Gas Laws Gas Pressure Just means that gas
























- Slides: 34

Gas Laws

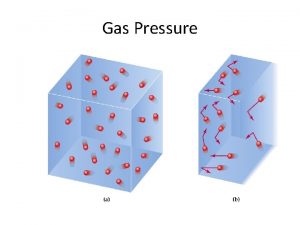
Gas Pressure Just means that gas is “pushing” on something.

Gas Pressure What’s going on inside? Air: Nitrogen 78% Oxygen 21% Argon ~1% Carbon Dioxide <1% Each of these particles are constantly flying around. Like a lotto ball! Tire They slam against the container and keep the tire “full”. The particles press against the walls.

Measuring Gas Pressure Air: Nitrogen 78% Oxygen 21% Argon ~1% Carbon Dioxide <1% Think of a giant ball pit miles and miles up. At the bottom of the ball pit, is like us walking around. That’s the atmospheric pressure.

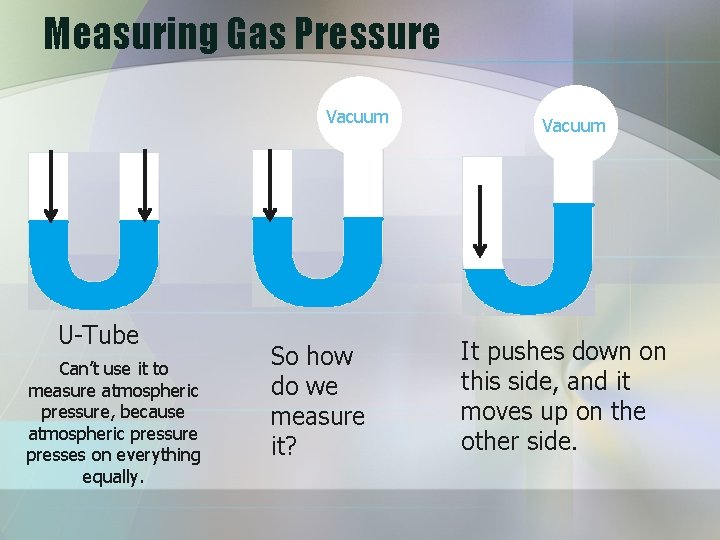
Measuring Gas Pressure Vacuum U-Tube Can’t use it to measure atmospheric pressure, because atmospheric pressure presses on everything equally. So how do we measure it? Vacuum It pushes down on this side, and it moves up on the other side.

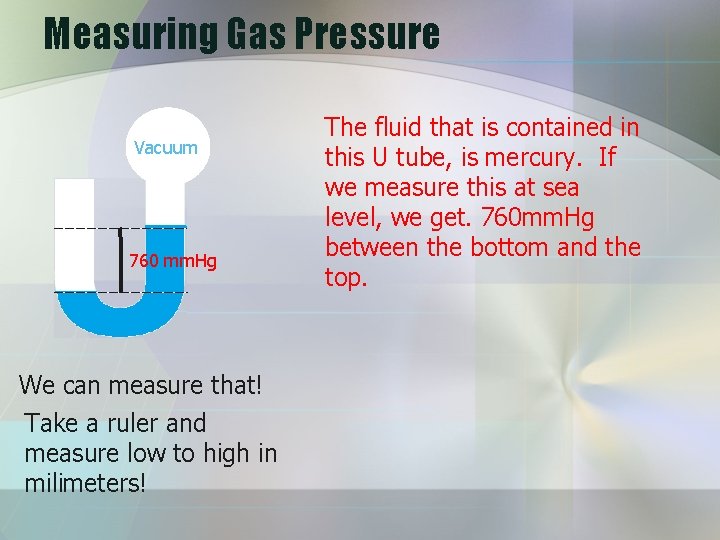
Measuring Gas Pressure Vacuum 760 mm. Hg We can measure that! Take a ruler and measure low to high in milimeters! The fluid that is contained in this U tube, is mercury. If we measure this at sea level, we get. 760 mm. Hg between the bottom and the top.

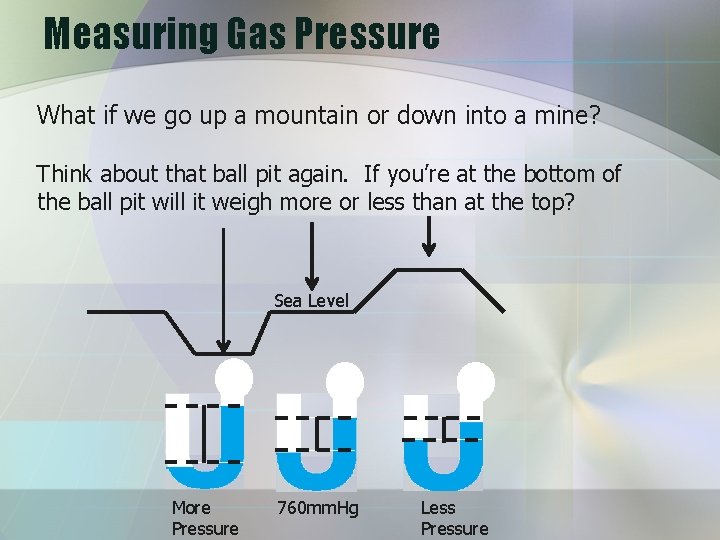
Measuring Gas Pressure What if we go up a mountain or down into a mine? Think about that ball pit again. If you’re at the bottom of the ball pit will it weigh more or less than at the top? Sea Level More Pressure 760 mm. Hg Less Pressure

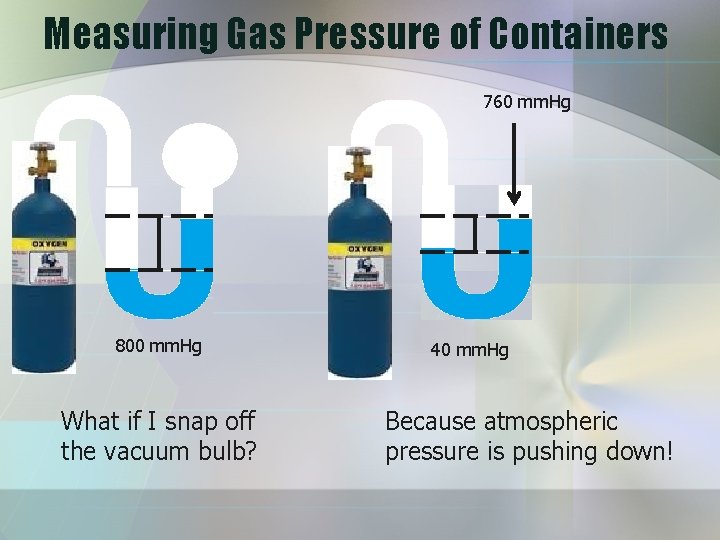
Measuring Gas Pressure of Containers 760 mm. Hg 800 mm. Hg What if I snap off the vacuum bulb? 40 mm. Hg Because atmospheric pressure is pushing down!

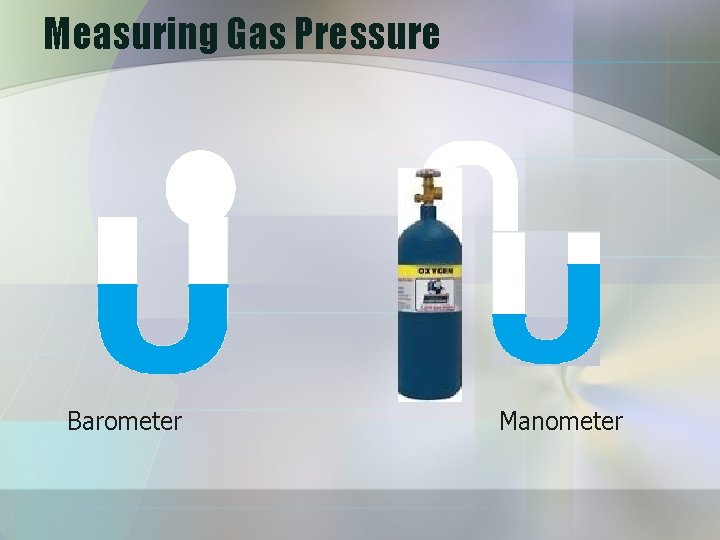
Measuring Gas Pressure Barometer Manometer

Gas Pressure Conversions How do we measure things? Lots of ways! Same goes with gas pressure. Gas Pressure Units mm. Hg atmosphere Torr atm Conversions 760 mm. Hg = 1 atm = 101. 3 kpa kilopascal k. Pa

Gas Pressure Conversions The pressure inside a car tire is 225 k. Pa. Express this value in both atm and mm. Hg. 760 mm. Hg = 1 atm = 101. 3 k. Pa 225 k. Pa x 1 atm =2. 22 atm 101. 3 k. Pa 225 k. Pa x 760 mm. Hg =1688 mm. Hg 101. 3 k. Pa

Boyle’s Law If we keep the temperature the same, we can predict what pressure and volume will do.

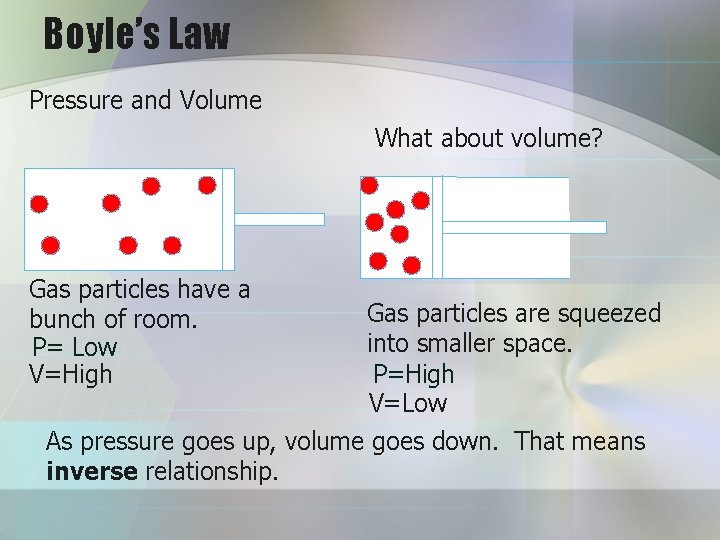
Boyle’s Law Pressure and Volume What about volume? Gas particles have a bunch of room. P= Low V=High Gas particles are squeezed into smaller space. P=High V=Low As pressure goes up, volume goes down. That means inverse relationship.

Boyle’s Teeter Totter Volume Pressure • When volume is high, pressure is low • When the volume is low, pressure is high • An Inverse relationship. Like when I buy clothes

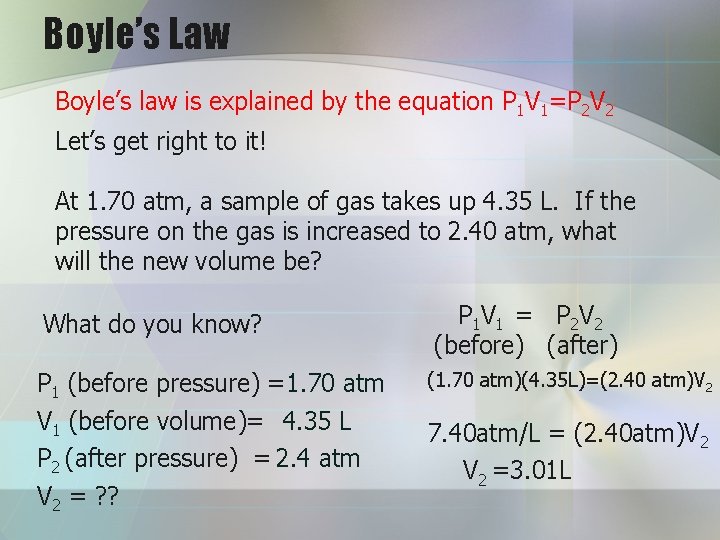
Boyle’s Law Boyle’s law is explained by the equation P 1 V 1=P 2 V 2 Let’s get right to it! At 1. 70 atm, a sample of gas takes up 4. 35 L. If the pressure on the gas is increased to 2. 40 atm, what will the new volume be? What do you know? P 1 V 1 = P 2 V 2 (before) (after) P 1 (before pressure) = 1. 70 atm (1. 70 atm)(4. 35 L)=(2. 40 atm)V 2 V 1 (before volume)= 4. 35 L P 2 (after pressure) = 2. 4 atm 7. 40 atm/L = (2. 40 atm)V 2 = ? ? V 2 =3. 01 L

Boyle’s Law Does that answer make sense? At 1. 70 atm, a sample of gas takes up 4. 35 L. If the pressure on the gas is increased to 2. 40 atm, what will the new volume be? We increased the pressure, so we pushed down that piston. We squeezed the molecules into a smaller space. So the volume should go down!

Boyle’s Law If I have 5. 6 liters of gas in a piston at a pressure of 1. 5 atm and compress the gas until its volume is 4. 8 L, what will the new pressure inside the piston be? P 1 V 1 = P 2 V 2 (before) (after) P 1 (before pressure) = 1. 5 atm V 1 (before volume)= 5. 6 L (1. 5 atm)(5. 6 L) = (P 2)(4. 8 L) ? P 2 (after pressure) = 8. 4 atm/L = (4. 8 L)P 2 V 2 = 4. 8 L 1. 8 atm = P 2

Charles’ Law Charles’ law relates volume and temperature, while keeping pressure the same V 1 = V 2 T 1 T 2

Charles’ Law How could we test theory that temperature and volume are related? Think about kinetic theory and molecules.

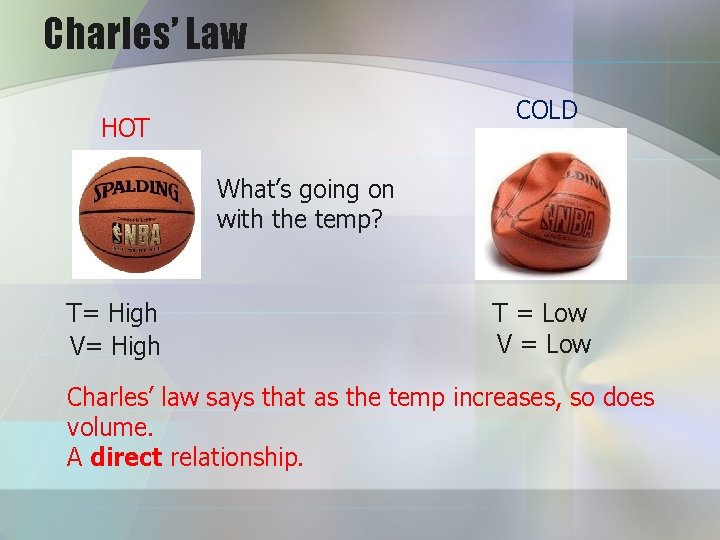
Charles’ Law COLD HOT What’s going on with the temp? T= High V= High T = Low V = Low Charles’ law says that as the temp increases, so does volume. A direct relationship.

Charles’ Law So now we can relate volume and temperature. V 1 = V 2 T 1 T 2 MUST ALWAYS USE KELVIN TEMPERATURE in gas laws A balloon takes up 625 L at 0°C. If it is heated to 80°C, what will its new volume be? V 1 = 625 L T 1 = 0 °C T 2 = 80 °C V 2 = ? ? Must convert to Kelvin. 0 °C + 273 = 273 K 80 °C + 273 = 353 K

Charles’ Law A balloon takes up 625 L at 0°C. If it is heated to 80°C, what will its new volume be? V 1 = V 2 T 1 T 2 V 1 = 625 L T 1 = 273 K T 2 = 353 K V 2 = ? ? L 625 L = V 2 273 K 353 K 2. 29 L/K= V 2 353 K 808 L = V 2

Charles’ Law At 27. 00 °C a gas has a volume of 6. 00 L. What will the volume be at 150. 0 °C? V 1 = V 2 What’s the equation? T 1 T 2 V 1= 6. 00 L T 1= 27 °C V 2= ? ? T 2= 150. 0 °C Must convert to Kelvin. 27 °C + 273 = 300 K 150°C + 273 = 423 K

Charles’ Law At 27. 00 °C a gas has a volume of 6. 00 L. What will the volume be at 150. 0 °C? V 1 = V 2 T 1 T 2 V 1= 6. 00 L T 1= 300 K V 2= ? ? T 2= 423 K 6. 00 L = V 2 300 K 423 K 0. 02 L/K = V 2 423 K 8. 46 L = V 2

Avogadro’s Law Relationship between: Amount of gas (n) and the Volume. What happens to one, when I change the other? I start with the first balloon, and then blow more air into it…will the volume increase? Yes, a direct relationship

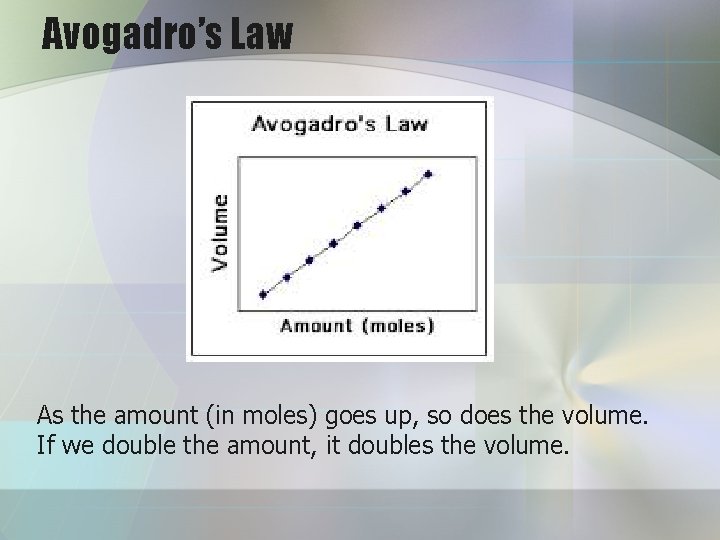
Avogadro’s Law As the amount (in moles) goes up, so does the volume. If we double the amount, it doubles the volume.

Avogadro’s Law We only changed TWO things. The volume and the amount of particles. We didn’t mess with the pressure or the temperature, they were held constant. n 1 = n 2 V 1 V 2

Avogadro’s Law n 1 = n 2 V 1 V 2 n 1= 50 g V 1 = 40 L Let’s try! In a sample of gas, 50. 0 g of oxygen gas (O 2) take up 48 L of volume. Keeping the pressure constant, the amount of gas is changed until the volume is 79 L. How many mols of gas are now in the container? n 2 = mol? V 2 = 79 L When doing Avogadro's law, “n” MUST be in moles!

Avogadro’s Law n 1 = n 2 V 1 V 2 Before 1. 6 mol n 1=50 g V 1 = 48 L After n 2 = g? V 2 = 79 L When doing Avogadro's law, “n” MUST be in moles! 50 g O 2 x 1 mol O 2 = 1. 6 mol O 2 32 g O 2 1. 6 mol O 2 = n 2 48 L 79 L 0. 03 = n 2 79 L 2. 6 mol = n 2

Gay Lussac’s Law This law only applies to gases held at a constant volume. Only the pressure and temperature will change. Pi = Pf Ti Tf Pi =initial pressure Pf = final pressure Ti = initial temperature (kelvin) Tf = final temperature (kelvin) The pressure in a sealed can of gas is 235 k. Pa when it sits at room temperature (20 C). If the can is warmed to 48 C, what will the new pressure inside the can be?

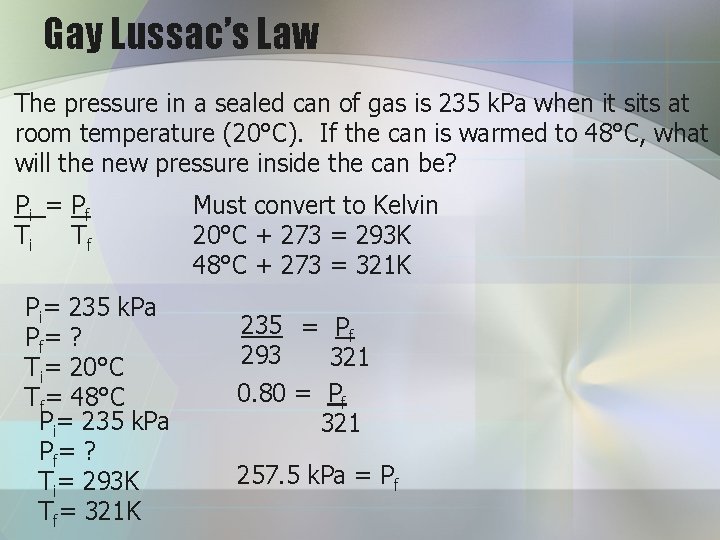
Gay Lussac’s Law The pressure in a sealed can of gas is 235 k. Pa when it sits at room temperature (20°C). If the can is warmed to 48°C, what will the new pressure inside the can be? Pi = Pf Ti Tf Pi= 235 k. Pa Pf= ? Ti= 20°C Tf= 48°C Pi= 235 k. Pa Pf= ? Ti= 293 K Tf= 321 K Must convert to Kelvin 20°C + 273 = 293 K 48°C + 273 = 321 K 235 = Pf 293 321 0. 80 = Pf 321 257. 5 k. Pa = Pf

How to use these formulas Charle’s Law V 1 = V 2 T 1 T 2 Avogadro’s Law V 1 = V 2 n 1 n 2 Gay Lussac’s Law P 1 = P 2 T 1 T 2 They are all pretty much the same equation, just different variables! Write the equations for each variable and for each law.

Combined Gas Law Charle’s Law V 1 = V 2 T 1 T 2 Boyle’s Law (P 1)(V 1) = (P 2)(V 2) Gay Lussac’s Law P 1 = P 2 T 1 T 2 What if I had a balloon. I wanted to increase the pressure and cool it down. What is the volume? Do we have an equation for that? P, T, V. I can combine the laws! Combined Gas Law (P 1)(V 1) = (P 2)(V 2) T 1 T 2

A 40. 0 L balloon is filled with air at sea level (1. 00 atm, 25. 0 °C). It's tied to a rock and thrown in a a cold body of water, and it sinks to the point where the temperature is 4. 0 ° C and the pressure is 11. 00 atm. What will its new volume be? Convert to Kelvin (P 1)(V 1) = (P 2)(V 2) 25°C + 273 = 298 K T 1 T 2 4°C + 273 = 277 K P 1= 1 atm P 2= 11 Patm 2= 11 atm V 1= 40 VL 1= 40 L V 2= ? ? T 1= 25 T°C 1= 298 K T 2= 4 °C T 2= 277 K (1)(40) = (11)(V 2) 298 K 277 K 0. 13 = (11)(V 2) 277 K 36. 01 = (11)(V 2) 3. 27 L = V 2
 Phân độ lown
Phân độ lown Block av độ 1
Block av độ 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Who is present when juliet awakens
Who is present when juliet awakens Charles de secondat
Charles de secondat Pressure tolerant vs pressure sensitive
Pressure tolerant vs pressure sensitive Pressure support vs pressure control
Pressure support vs pressure control Pressure mapping for pressure ulcers
Pressure mapping for pressure ulcers Intrapulmonary pressure vs intrapleural pressure
Intrapulmonary pressure vs intrapleural pressure Starling's equation
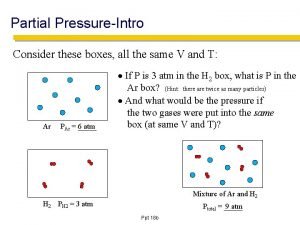
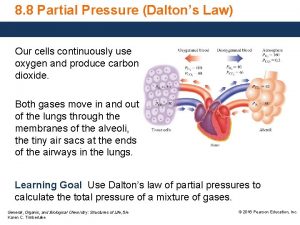
Starling's equation Partial pressure
Partial pressure Intrapleural pressure
Intrapleural pressure Confining pressure vs directed pressure
Confining pressure vs directed pressure Sore throat after surgery
Sore throat after surgery Stream line equation
Stream line equation Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Oncotic pressure
Oncotic pressure Difference between osmotic and oncotic pressure
Difference between osmotic and oncotic pressure Hydrostatic oncotic pressure
Hydrostatic oncotic pressure Dynamothermal
Dynamothermal How is blood pressure regulated
How is blood pressure regulated How to find partial pressure from total pressure
How to find partial pressure from total pressure Ventilator pressure support
Ventilator pressure support Bevel of et tube
Bevel of et tube What is low pressure
What is low pressure Poly means many and gon means
Poly means many and gon means Meta means and morphe means
Meta means and morphe means Meta and morph means
Meta and morph means