease severity with any history of vaccination However, best protection if vaccinated <3 -5 yrs ago; we cannot rely on previous vaccinations to protect our population and we should consider the population to lack immunity to smallpox.

Smallpox Vaccine Indications: Non-Emergency n Current Indications: – Laboratory workers who handle cultures or animals infected with non-highly attenuated vaccinia or other Orthopoxviruses n New Recommendations: – Public health, hospital, and other personnel, generally 18 -65 years of age, who may have to respond to a smallpox case or outbreak

Smallpox Vaccine Indications: Emergency Situations n Ring Vaccination – Persons exposed to initial release – Close contact with confirmed or suspected case – Direct care or transportation of confirmed or suspected case – Laboratory personnel – Persons with risk of contact with infectious materials from case n Mass Vaccination of entire populations?

Contraindications: Non-Emergency Situations n n n n Eczema/atopic dermatitis (active or history of) or household contact with eczema/atopic dermatitis Other active skin conditions (allergic rash, burns, impetigo, chickenpox, shingles, herpes, psoriasis, severe acne, etc. ) or household contact with acitve skin condition Immunosuppression or household contact with immunosuppression Pregnancy or pregnant household contact Breastfeeding Infants (not advised in children < 18) Severe allergic reaction to prior vaccination or vaccine component

Contraindications: Immunodeficiency n Conditions causing immunodeficiency: – HIV, leukemia, lymphoma, other cancers, agammaglobulinemia, certain autoimmune disorders (e. g. , SLE), other immune disorders n Treatments causing immunodeficiency: – Chemotherapy, radiation treatment, antimetabolites, alkyltating agents, organ transplant meds, high-dose corticosteroids – Immunomodulatory medications? Unknown

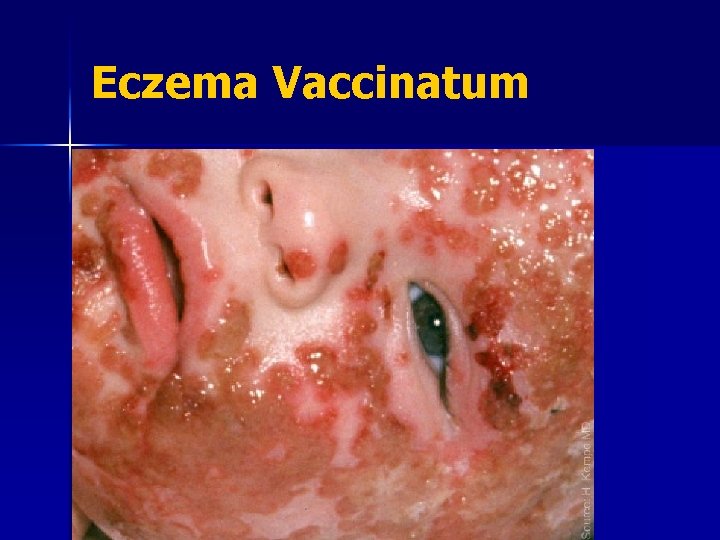
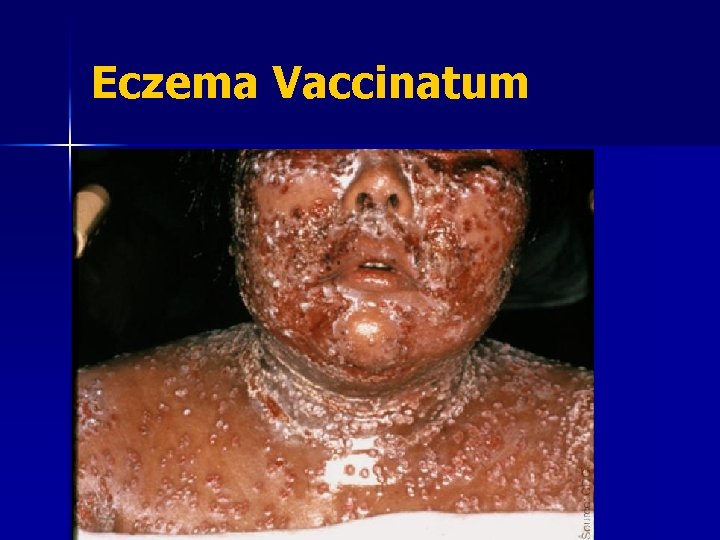
Contraindications: Eczema/Atopic Dermatitis n Eczema: a red, itchy rash that lasts at least two weeks and then comes and goes It is estimated that at least 15 million people in U. S. have atopic dermatitis n These people are at risk of a serious complication, eczema vaccinatum n

Contraindications: Emergency Situations n Exposed persons – no contraindications n Unexposed persons – generally same as non-emergency situations w/ some modifications, depending on situation

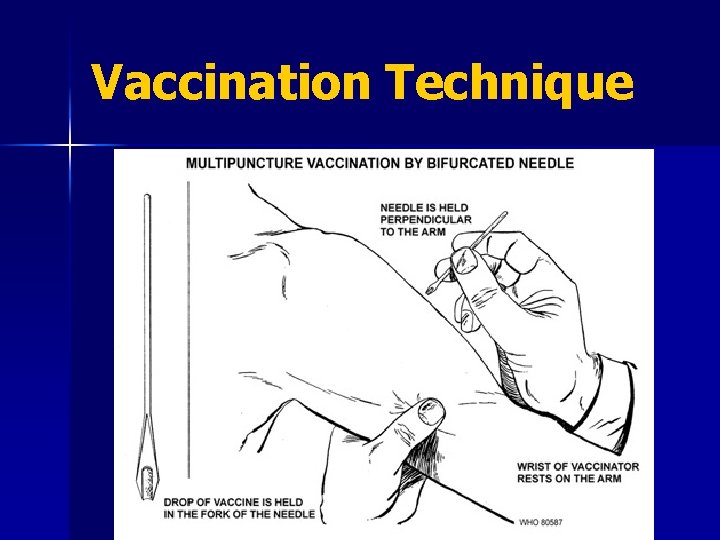
Vaccine Administration Surgical needle n Vaccinostyle n Rotary lancet n Jet injector n Bifurcated needle* n *Only administration technique currently in use.

Vaccination Technique



Vaccination Site Care Remember – live vaccinia virus is present at site of vaccination until scab falls off on its own, usually 2 -3 weeks. n Dressing n ØHealth care setting: 3 layers of protection – gauze, semipermeable dressing, shirt ØNon-health care setting: 2 layers of protection – gauze & shirt Avoid salves and ointments n Avoid touching/scratching site and picking scab n

Post-Vaccination Follow-up n n Semipermeable dressing: change dressing at least every 3 -5 days and as needed Gauze dressing secured by tape: change dressing every 1 -3 days and as needed “Take” evaluation: 7 days after vaccination (+/- 1 day) If significant side effects or adverse event, follow-up with designated health care provider

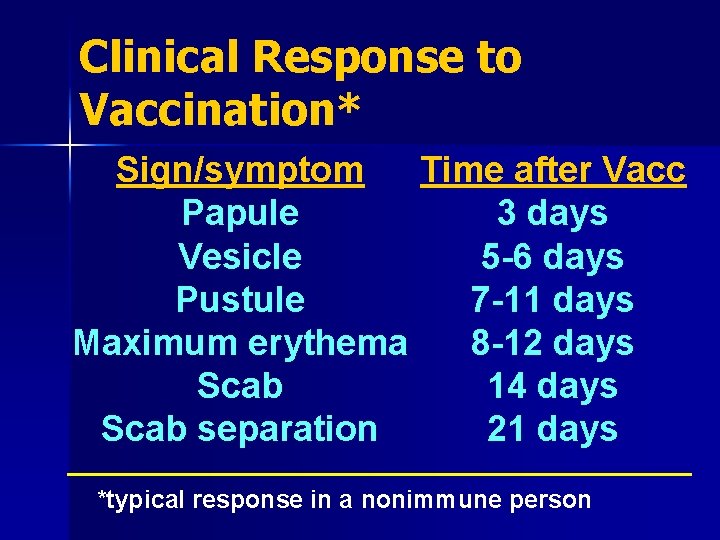
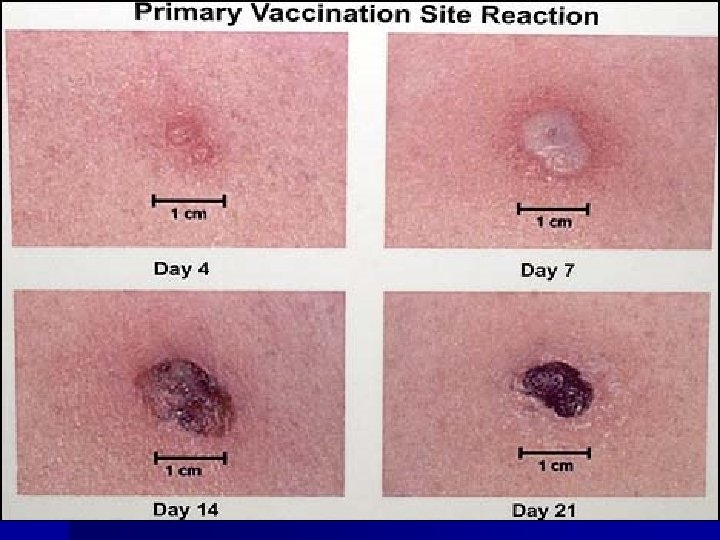
Clinical Response to Vaccination* Sign/symptom Time after Vacc Papule 3 days Vesicle 5 -6 days Pustule 7 -11 days Maximum erythema 8 -12 days Scab 14 days Scab separation 21 days *typical response in a nonimmune person

Clinical Response to Vaccination n Major (primary) reaction – Indicates viral replication has occurred and vaccination was successful n No reaction or equivocal reaction – No immunity and vaccination must be repeated


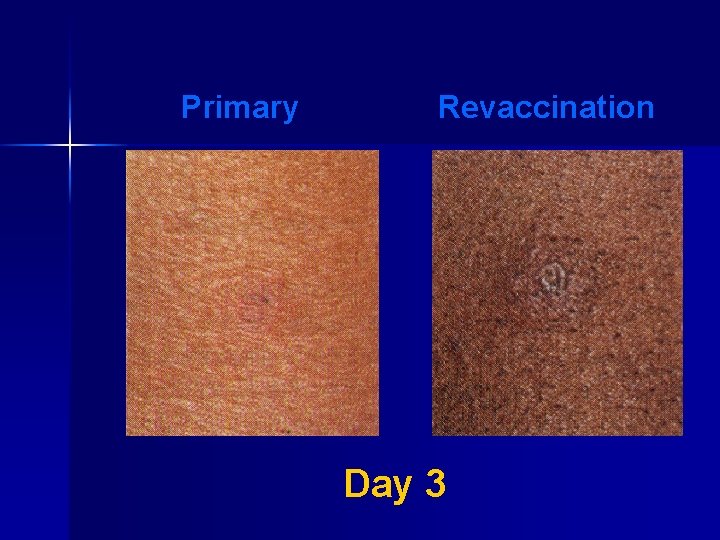
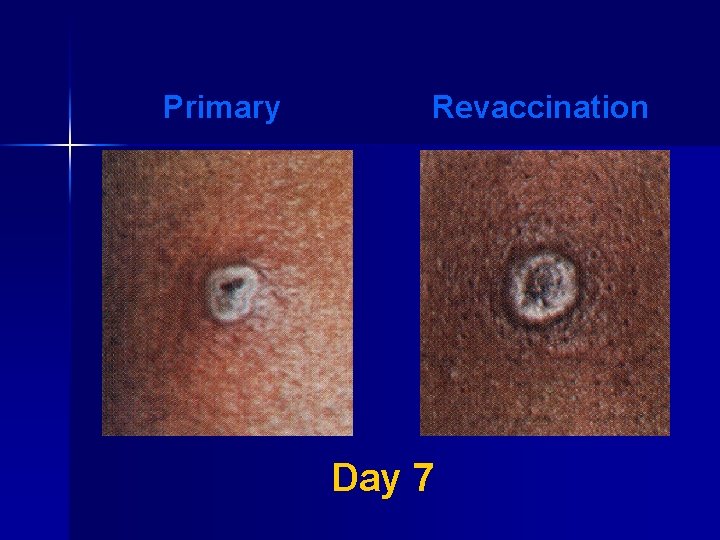
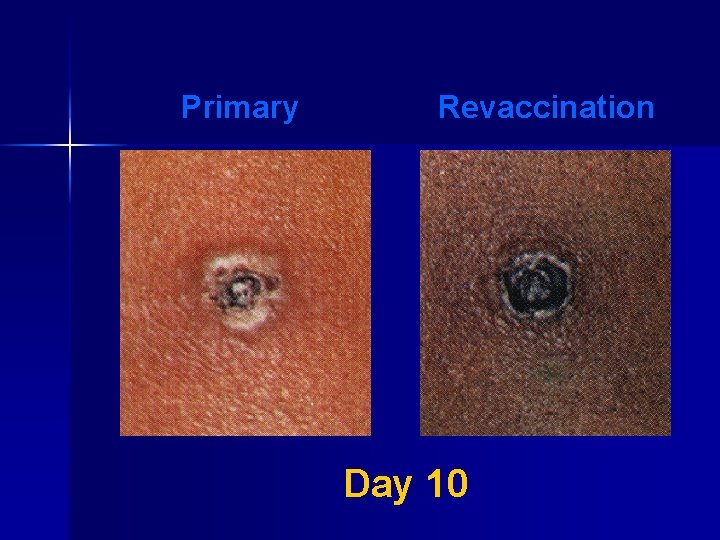
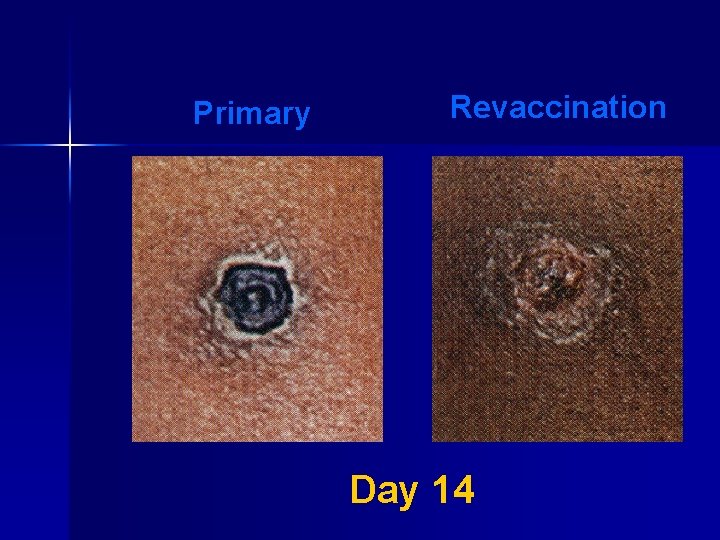
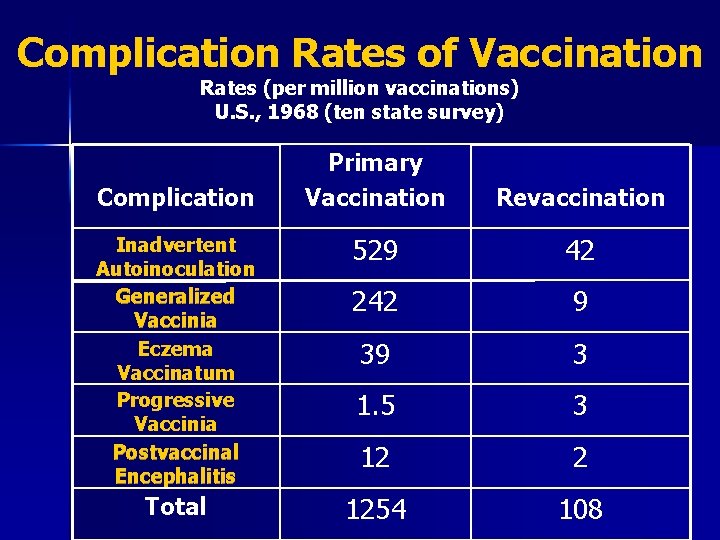
Major Reaction* (6 -8 days after vaccination) n Primary vaccination – Vesicular or pustular lesion – Area of definite palpable induration surrounding a central crust or ulcer n Revaccination – Less pronounced and more rapid progression – Pustular lesion or induration surrounding a central crust or ulcer *WHO Expert Committee on Smallpox, 1964

Primary Revaccination Day 3

Primary Revaccination Day 7

Primary Revaccination Day 10

Primary Revaccination Day 14

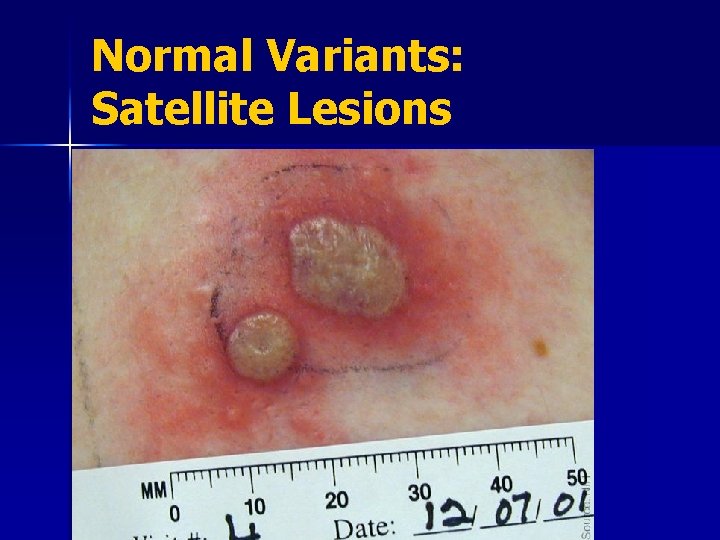
Normal Variants: Satellite Lesions

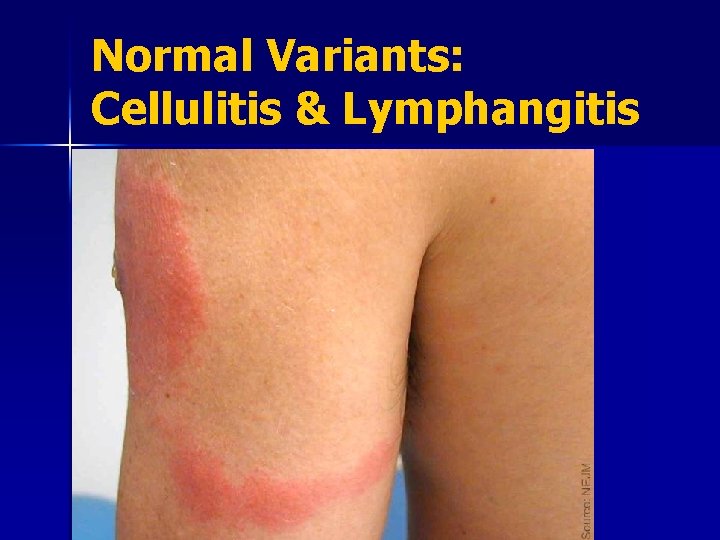
Normal Variants: Cellulitis & Lymphangitis

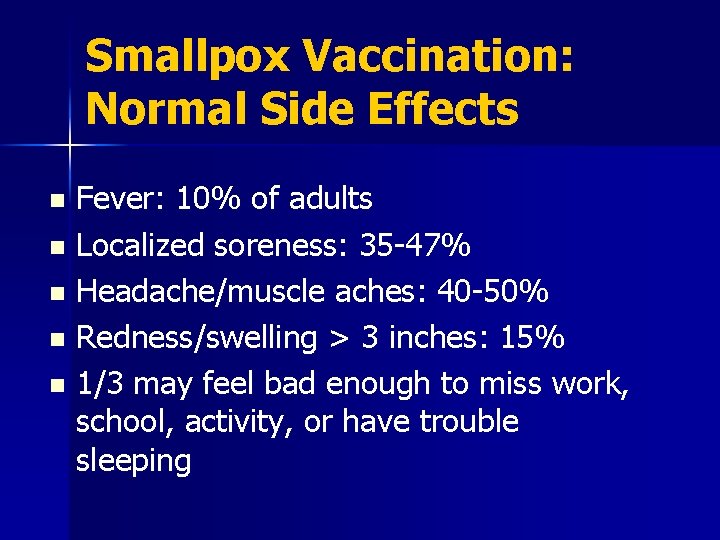
Smallpox Vaccination: Normal Side Effects Fever: 10% of adults n Localized soreness: 35 -47% n Headache/muscle aches: 40 -50% n Redness/swelling > 3 inches: 15% n 1/3 may feel bad enough to miss work, school, activity, or have trouble sleeping n

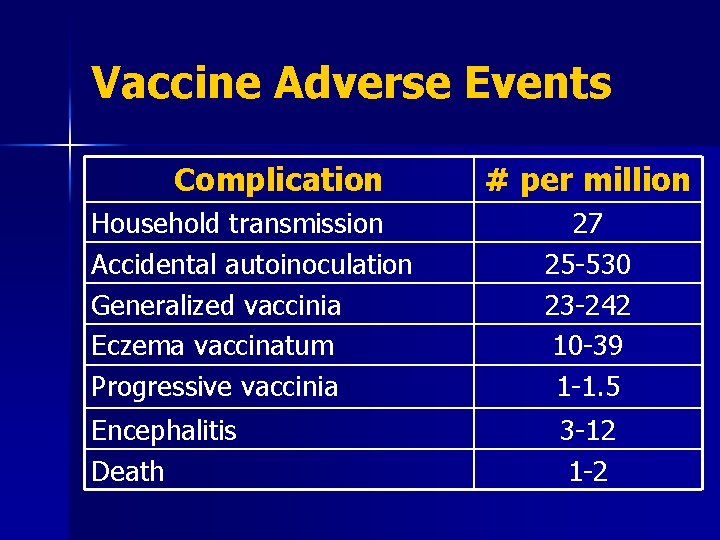
Smallpox Vaccination: Adverse Events n n n n Contact transmission: spread vaccinia to others Inadvertent autoinoculation: spread to other sites on body Generalized vaccinia: spread throughout body Eczema vaccinatum: severe skin reaction Progressive vaccinia (vaccinia necrosum) Postvaccinial encephalitis Death

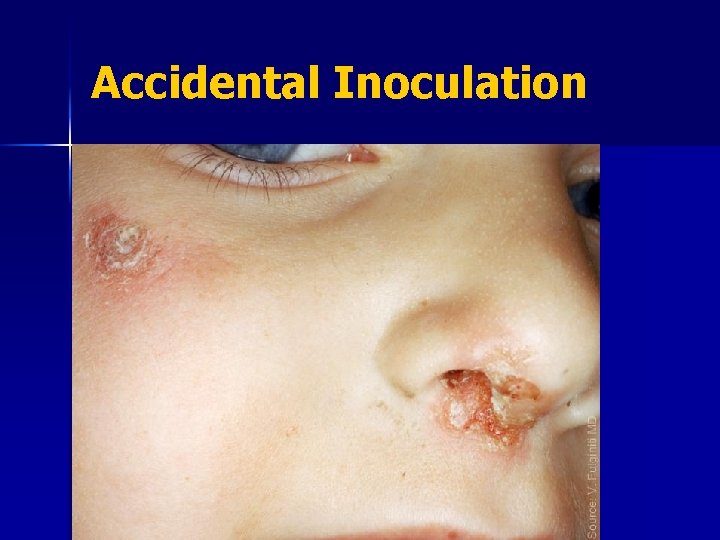
Accidental Inoculation Accidental auto-inoculation of cheek with vaccinia virus, approximately 5 days old. Primary take on arm, 10 -12 days old. Photo courtesy of John M. Leedom, MD.

Accidental Inoculation

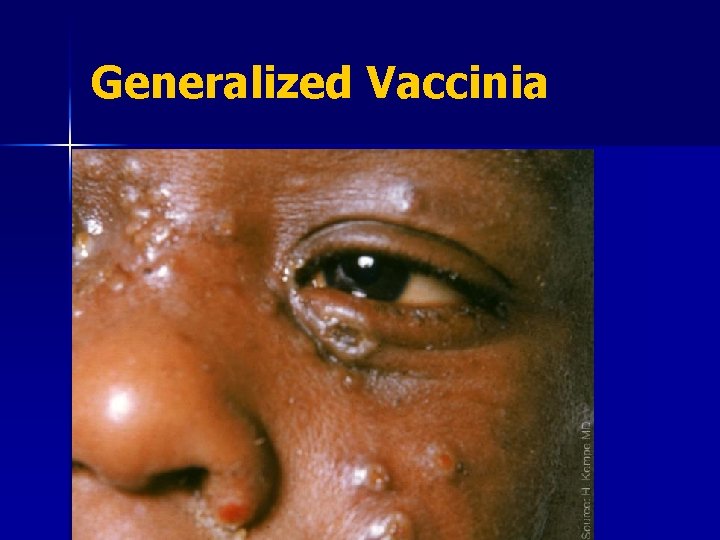
Generalized Vaccinia Generalized vaccinia in an apparently normal child. Recovered without sequelae. Photo courtesy of John M. Leedom, M. D.

Generalized Vaccinia

Eczema Vaccinatum

Eczema Vaccinatum

Progressive Vaccinia

Post-Vaccinial Encephalitis Autoimmune process n No predictors of susceptibility n Supportive care; no specific therapy n Vaccinia Immune Globulin is not effective and is not recommended. n 15 -25% mortality; and n 25% had permanent neurological sequelae n

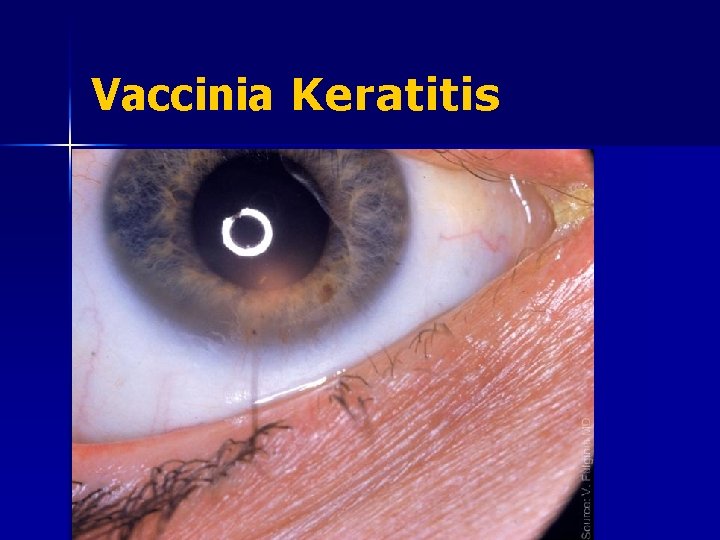
Vaccinia Keratitis

Vaccine Adverse Events Complication Household transmission Accidental autoinoculation Generalized vaccinia Eczema vaccinatum Progressive vaccinia Encephalitis Death # per million 27 25 -530 23 -242 10 -39 1 -1. 5 3 -12 1 -2

Complication Rates of Vaccination Rates (per million vaccinations) U. S. , 1968 (ten state survey) Primary Vaccination Revaccination Inadvertent Autoinoculation Generalized Vaccinia Eczema Vaccinatum Progressive Vaccinia Postvaccinal Encephalitis 529 42 242 9 39 3 1. 5 3 12 2 Total 1254 108 Complication

VIG: Vaccinia Immune Globulin n Indicated: – – Eczema vaccinatum Progressive vaccinia Generalized vaccinia (if severe or recurrent) Accidental implantation (ocular or extensive lesions) – – Accidental implantation (mild instances) Generalized vaccinia (mild or limited - most instances) Erythema multiforme Encephalitis Not Recommended: Contraindicated: – Vaccinia keratitis

Issues for Discussion n HIV testing n Pregnancy testing n Vaccination site care – who, how often? n Should healthcare provider continue to work? n Liability & workers’ compensation

“…it now becomes too manifest to admit of controversy, that the annihilation of the Small Pox, the most dreadful scourge of the human species, must be the final result of this practice. ” -Edward Jenner, 1801
 Smallpox vaccine inventor industrial revolution
Smallpox vaccine inventor industrial revolution Jennerian vesicle
Jennerian vesicle Overview of education in health care
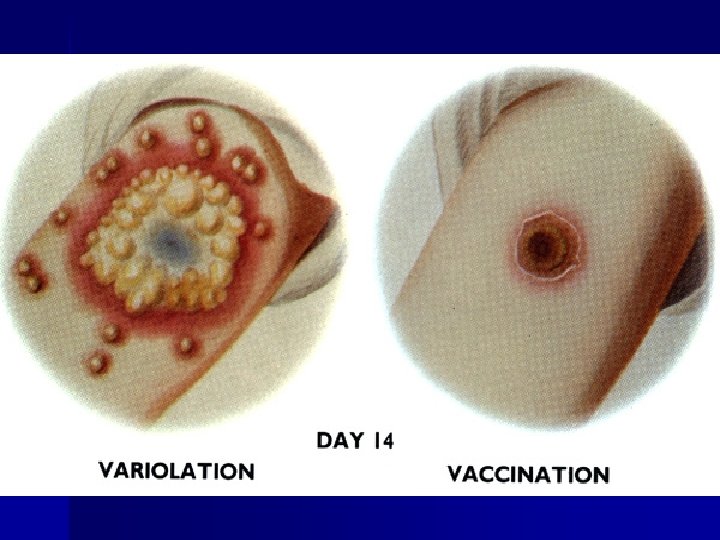
Overview of education in health care Smallpox vs chickenpox
Smallpox vs chickenpox Smallpox
Smallpox Smallpox disfigurement
Smallpox disfigurement Why was the cherokees last treaty a sham
Why was the cherokees last treaty a sham Smallpox endemic
Smallpox endemic Smallpox definition ap world history
Smallpox definition ap world history Edward jenner
Edward jenner Hazardous area response team
Hazardous area response team Primary secondary tertiary medical care
Primary secondary tertiary medical care Health and social care values unit 2
Health and social care values unit 2 Health and social care component 3 health and wellbeing
Health and social care component 3 health and wellbeing Natural and forced response
Natural and forced response Natural response and forced response example
Natural response and forced response example Primary immune response and secondary immune response
Primary immune response and secondary immune response Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Personlig tidbok för yrkesförare
Personlig tidbok för yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Att skriva en debattartikel
Att skriva en debattartikel För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Tryck formel
Tryck formel Offentlig förvaltning
Offentlig förvaltning Jag har nigit för nymånens skära text
Jag har nigit för nymånens skära text Presentera för publik crossboss
Presentera för publik crossboss Argument för teckenspråk som minoritetsspråk
Argument för teckenspråk som minoritetsspråk Vem räknas som jude
Vem räknas som jude Treserva lathund
Treserva lathund Fimbrietratt
Fimbrietratt Bästa kameran för astrofoto
Bästa kameran för astrofoto Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Lågenergihus nyproduktion
Lågenergihus nyproduktion Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referatmarkering
Referatmarkering Redogör för vad psykologi är
Redogör för vad psykologi är Matematisk modellering eksempel
Matematisk modellering eksempel Tack för att ni har lyssnat
Tack för att ni har lyssnat Borra hål för knoppar
Borra hål för knoppar Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Formel för standardavvikelse
Formel för standardavvikelse Tack för att ni har lyssnat
Tack för att ni har lyssnat Rita perspektiv
Rita perspektiv Verksamhetsanalys exempel
Verksamhetsanalys exempel Tobinskatten för och nackdelar
Tobinskatten för och nackdelar