Section 13 2 Using Gas Laws to Solve






















- Slides: 50

Section 13. 2 Using Gas Laws to Solve Problems Objectives 1. To understand the Ideal Gas Law and use it in calculations 2. To understand the relationship between the partial and total pressure of a gas mixture 3. To do calculations involving Dalton’s Law of partial pressures 4. To prepare for the Universal Gas Constant lab

Section 13. 2 Using Gas Laws to Solve Problems • • So far we have considered “what happens, ” but not “why. ” In science, “what” always comes before “why. ”

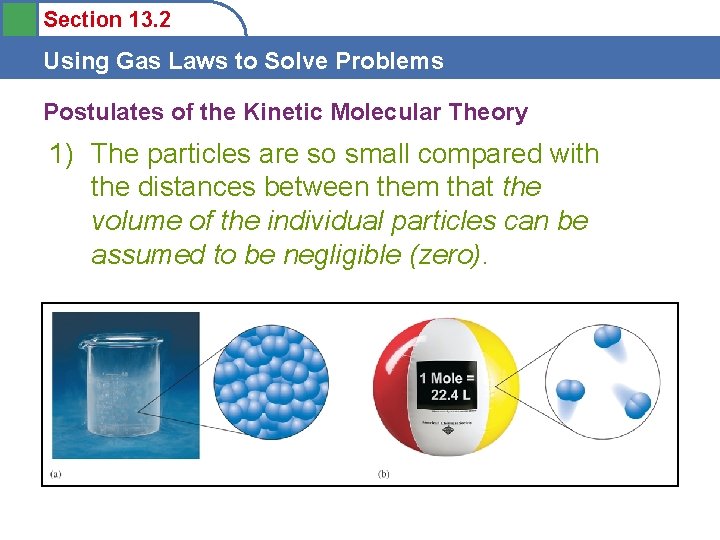
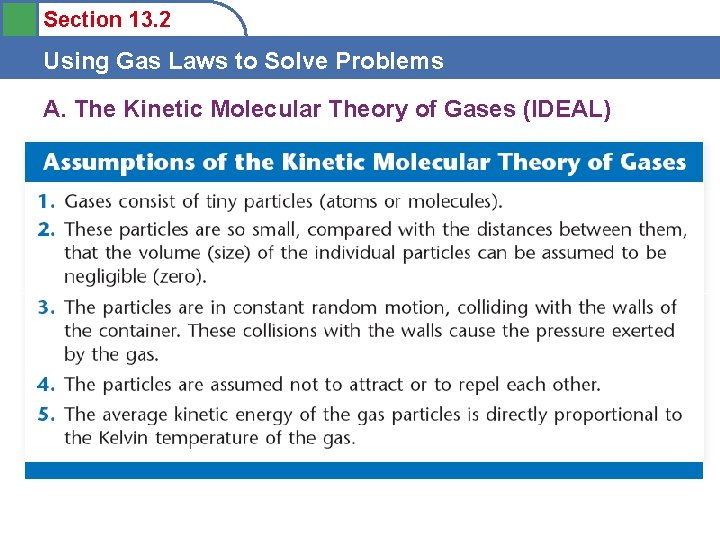
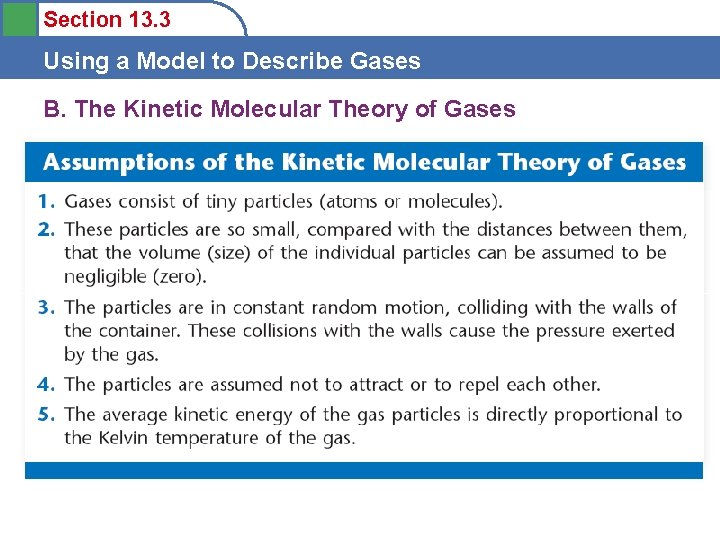
Section 13. 2 Using Gas Laws to Solve Problems Postulates of the Kinetic Molecular Theory 1) The particles are so small compared with the distances between them that the volume of the individual particles can be assumed to be negligible (zero).

Section 13. 2 Using Gas Laws to Solve Problems Postulates of the Kinetic Molecular Theory 2) The particles are in constant motion. The collisions of the particles with the walls of the container are the cause of the pressure exerted by the gas.

Section 13. 2 Using Gas Laws to Solve Problems Kinetic Molecular Theory

Section 13. 2 Using Gas Laws to Solve Problems Postulates of the Kinetic Molecular Theory 3) The particles are assumed to exert no forces on each other; they are assumed neither to attract nor to repel each other.

Section 13. 2 Using Gas Laws to Solve Problems Postulates of the Kinetic Molecular Theory 4) The average internal kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas. PHet gas behavior

Section 13. 2 Using Gas Laws to Solve Problems A. The Kinetic Molecular Theory of Gases (IDEAL)

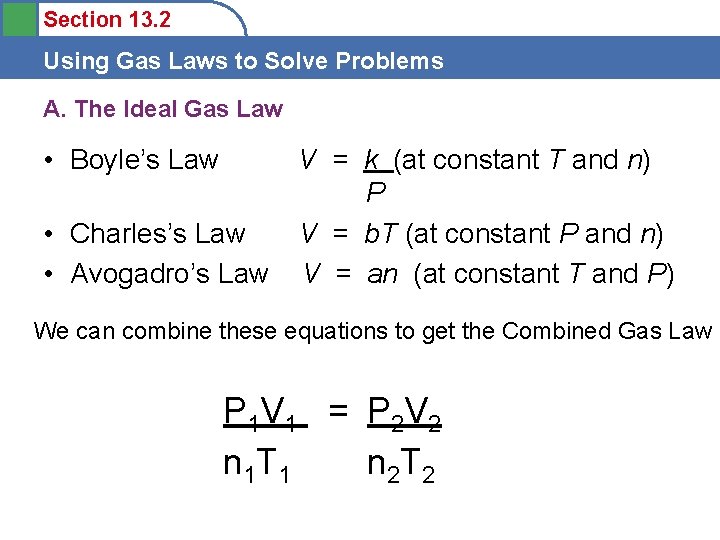
Section 13. 2 Using Gas Laws to Solve Problems A. The Ideal Gas Law • Boyle’s Law • Charles’s Law • Avogadro’s Law V = k (at constant T and n) P V = b. T (at constant P and n) V = an (at constant T and P) We can combine these equations to get the Combined Gas Law P 1 V 1 = P 2 V 2 n 1 T 1 n 2 T 2

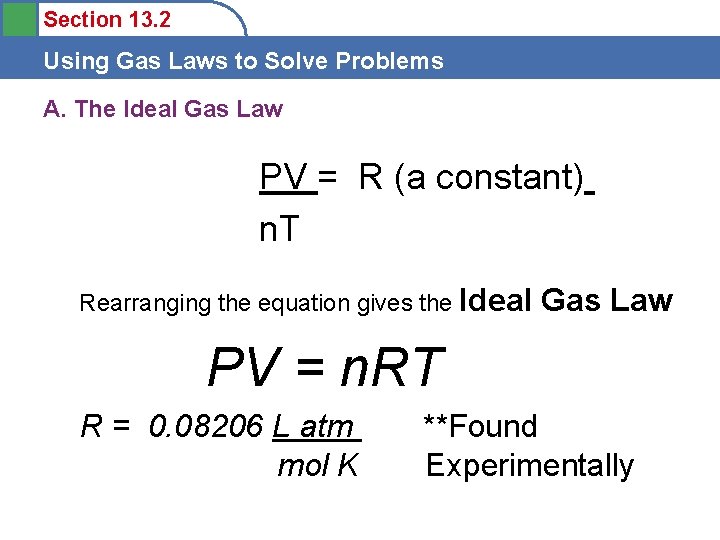
Section 13. 2 Using Gas Laws to Solve Problems A. The Ideal Gas Law PV = R (a constant) n. T Rearranging the equation gives the Ideal Gas Law PV = n. RT R = 0. 08206 L atm mol K **Found Experimentally

Section 13. 2 Using Gas Laws to Solve Problems A sample of hydrogen gas, H 2, has a volume of 8. 56 L at a temperature of 0 o. C and a pressure of 1. 5 atm. Calculate the number of moles of H 2 present in this gas sample. Assume that the gas behaves ideally. PV=n. RT P= V= n= R=

Section 13. 2 Using Gas Laws to Solve Problems PV=n. RT P= 1. 5 atm **UNITS** V= 8. 56 L n= ? R= 0. 08206 Latm/mol. K T= 0 o. C + 273 = 273 K n = PV RT n= (1. 5 atm)(8. 56 L) = 0. 57 mol (0. 08206 Latm/mol. K)(273 K)

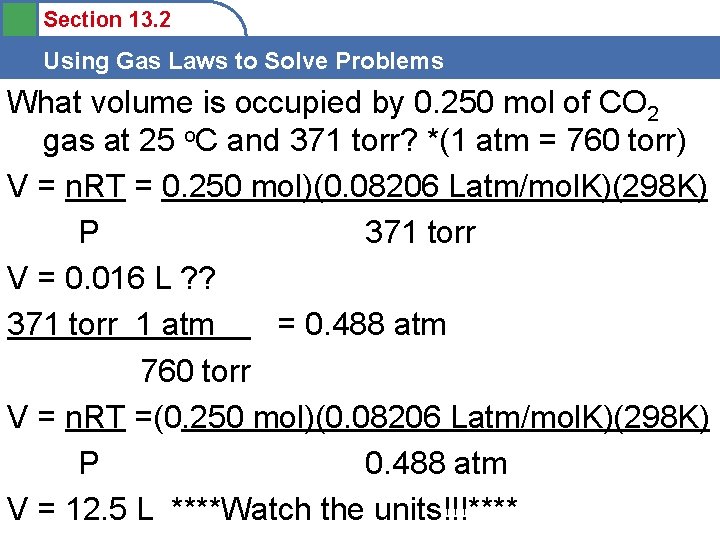
Section 13. 2 Using Gas Laws to Solve Problems What volume is occupied by 0. 250 mol of CO 2 gas at 25 o. C and 371 torr? PV=n. RT P= V= n= R= T= V=

Section 13. 2 Using Gas Laws to Solve Problems What volume is occupied by 0. 250 mol of CO 2 gas at 25 o. C and 371 torr? *(1 atm = 760 torr) PV=n. RT P= 371 torr V= ? n= 0. 250 mol R= 0. 08206 Latm/mol. K T= 25 o. C + 273 = 298 K V = n. RT P

Section 13. 2 Using Gas Laws to Solve Problems What volume is occupied by 0. 250 mol of CO 2 gas at 25 o. C and 371 torr? *(1 atm = 760 torr) V = n. RT = 0. 250 mol)(0. 08206 Latm/mol. K)(298 K) P 371 torr V = 0. 016 L ? ? 371 torr 1 atm = 0. 488 atm 760 torr V = n. RT =(0. 250 mol)(0. 08206 Latm/mol. K)(298 K) P 0. 488 atm V = 12. 5 L ****Watch the units!!!****

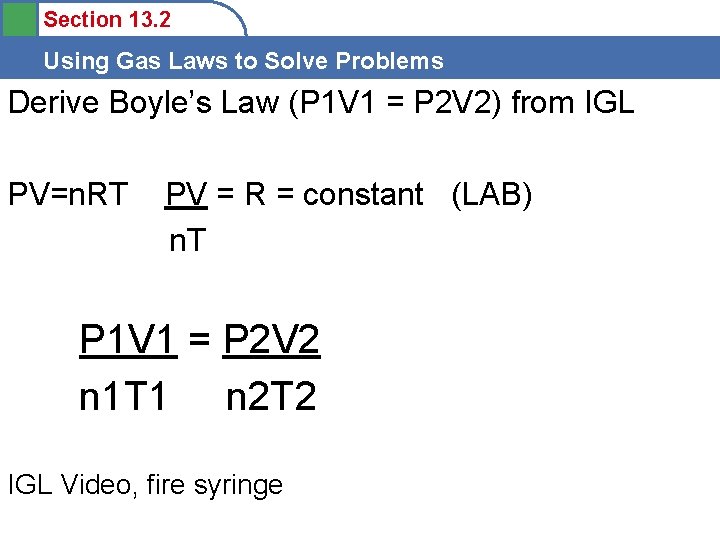
Section 13. 2 Using Gas Laws to Solve Problems Derive Boyle’s Law (P 1 V 1 = P 2 V 2) from IGL PV=n. RT PV = R = constant (LAB) n. T P 1 V 1 = P 2 V 2 n 1 T 1 n 2 T 2 IGL Video, fire syringe

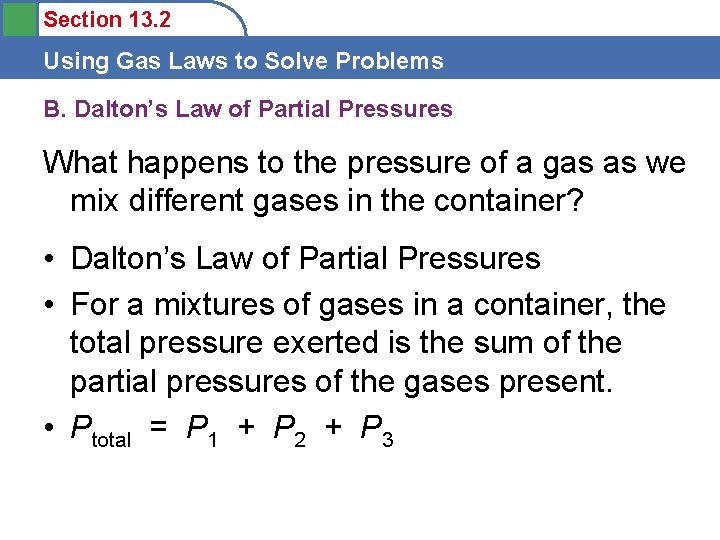
Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures What happens to the pressure of a gas as we mix different gases in the container? • Dalton’s Law of Partial Pressures • For a mixtures of gases in a container, the total pressure exerted is the sum of the partial pressures of the gases present. • Ptotal = P 1 + P 2 + P 3

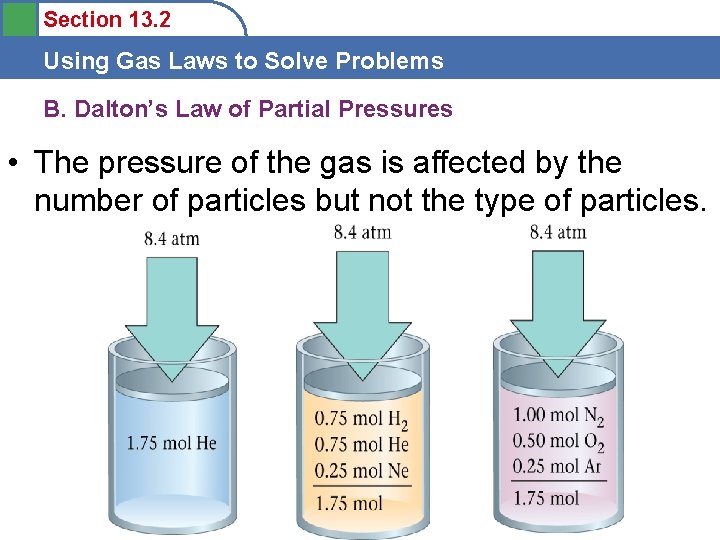
Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures • The pressure of the gas is affected by the number of particles but not the type of particles.

Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures Two crucial things we learn from this are: • The volume of the individual particles is not very important. • The forces among the particles must not be very important.

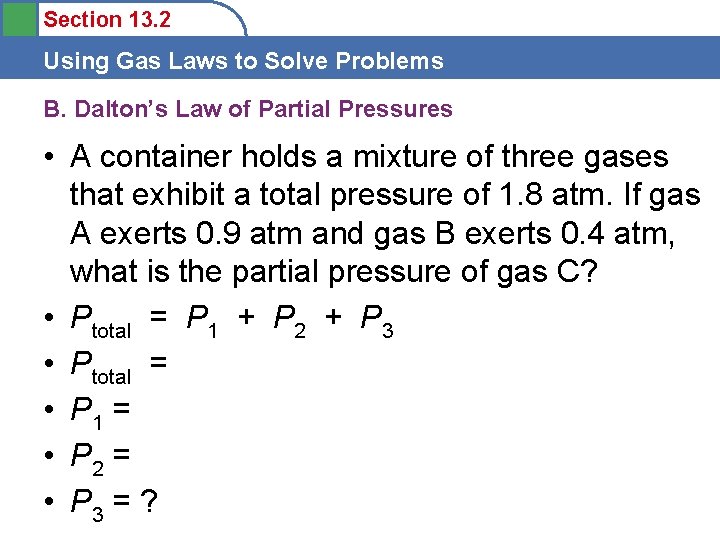
Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures • A container holds a mixture of three gases that exhibit a total pressure of 1. 8 atm. If gas A exerts 0. 9 atm and gas B exerts 0. 4 atm, what is the partial pressure of gas C? • Ptotal = P 1 + P 2 + P 3 • Ptotal = • P 1 = • P 2 = • P 3 = ?

Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures • • Ptotal = P 1 + P 2 + P 3 Ptotal = 1. 8 atm P 1 = 0. 9 atm P 2 = 0. 4 atm P 3 = ? P 3 = Ptotal - P 1 - P 2 P 3 = 1. 8 atm – 0. 9 atm – 0. 4 atm = 0. 5 atm

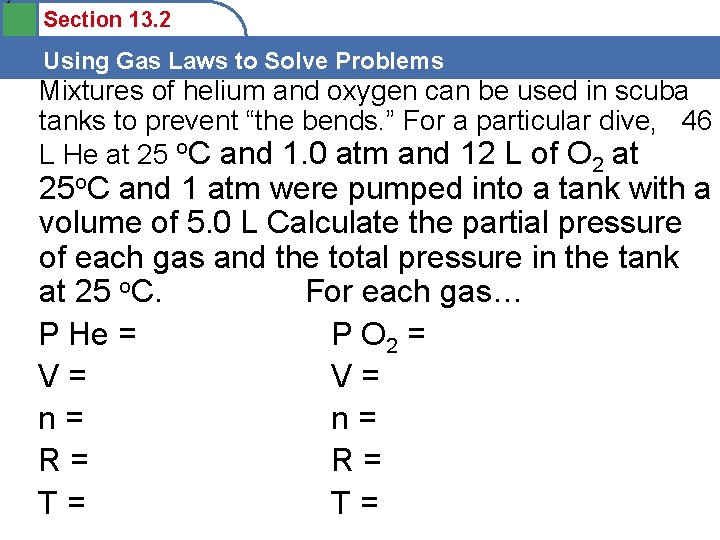
2 Section 13. 2 Using Gas Laws to Solve Problems Mixtures of helium and oxygen can be used in scuba tanks to prevent “the bends. ” For a particular dive, 46 L He at 25 o. C and 1. 0 atm and 12 L of O 2 at 25 o. C and 1 atm were pumped into a tank with a volume of 5. 0 L Calculate the partial pressure of each gas and the total pressure in the tank at 25 o. C. For each gas… P He = P O 2 = V= V= n= n= R= R= T= T=

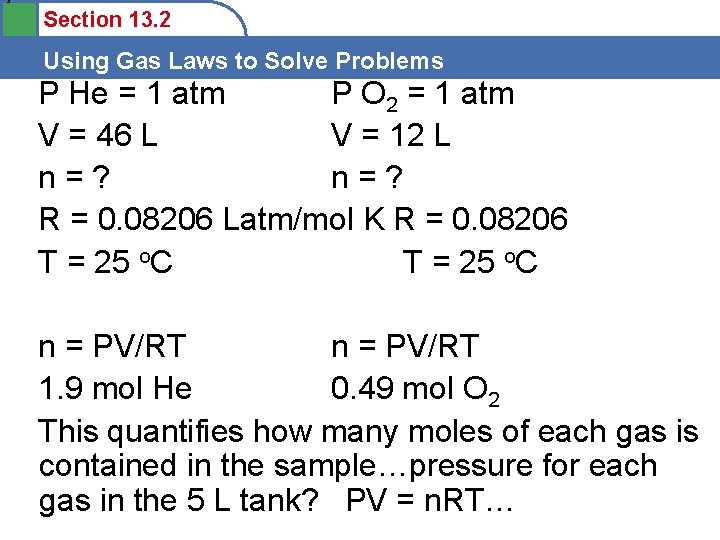
3 Section 13. 2 Using Gas Laws to Solve Problems P He = 1 atm P O 2 = 1 atm V = 46 L V = 12 L n=? R = 0. 08206 Latm/mol K R = 0. 08206 T = 25 o. C n = PV/RT 1. 9 mol He 0. 49 mol O 2 This quantifies how many moles of each gas is contained in the sample…pressure for each gas in the 5 L tank? PV = n. RT…

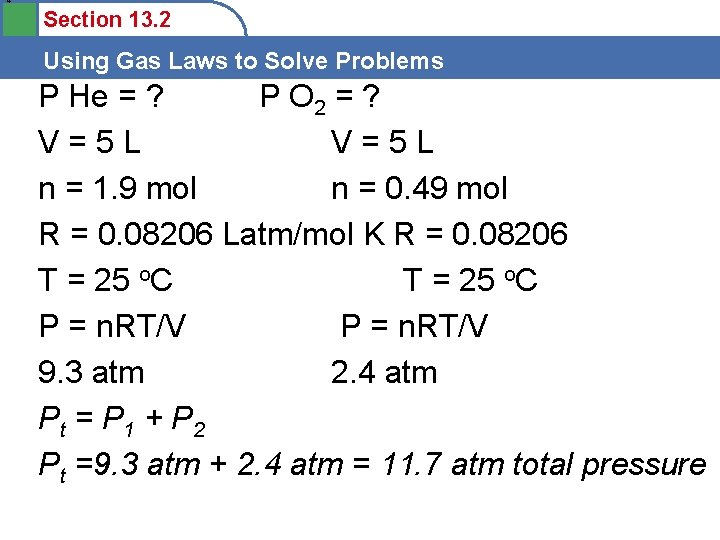
4 Section 13. 2 Using Gas Laws to Solve Problems P He = ? P O 2 = ? V=5 L n = 1. 9 mol n = 0. 49 mol R = 0. 08206 Latm/mol K R = 0. 08206 T = 25 o. C P = n. RT/V 9. 3 atm 2. 4 atm Pt = P 1 + P 2 Pt =9. 3 atm + 2. 4 atm = 11. 7 atm total pressure

5 Section 13. 2 Using Gas Laws to Solve Problems Exercise 27. 4 L of oxygen gas at 25. 0°C and 1. 30 atm, and 8. 50 L of helium gas at 25. 0°C and 2. 00 atm were pumped into a tank with a volume of 5. 81 L at 25°C. • Calculate the new partial pressure of oxygen. 6. 13 atm • Calculate the new partial pressure of helium. 2. 93 atm • Calculate the new total pressure of both gases. 9. 06 atm

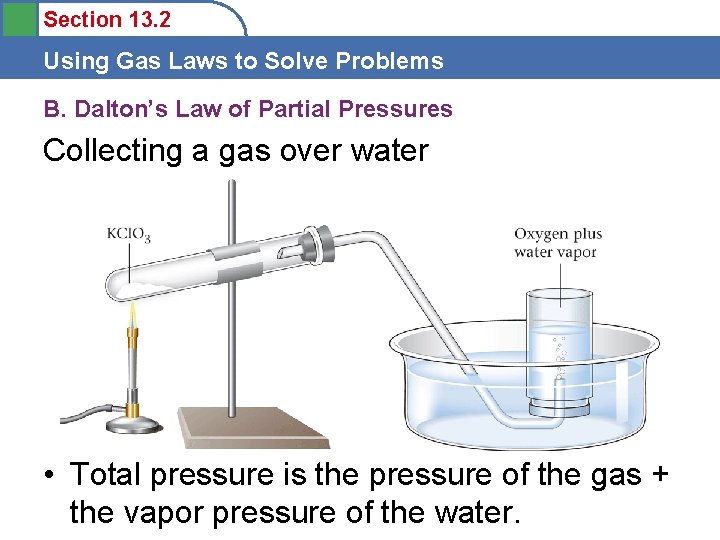
Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures Collecting a gas over water • Total pressure is the pressure of the gas + the vapor pressure of the water.

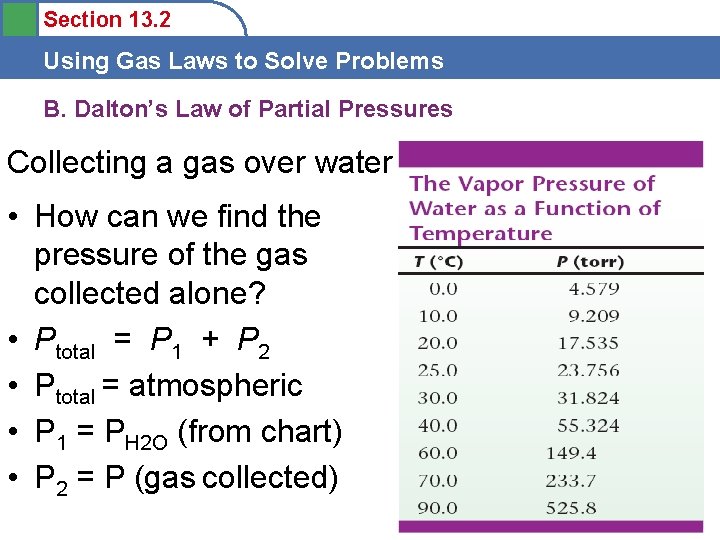
Section 13. 2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures Collecting a gas over water • How can we find the pressure of the gas collected alone? • Ptotal = P 1 + P 2 • Ptotal = atmospheric • P 1 = PH 2 O (from chart) • P 2 = P (gas collected)

Section 13. 2 Using Gas Laws to Solve Problems Objectives Review 1. To understand the ideal gas law and use it in calculations 2. To understand the relationship between the partial and total pressure of a gas mixture 3. To do calculations involving Dalton’s law of partial pressures 4. To prepare for the Universal Gas Constant lab 5. Work Session: 459 Practice Problem 13. 8 460 Practice Problem 13. 9 469 Practice Problem 13. 13 – PH 2 O ONLY 481 # 35, 36 PO 2 ONLY 473 13. 2 Review # 3

Section 13. 2 Using Gas Laws to Solve Problems Objectives 1. To double check for unit agreement when working the 5 -Step Problem Solving Method 2. To convert between pressure units using the unit analysis approach 3. To remember how to convert from g mol!!

Section 13. 2 Using Gas Laws to Solve Problems Pressure Unit Conversions, Unit Analysis Approach 1. 1 atm = 760 mm Hg 2. 760 mm Hg = 760 torr 3. 1 atm = 101, 325 Pa Convert 5. 2 atmospheres to mm Hg. 5. 2 atm = Convert 5. 2 atmospheres to torrs. 5. 2 atm =

Section 13. 2 Using Gas Laws to Solve Problems Pressure Unit Conversions, Unit Analysis Approach 1. 1 atm = 760 mm Hg 2. 760 mm Hg = 760 torr 3. 1 atm = 101, 325 Pa Convert 5. 2 atmospheres to Pa. 5. 2 atm = Convert 748 torrs to Pa 748 torr =

Section 13. 2 Using Gas Laws to Solve Problems g mol Conversions, Unit Analysis Approach Convert 5. 2 g CH 4 to mol CH 4 5. 2 g CH 4 = Convert 7. 48 g nitrogen gas to mol 7. 48 g =

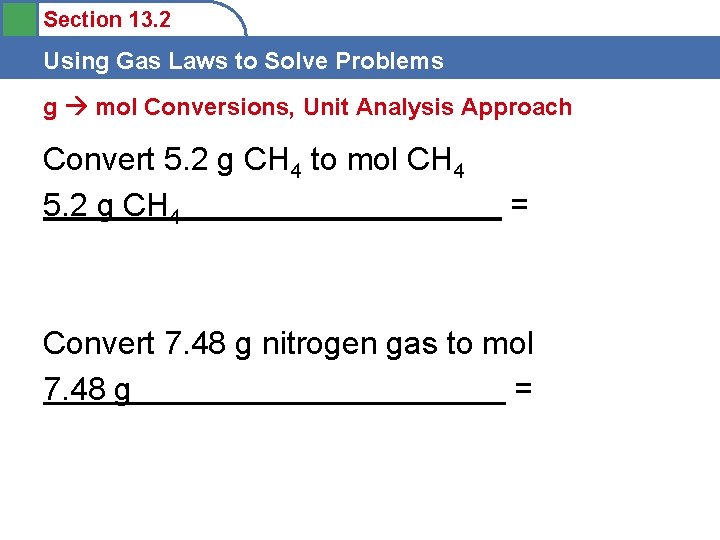
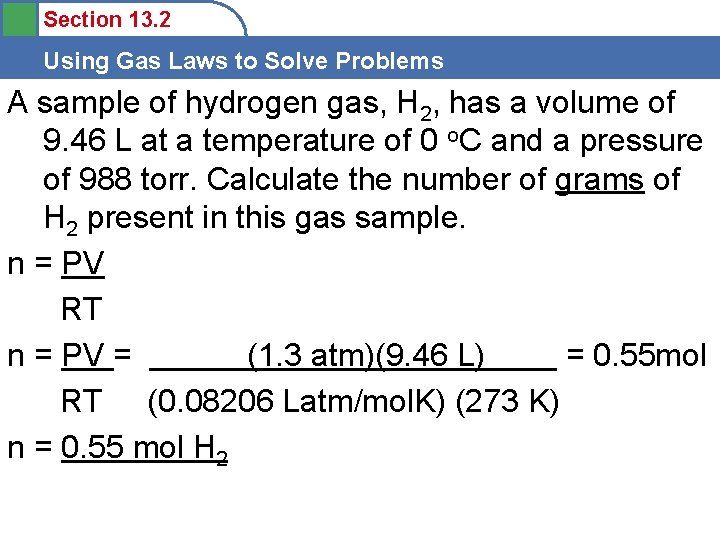
Section 13. 2 Using Gas Laws to Solve Problems A sample of hydrogen gas, H 2, has a volume of 9. 46 L at a temperature of 0 o. C and a pressure of 988 torr. Calculate the number of grams of H 2 present in this gas sample. PV=n. RT P= V= n= R= T=

Section 13. 2 Using Gas Laws to Solve Problems A sample of hydrogen gas, H 2, has a volume of 9. 46 L at a temperature of 0 o. C and a pressure of 988 torr. Calculate the number of grams of H 2 present in this gas sample. PV=n. RT P= 988 torr V= 9. 46 L n= ? R= 0. 08206 Latm/mol. K T= 0 o. C + 273 = 273 K

Section 13. 2 Using Gas Laws to Solve Problems A sample of hydrogen gas, H 2, has a volume of 9. 46 L at a temperature of 0 o. C and a pressure of 988 torr. Calculate the number of grams of H 2 present in this gas sample. n = PV RT n = PV = (1. 3 atm)(9. 46 L) = 0. 55 mol RT (0. 08206 Latm/mol. K) (273 K) n = 0. 55 mol H 2

Section 13. 2 Using Gas Laws to Solve Problems Objectives Review 1. To double check for unit agreement when working the 5 -Step Problem Solving Method 2. To convert between pressure units using the unit analysis approach 3. To remember how to convert from g mol!! 4. Work Session: Page 480 # 4 473 13. 2 Review # 2 (g mol)

Section 13. 3 Using a Model to Describe Gases Objectives 1. To understand the molar volume of an ideal gas 2. To learn the definition of STP 3. To understand the relationship between laws and models (theories) 4. To understand the postulates of the kinetic molecular theory 5. To understand temperature 6. To learn how the kinetic molecular theory explains the gas laws 7. To describe the properties of real gases

Section 13. 2 Using Gas Laws to Solve Problems A 1. Gas Stoichiometry Molar Volume • Standard temperature and pressure (STP) – 0 o. C and 1 atm • For one mole of a gas at STP • Molar volume of an ideal gas at STP 22. 4 L

9 Section 13. 2 Using Gas Laws to Solve Problems A sample of nitrogen gas has a volume of 1. 75 L at STP. How many moles of N 2 are present? PV=n. RT P= V= n= R= T= 0. 078 moles N 2

0 Section 13. 2 Using Gas Laws to Solve Problems Exercise A sample of oxygen gas has a volume of 2. 50 L at STP. How many grams of O 2 are present? 3. 57 g

Section 13. 3 Using a Model to Describe Gases A. Laws and Models (Theories) : A Review • A model (theory) is an approximation and is destined to be modified as we understand more or are able to better measure phenomena occurring around us. • A model (theory) can never be proved absolutely true.

Section 13. 3 Using a Model to Describe Gases B. The Kinetic Molecular Theory of Gases

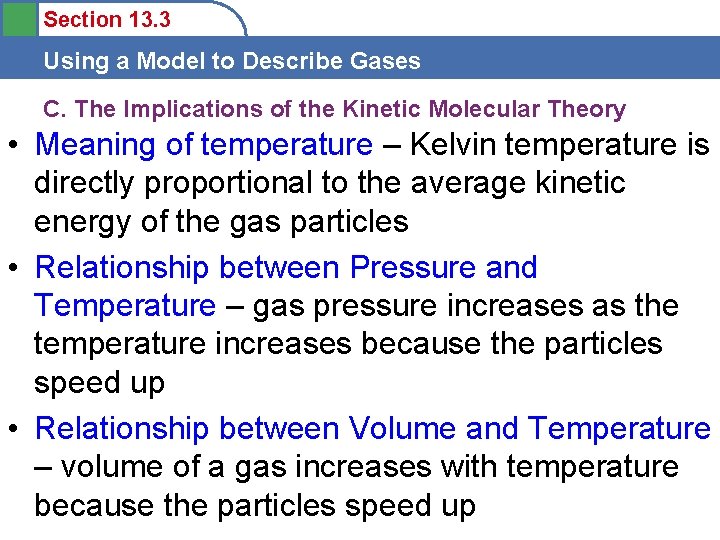
Section 13. 3 Using a Model to Describe Gases C. The Implications of the Kinetic Molecular Theory • Meaning of temperature – Kelvin temperature is directly proportional to the average kinetic energy of the gas particles • Relationship between Pressure and Temperature – gas pressure increases as the temperature increases because the particles speed up • Relationship between Volume and Temperature – volume of a gas increases with temperature because the particles speed up

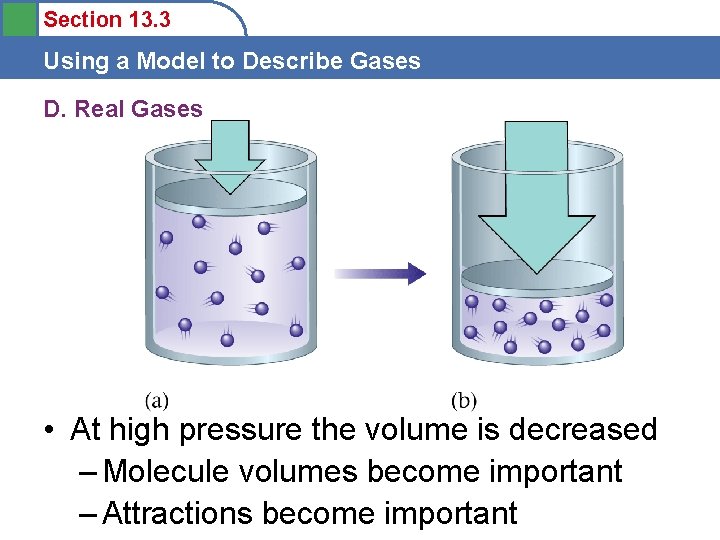
Section 13. 3 Using a Model to Describe Gases D. Real Gases • Gases do not behave ideally under conditions of high pressure and low temperature. • Why?

5 Section 13. 3 Using a Model to Describe Gases 1) The particles are so small compared with the distances between them that the volume of the individual particles can be assumed to be negligible (zero). 2) The particles are in constant motion. The collisions of the particles with the walls of the container are the cause of the pressure exerted by the gas. 3) 3) The particles are assumed to exert no forces on each other; they are assumed neither to attract nor to repel each other. 4) The average internal kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas.

Section 13. 3 Using a Model to Describe Gases B. The Kinetic Molecular Theory of Gases

Section 13. 3 Using a Model to Describe Gases D. Real Gases • At high pressure the volume is decreased – Molecule volumes become important – Attractions become important

Section 13. 3 Using a Model to Describe Gases D. Real Gases • Math Laws are still approximations…. . • PV = n. RT ……. • …. . or does it?

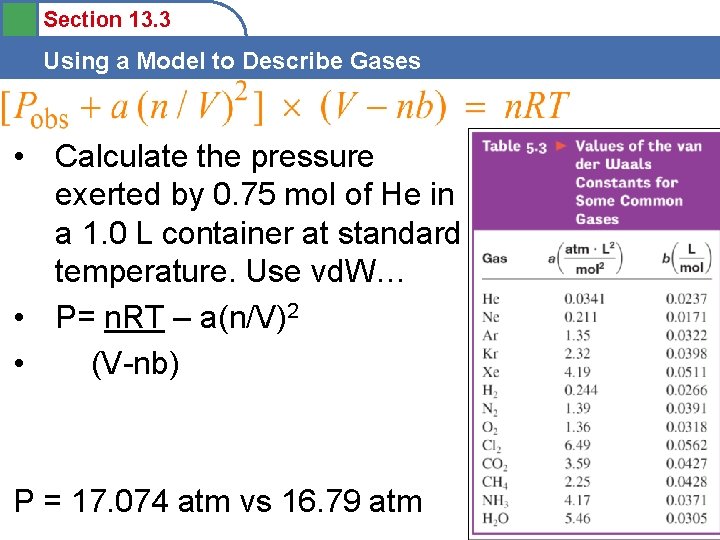
Section 13. 3 Using a Model to Describe Gases • Calculate the pressure exerted by 0. 75 mol of He in a 1. 0 L container at standard temperature. Use vd. W… • P= n. RT – a(n/V)2 • (V-nb) P = 17. 074 atm vs 16. 79 atm 49

Section 13. 3 Using a Model to Describe Gases Objectives Review 1. To understand the molar volume of an ideal gas 2. To learn the definition of STP 3. To understand the relationship between laws and models (theories) 4. To understand the postulates of the kinetic molecular theory 5. To understand temperature 6. To learn how the kinetic molecular theory explains the gas laws 7. To describe the properties of real gases (PHet) 8. Work session: Page 478 13. 3 Review # 1 - 5