Please note these are the actual videorecorded proceedings











![J Clin Oncol 2019; [Epub ahead of print]. J Clin Oncol 2019; [Epub ahead of print].](https://slidetodoc.com/presentation_image/4b785af8faedb3c82f97ed92b7616f92/image-36.jpg)


- Slides: 44

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Addressing Current Questions and Controversies in the Management of Gastrointestinal Cancers Saturday, June 1, 2019 7: 00 PM – 9: 30 PM Chicago, Illinois Faculty Professor Dirk Arnold, MD, Ph. D Tanios Bekaii-Saab, MD Joseph Chao, MD Anthony El-Khoueiry, MD Richard S Finn, MD Yelena Y Janjigian, MD Scott Kopetz, MD, Ph. D Eileen M O’Reilly, MD Moderator Neil Love, MD

Module 4 – Colorectal Cancer (CRC)

Professor Dirk Arnold, MD, Ph. D Director Asklepios Tumorzentrum Hamburg AK Altona Hamburg, Germany

Disclosures for Professor Dirk Arnold, MD, Ph. D No financial interests or affiliations to disclose.

Scott Kopetz, MD, Ph. D Associate Professor Department of Gastrointestinal Medical Oncology Division of Cancer Medicine The University of Texas MD Anderson Cancer Center Houston, Texas

Disclosures for Scott Kopetz, MD, Ph. D Consulting Agreements Amgen Inc, Bayer Health. Care Pharmaceuticals, Genentech

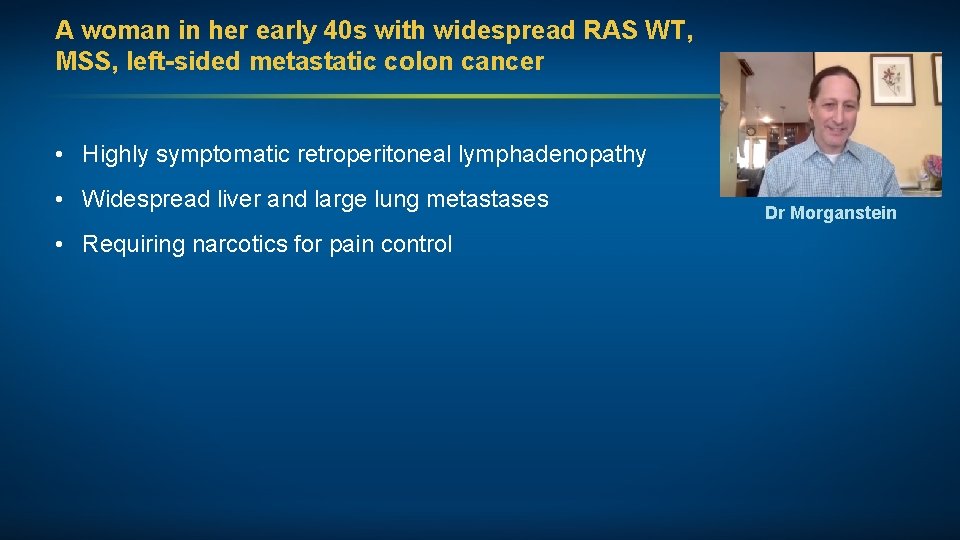
A woman in her early 40 s with widespread RAS WT, MSS, left-sided metastatic colon cancer • Highly symptomatic retroperitoneal lymphadenopathy • Widespread liver and large lung metastases • Requiring narcotics for pain control Dr Morganstein

2/2019: Before FOLFIRI/cetuximab 4/2019: After FOLFIRI/cetuximab

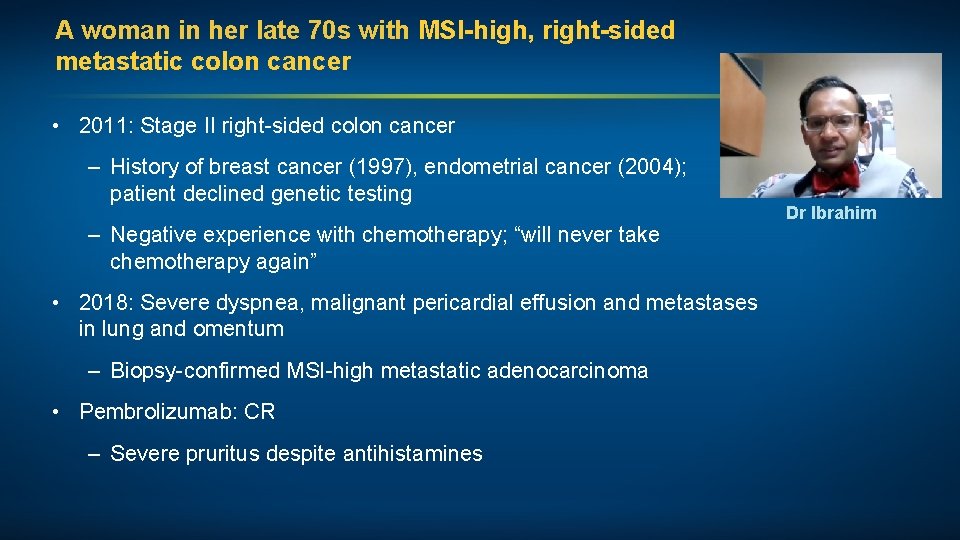
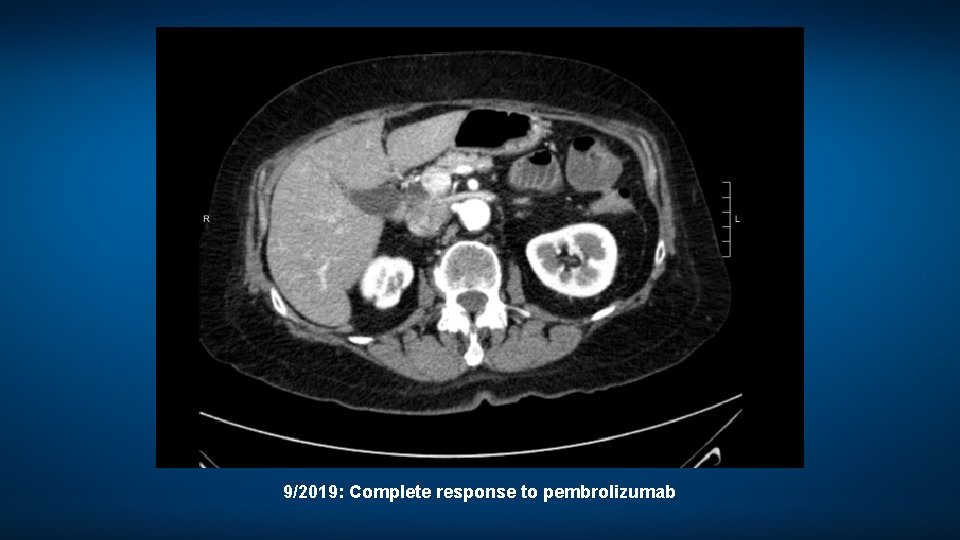
A woman in her late 70 s with MSI-high, right-sided metastatic colon cancer • 2011: Stage II right-sided colon cancer – History of breast cancer (1997), endometrial cancer (2004); patient declined genetic testing – Negative experience with chemotherapy; “will never take chemotherapy again” • 2018: Severe dyspnea, malignant pericardial effusion and metastases in lung and omentum – Biopsy-confirmed MSI-high metastatic adenocarcinoma • Pembrolizumab: CR – Severe pruritus despite antihistamines Dr Ibrahim

5/2018: Malignant pericardial effusion

9/2019: Complete response to pembrolizumab

A woman in her late 80 s with RAS WT, BRAF V 600 Emutated, MSS, right-sided metastatic colon cancer • 7/2018: Two separate colon tumors with mucinous differentiation (p. T 3 N 0, 0/40 positive nodes) – History of Stage I breast cancer in 2008; strong family history of cancer in grandmothers and aunts (genetic testing pending) – CHF with pacemaker • 10/2018: Metastatic colon cancer to the mesentery • FOLFOX: Partial response disease progression after 5 cycles • 1/2019: Cetuximab, encorafenib and binimetinib Dr Favaro

Module 4 – Colorectal Cancer (CRC)

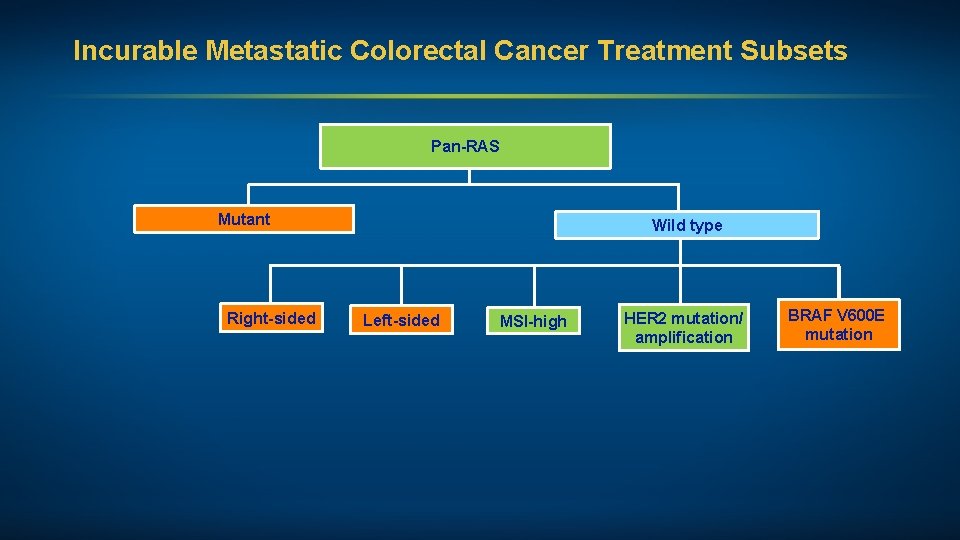
Incurable Metastatic Colorectal Cancer Treatment Subsets Pan-RAS Mutant Right-sided Wild type Left-sided MSI-high HER 2 mutation/ amplification BRAF V 600 E mutation

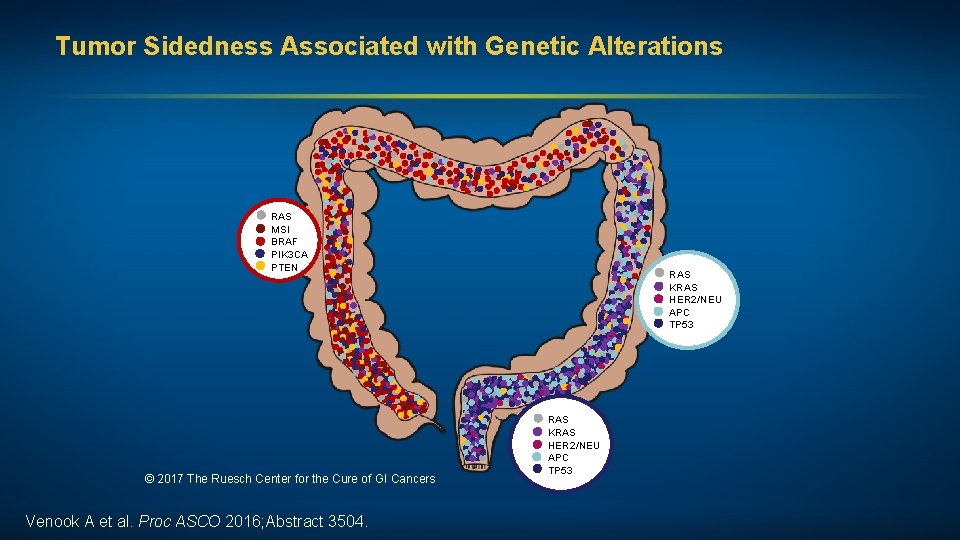
Tumor Sidedness Associated with Genetic Alterations RAS MSI BRAF PIK 3 CA PTEN © 2017 The Ruesch Center for the Cure of GI Cancers Venook A et al. Proc ASCO 2016; Abstract 3504. RAS KRAS HER 2/NEU APC TP 53

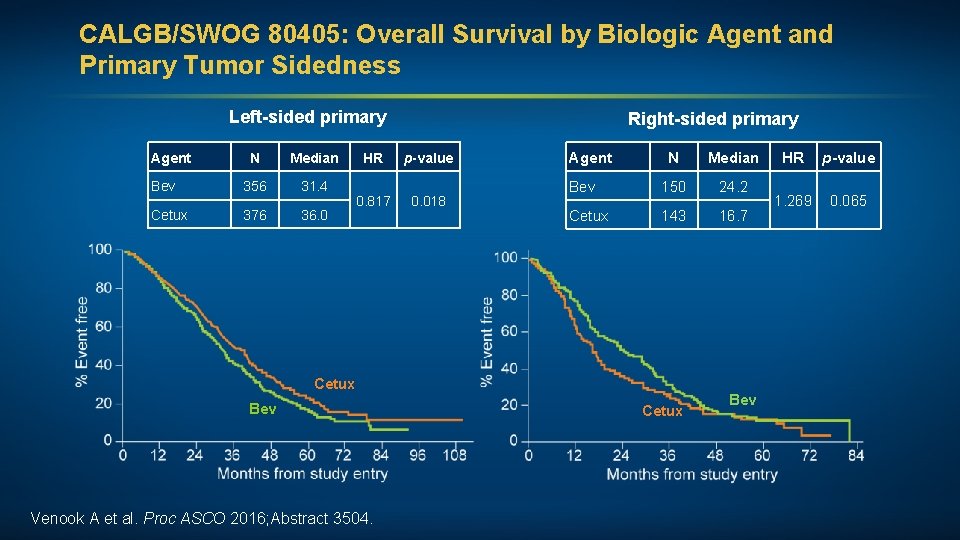
CALGB/SWOG 80405: Overall Survival by Biologic Agent and Primary Tumor Sidedness Left-sided primary Agent N Median Bev 356 31. 4 Cetux 376 36. 0 HR 0. 817 Right-sided primary p-value 0. 018 Agent N Median Bev 150 24. 2 Cetux 143 16. 7 Cetux Bev Venook A et al. Proc ASCO 2016; Abstract 3504. Cetux Bev HR p-value 1. 269 0. 065

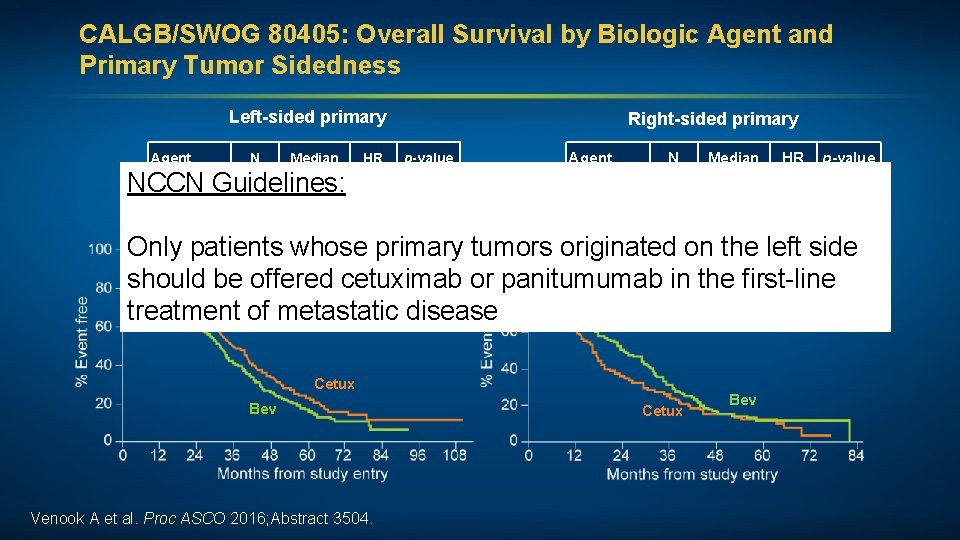
CALGB/SWOG 80405: Overall Survival by Biologic Agent and Primary Tumor Sidedness Left-sided primary Agent N Median HR Right-sided primary p-value Agent N Median HR p-value Bev 356 31. 4 Bev 150 24. 2 NCCN Guidelines: 0. 817 0. 018 1. 269 0. 065 376 36. 0 Cetux 143 16. 7 Cetux Only patients whose primary tumors originated on the left side should be offered cetuximab or panitumumab in the first-line treatment of metastatic disease Cetux Bev Venook A et al. Proc ASCO 2016; Abstract 3504. Cetux Bev

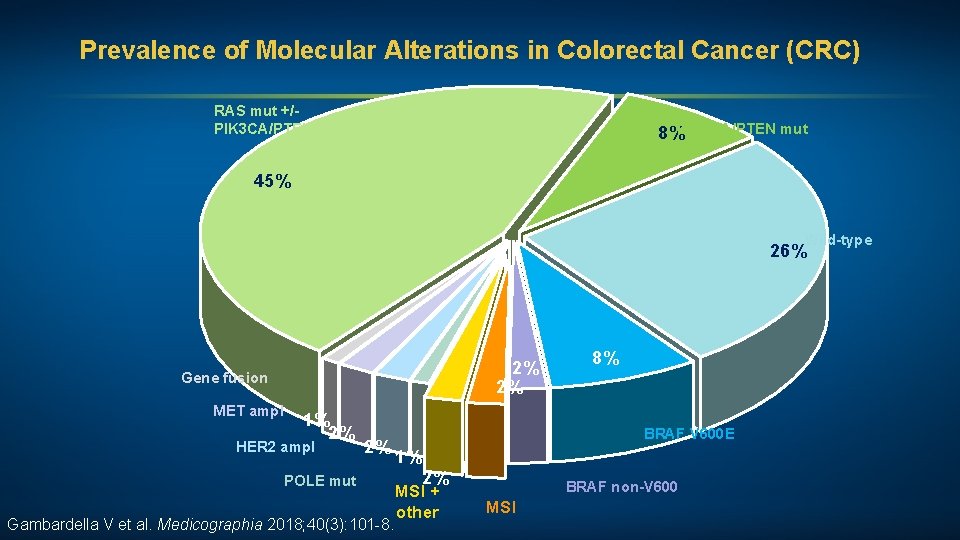
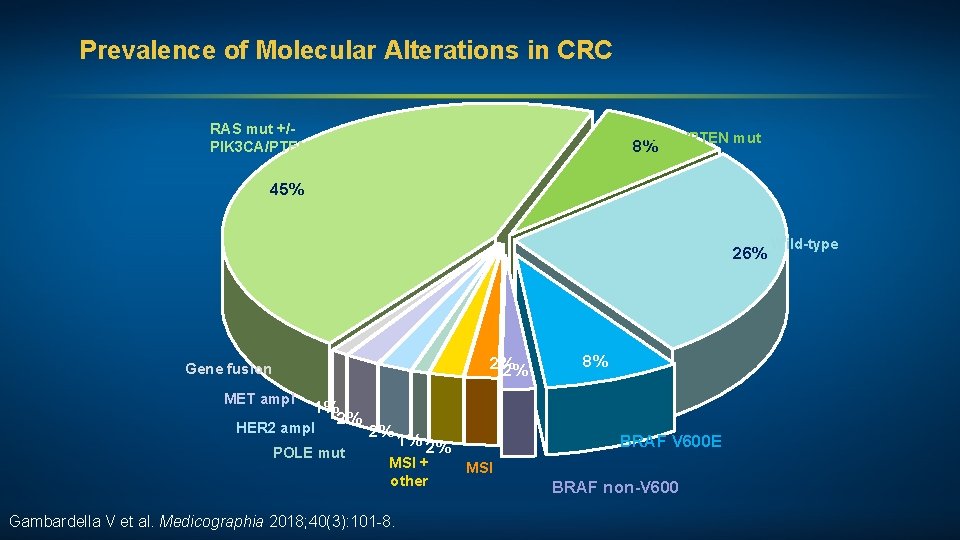
Prevalence of Molecular Alterations in Colorectal Cancer (CRC) RAS mut +/PIK 3 CA/PTEN mut 8%PIK 3 CA/PTEN mut 45% Wild-type 26% 2% 2% Gene fusion MET ampl 1% 2% HER 2 ampl 2% POLE mut Gambardella V et al. Medicographia 2018; 40(3): 101 -8. 8% BRAF V 600 E 1% 2% MSI + other BRAF non-V 600 MSI

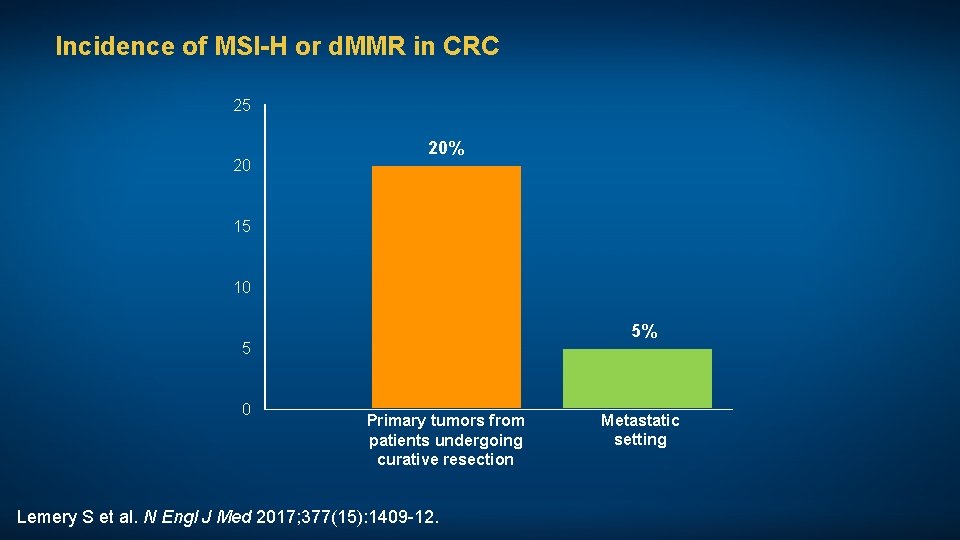
Incidence of MSI-H or d. MMR in CRC 25 20 20% 15 10 5% 5 0 Primary tumors from patients undergoing curative resection Lemery S et al. N Engl J Med 2017; 377(15): 1409 -12. Metastatic setting

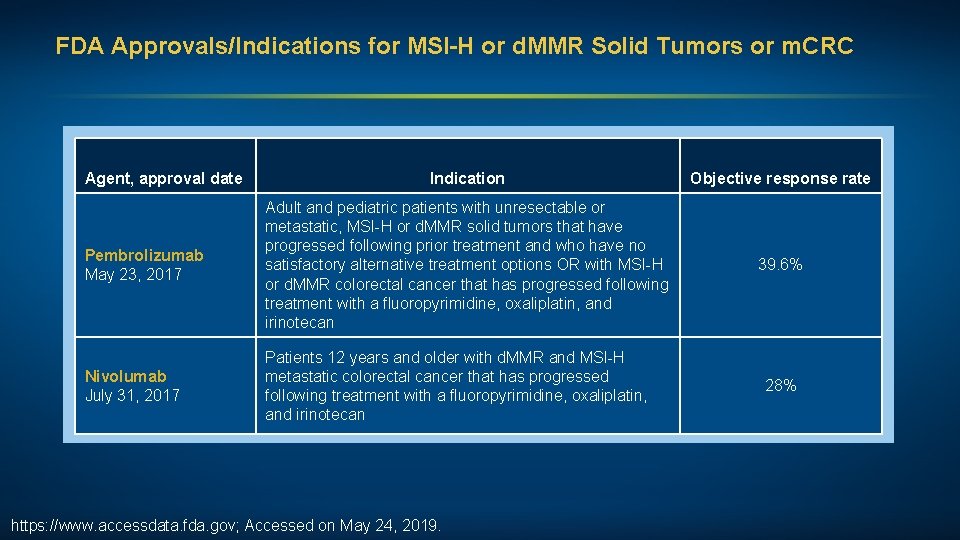
FDA Approvals/Indications for MSI-H or d. MMR Solid Tumors or m. CRC Agent, approval date Indication Objective response rate Pembrolizumab May 23, 2017 Adult and pediatric patients with unresectable or metastatic, MSI-H or d. MMR solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options OR with MSI-H or d. MMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan 39. 6% Nivolumab July 31, 2017 Patients 12 years and older with d. MMR and MSI-H metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan https: //www. accessdata. fda. gov; Accessed on May 24, 2019. 28%

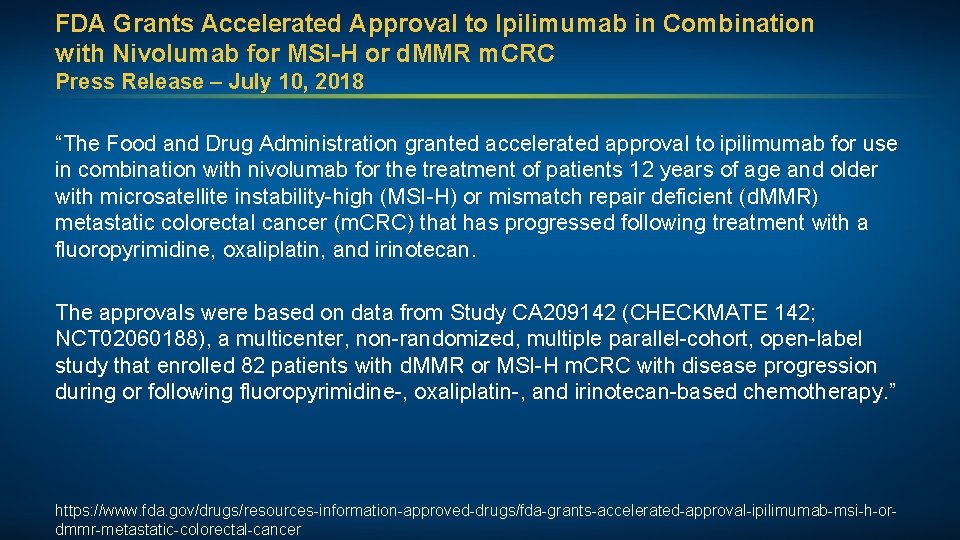
FDA Grants Accelerated Approval to Ipilimumab in Combination with Nivolumab for MSI-H or d. MMR m. CRC Press Release – July 10, 2018 “The Food and Drug Administration granted accelerated approval to ipilimumab for use in combination with nivolumab for the treatment of patients 12 years of age and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (d. MMR) metastatic colorectal cancer (m. CRC) that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. The approvals were based on data from Study CA 209142 (CHECKMATE 142; NCT 02060188), a multicenter, non-randomized, multiple parallel-cohort, open-label study that enrolled 82 patients with d. MMR or MSI-H m. CRC with disease progression during or following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. ” https: //www. fda. gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-ipilimumab-msi-h-ordmmr-metastatic-colorectal-cancer

J Clin Oncol 2018; 36(8): 773 -9.

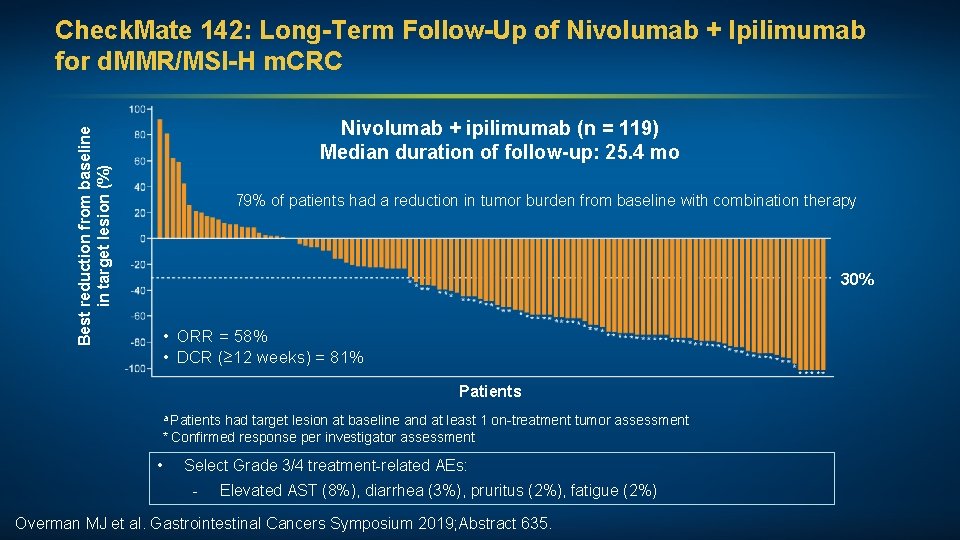
Best reduction from baseline in target lesion (%) Check. Mate 142: Long-Term Follow-Up of Nivolumab + Ipilimumab for d. MMR/MSI-H m. CRC Nivolumab + ipilimumab (n = 119) Median duration of follow-up: 25. 4 mo 79% of patients had a reduction in tumor burden from baseline with combination therapy 30% • ORR = 58% • DCR (≥ 12 weeks) = 81% Patients a Patients had target lesion at baseline and at least 1 on-treatment tumor assessment * Confirmed response per investigator assessment • Select Grade 3/4 treatment-related AEs: - Elevated AST (8%), diarrhea (3%), pruritus (2%), fatigue (2%) Overman MJ et al. Gastrointestinal Cancers Symposium 2019; Abstract 635.

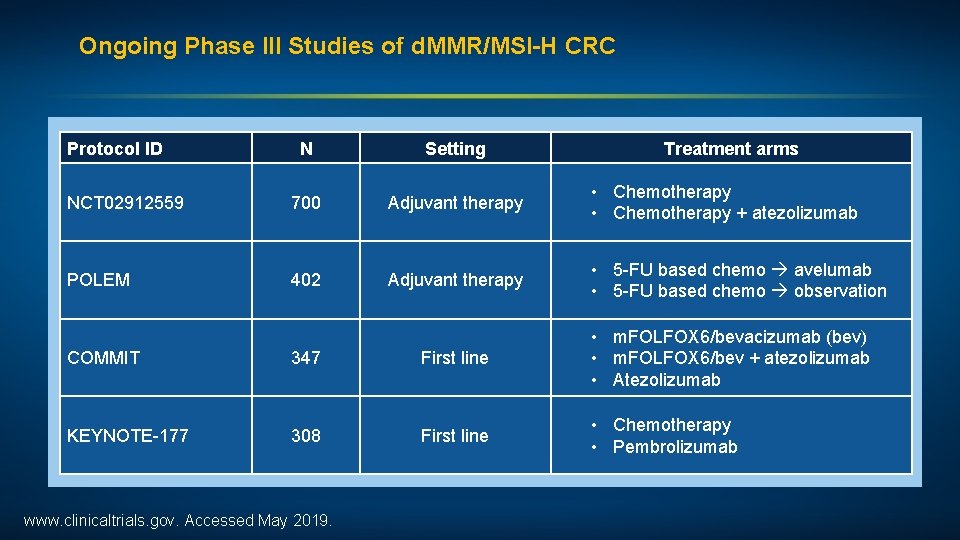
Ongoing Phase III Studies of d. MMR/MSI-H CRC Protocol ID N Setting Treatment arms NCT 02912559 700 Adjuvant therapy • Chemotherapy + atezolizumab POLEM 402 Adjuvant therapy • 5 -FU based chemo avelumab • 5 -FU based chemo observation COMMIT 347 First line • m. FOLFOX 6/bevacizumab (bev) • m. FOLFOX 6/bev + atezolizumab • Atezolizumab KEYNOTE-177 308 First line • Chemotherapy • Pembrolizumab www. clinicaltrials. gov. Accessed May 2019.


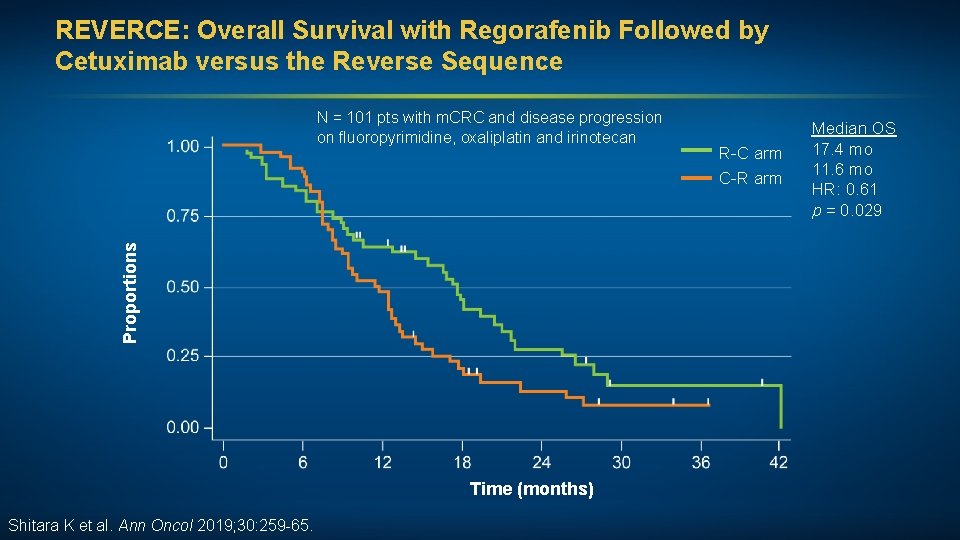
REVERCE: Overall Survival with Regorafenib Followed by Cetuximab versus the Reverse Sequence Proportions N = 101 pts with m. CRC and disease progression on fluoropyrimidine, oxaliplatin and irinotecan Time (months) Shitara K et al. Ann Oncol 2019; 30: 259 -65. R-C arm C-R arm Median OS 17. 4 mo 11. 6 mo HR: 0. 61 p = 0. 029

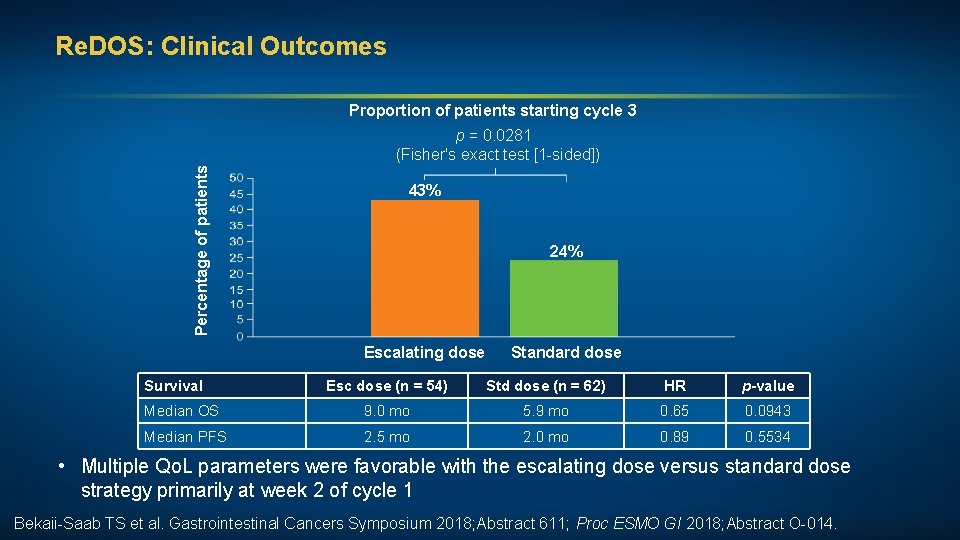
Regorafenib Dose Optimization Study (Re. DOS): Randomized Phase II Trial to Evaluate Dosing Strategies for Regorafenib in Refractory Metastatic CRC (m. CRC) — An ACCRU Network Study Regorafenib Dose Optimization Study (Re. DOS): Randomized Phase II Trial to Evaluate Escalating Dosing Strategy and Pre-emptive Topical Steroids for Regorafenib in Refractory Metastatic Colorectal Cancer (m. CRC) — An ACCRU Network Study Bekaii-Saab TS et al. Gastrointestinal Cancers Symposium 2018; Abstract 611. Bekaii-Saab TS et al. Proc ESMO GI 2018; Abstract O-014.

Re. DOS: Clinical Outcomes Proportion of patients starting cycle 3 Percentage of patients p = 0. 0281 (Fisher's exact test [1 -sided]) 43% 24% Escalating dose Survival Standard dose Esc dose (n = 54) Std dose (n = 62) HR p-value Median OS 9. 0 mo 5. 9 mo 0. 65 0. 0943 Median PFS 2. 5 mo 2. 0 mo 0. 89 0. 5534 • Multiple Qo. L parameters were favorable with the escalating dose versus standard dose strategy primarily at week 2 of cycle 1 Bekaii-Saab TS et al. Gastrointestinal Cancers Symposium 2018; Abstract 611; Proc ESMO GI 2018; Abstract O-014.

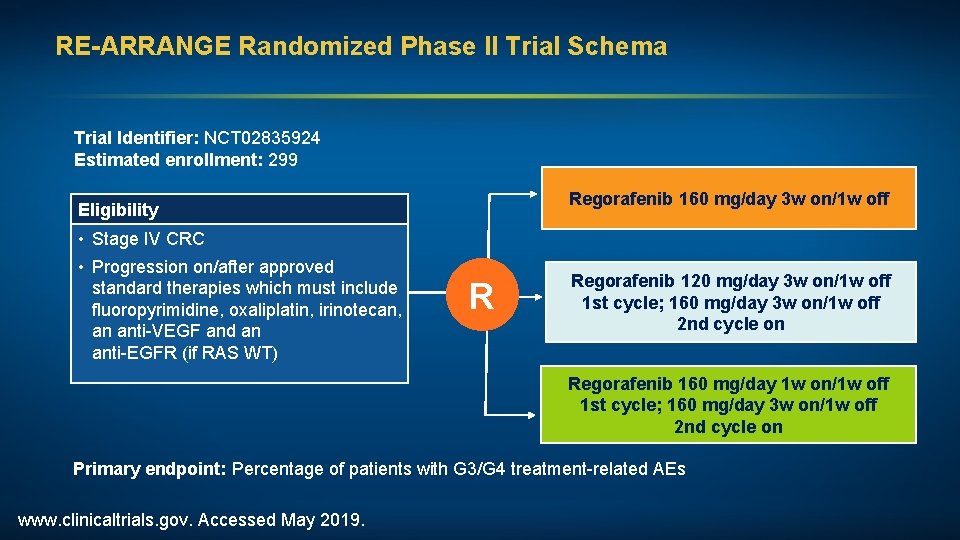
RE-ARRANGE Randomized Phase II Trial Schema Trial Identifier: NCT 02835924 Estimated enrollment: 299 Regorafenib 160 mg/day 3 w on/1 w off Eligibility • Stage IV CRC • Progression on/after approved standard therapies which must include fluoropyrimidine, oxaliplatin, irinotecan, an anti-VEGF and an anti-EGFR (if RAS WT) R Regorafenib 120 mg/day 3 w on/1 w off 1 st cycle; 160 mg/day 3 w on/1 w off 2 nd cycle on Regorafenib 160 mg/day 1 w on/1 w off 1 st cycle; 160 mg/day 3 w on/1 w off 2 nd cycle on Primary endpoint: Percentage of patients with G 3/G 4 treatment-related AEs www. clinicaltrials. gov. Accessed May 2019.

J Clin Oncol 2018; 36(4): 350 -8. Eur J Cancer 2018; 90: 63 -72.

Safety and Efficacy of Trifluridine/Tipiracil in Previously Treated Metastatic Colorectal Cancer (m. CRC): Preliminary Results from the Phase IIIb, International, Open-Label, Early-Access PRECONNECT Study 1 Safety and Efficacy of Trifluridine/Tipiracil (FTD/TPI) in Metastatic Colorectal Cancer (m. CRC) Patients According to Previous Treatment with Regorafenib in the International Phase 3 b PRECONNECT Study 2 1 Falcone A et al. Proc ESMO GI 2018; Abstract O-013. 2 Taieb J et al. Proc ESMO 2018; Abstract 464 P.

Prevalence of Molecular Alterations in CRC RAS mut +/PIK 3 CA/PTEN mut 8% 45% 26% 2% 2% Gene fusion MET ampl 1% 2% HER 2 ampl POLE mut 2% 1% 2% MSI + other Gambardella V et al. Medicographia 2018; 40(3): 101 -8. 8% BRAF V 600 E MSI BRAF non-V 600 Wild-type

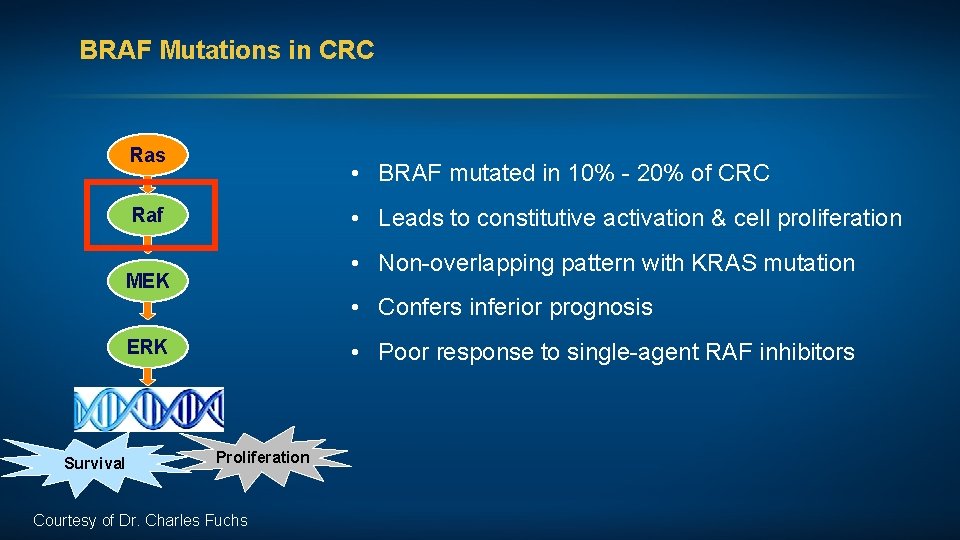
BRAF Mutations in CRC Ras • BRAF mutated in 10% - 20% of CRC • Leads to constitutive activation & cell proliferation Raf • Non-overlapping pattern with KRAS mutation MEK • Confers inferior prognosis ERK Survival • Poor response to single-agent RAF inhibitors Proliferation Courtesy of Dr. Charles Fuchs

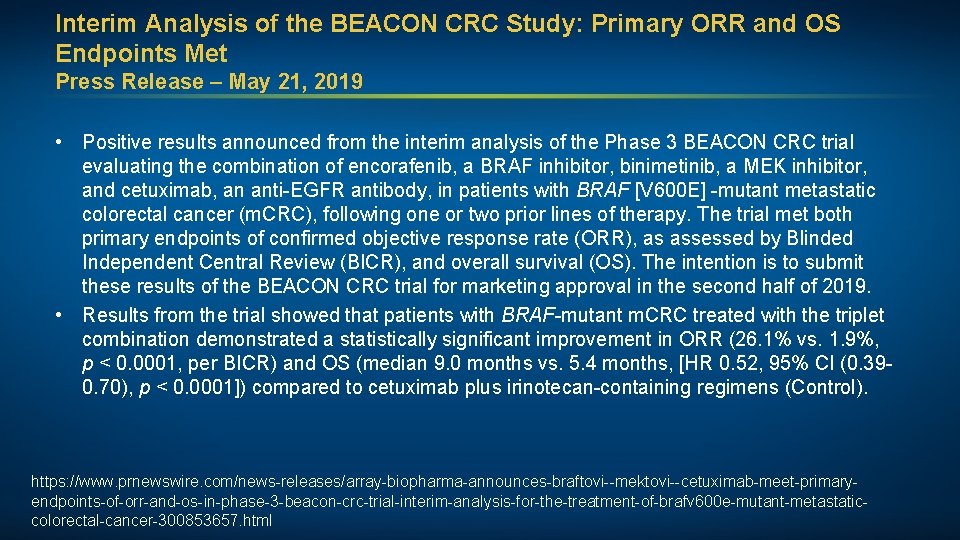
Interim Analysis of the BEACON CRC Study: Primary ORR and OS Endpoints Met Press Release – May 21, 2019 • Positive results announced from the interim analysis of the Phase 3 BEACON CRC trial evaluating the combination of encorafenib, a BRAF inhibitor, binimetinib, a MEK inhibitor, and cetuximab, an anti-EGFR antibody, in patients with BRAF [V 600 E] -mutant metastatic colorectal cancer (m. CRC), following one or two prior lines of therapy. The trial met both primary endpoints of confirmed objective response rate (ORR), as assessed by Blinded Independent Central Review (BICR), and overall survival (OS). The intention is to submit these results of the BEACON CRC trial for marketing approval in the second half of 2019. • Results from the trial showed that patients with BRAF-mutant m. CRC treated with the triplet combination demonstrated a statistically significant improvement in ORR (26. 1% vs. 1. 9%, p < 0. 0001, per BICR) and OS (median 9. 0 months vs. 5. 4 months, [HR 0. 52, 95% CI (0. 390. 70), p < 0. 0001]) compared to cetuximab plus irinotecan-containing regimens (Control). https: //www. prnewswire. com/news-releases/array-biopharma-announces-braftovi--mektovi--cetuximab-meet-primaryendpoints-of-orr-and-os-in-phase-3 -beacon-crc-trial-interim-analysis-for-the-treatment-of-brafv 600 e-mutant-metastaticcolorectal-cancer-300853657. html
![J Clin Oncol 2019 Epub ahead of print J Clin Oncol 2019; [Epub ahead of print].](https://slidetodoc.com/presentation_image/4b785af8faedb3c82f97ed92b7616f92/image-36.jpg)
J Clin Oncol 2019; [Epub ahead of print].

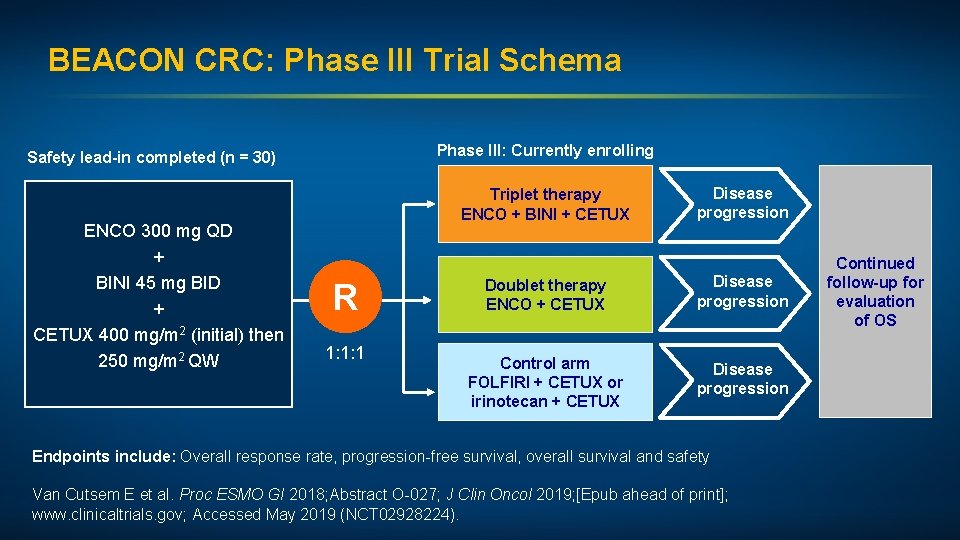
BEACON CRC: Phase III Trial Schema Phase III: Currently enrolling Safety lead-in completed (n = 30) ENCO 300 mg QD + BINI 45 mg BID + CETUX 400 mg/m 2 (initial) then 250 mg/m 2 QW Triplet therapy ENCO + BINI + CETUX R 1: 1: 1 Disease progression Doublet therapy ENCO + CETUX Disease progression Control arm FOLFIRI + CETUX or irinotecan + CETUX Disease progression Endpoints include: Overall response rate, progression-free survival, overall survival and safety Van Cutsem E et al. Proc ESMO GI 2018; Abstract O-027; J Clin Oncol 2019; [Epub ahead of print]; www. clinicaltrials. gov; Accessed May 2019 (NCT 02928224). Continued follow-up for evaluation of OS

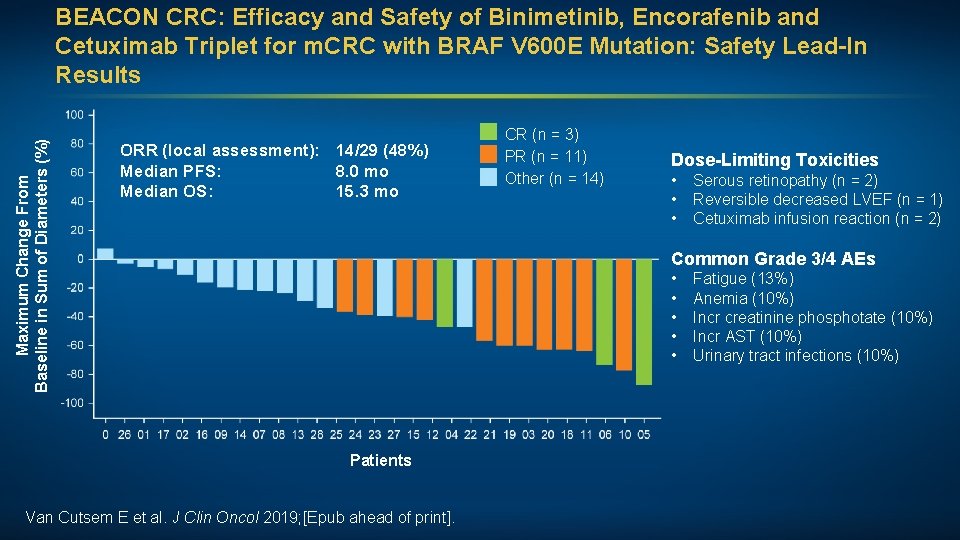
Maximum Change From Baseline in Sum of Diameters (%) BEACON CRC: Efficacy and Safety of Binimetinib, Encorafenib and Cetuximab Triplet for m. CRC with BRAF V 600 E Mutation: Safety Lead-In Results ORR (local assessment): 14/29 (48%) Median PFS: 8. 0 mo Median OS: 15. 3 mo CR (n = 3) PR (n = 11) Other (n = 14) Dose-Limiting Toxicities • • • Serous retinopathy (n = 2) Reversible decreased LVEF (n = 1) Cetuximab infusion reaction (n = 2) Common Grade 3/4 AEs • • • Patients Van Cutsem E et al. J Clin Oncol 2019; [Epub ahead of print]. Fatigue (13%) Anemia (10%) Incr creatinine phosphotate (10%) Incr AST (10%) Urinary tract infections (10%)

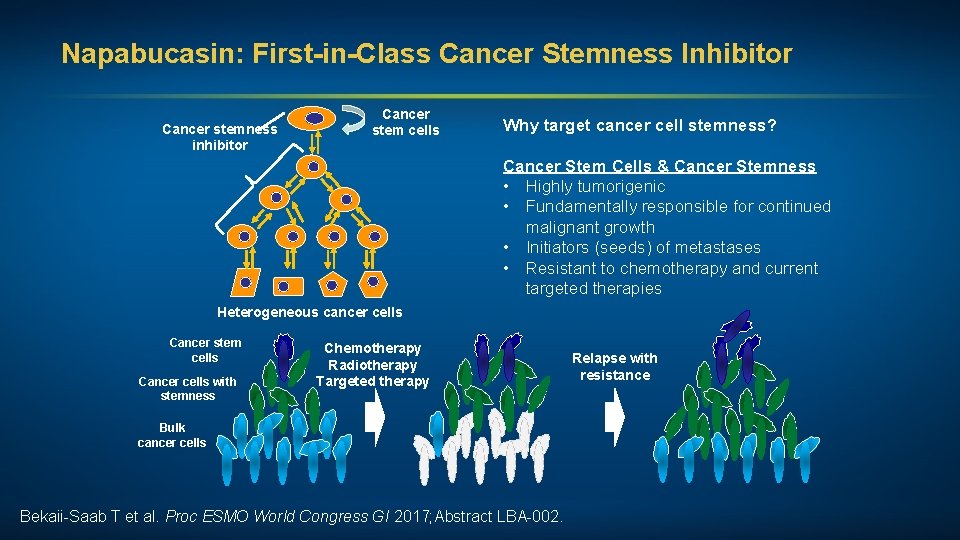
Napabucasin: First-in-Class Cancer Stemness Inhibitor Cancer stemness inhibitor Cancer stem cells Why target cancer cell stemness? Cancer Stem Cells & Cancer Stemness • Highly tumorigenic • Fundamentally responsible for continued malignant growth • Initiators (seeds) of metastases • Resistant to chemotherapy and current targeted therapies Heterogeneous cancer cells Cancer stem cells Cancer cells with stemness Chemotherapy Radiotherapy Targeted therapy Bulk cancer cells Bekaii-Saab T et al. Proc ESMO World Congress GI 2017; Abstract LBA-002. Relapse with resistance

Phase Ib/II Study of Cancer Stemness Inhibitor Napabucasin in Combination with FOLFIRI +/- Bevacizumab (bev) in Metastatic Colorectal Cancer (m. CRC) Patients (pts) Bendell J et al. Proc ESMO World Congress GI 2017; Abstract LBA-003.

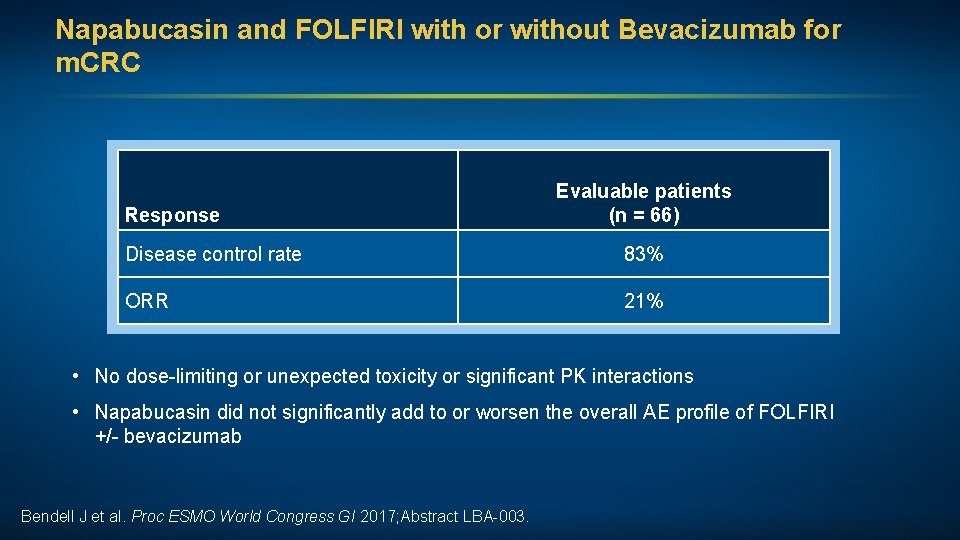
Napabucasin and FOLFIRI with or without Bevacizumab for m. CRC Response Evaluable patients (n = 66) Disease control rate 83% ORR 21% • No dose-limiting or unexpected toxicity or significant PK interactions • Napabucasin did not significantly add to or worsen the overall AE profile of FOLFIRI +/- bevacizumab Bendell J et al. Proc ESMO World Congress GI 2017; Abstract LBA-003.

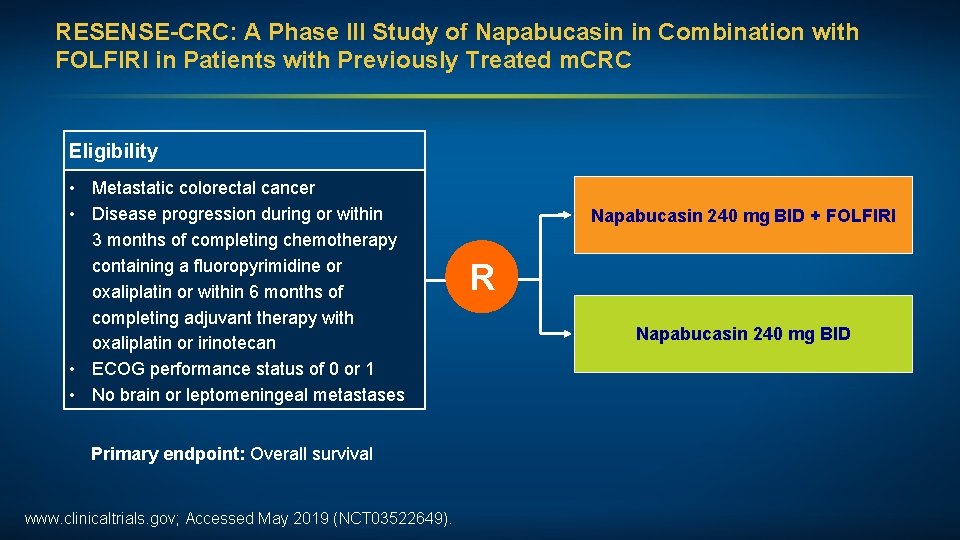
RESENSE-CRC: A Phase III Study of Napabucasin in Combination with FOLFIRI in Patients with Previously Treated m. CRC Eligibility • Metastatic colorectal cancer • Disease progression during or within 3 months of completing chemotherapy containing a fluoropyrimidine or oxaliplatin or within 6 months of completing adjuvant therapy with oxaliplatin or irinotecan • ECOG performance status of 0 or 1 • No brain or leptomeningeal metastases Primary endpoint: Overall survival www. clinicaltrials. gov; Accessed May 2019 (NCT 03522649). Napabucasin 240 mg BID + FOLFIRI R Napabucasin 240 mg BID

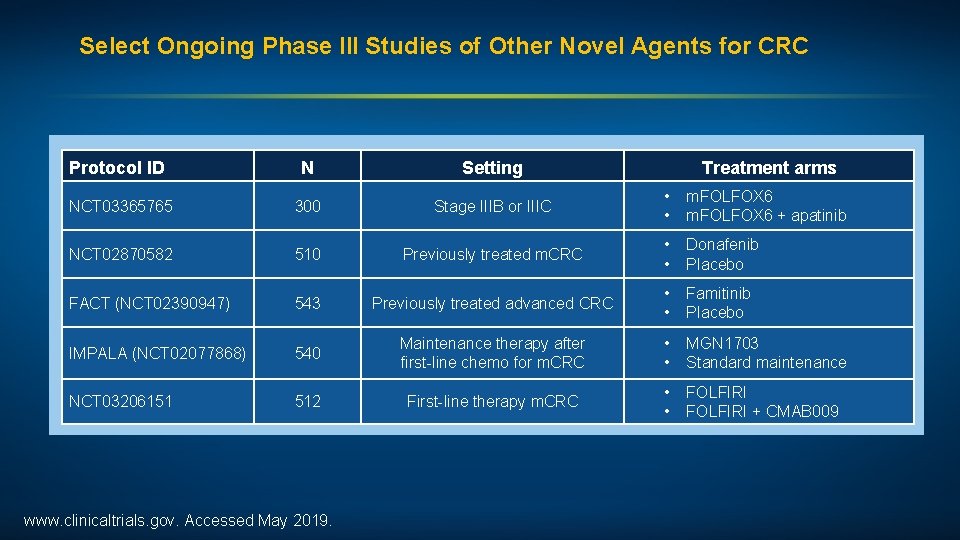
Select Ongoing Phase III Studies of Other Novel Agents for CRC Protocol ID N Setting NCT 03365765 300 Stage IIIB or IIIC • • m. FOLFOX 6 + apatinib NCT 02870582 510 Previously treated m. CRC • • Donafenib Placebo FACT (NCT 02390947) 543 Previously treated advanced CRC • • Famitinib Placebo IMPALA (NCT 02077868) 540 Maintenance therapy after first-line chemo for m. CRC • • MGN 1703 Standard maintenance NCT 03206151 512 First-line therapy m. CRC • • FOLFIRI + CMAB 009 www. clinicaltrials. gov. Accessed May 2019. Treatment arms

What is your usual first-line treatment strategy for a 65 -year-old patient with left-sided, MSS, pan-RAS wild-type metastatic colorectal cancer (m. CRC)? a. b. c. d. Chemotherapy + bevacizumab Chemotherapy + EGFR antibody Chemotherapy Other