PGT 120 Engineering Materials Lecture 3 Crystal Structure



![Example: Draw the following direction vectors in cubic unit cells. a)[100] and [110] b) Example: Draw the following direction vectors in cubic unit cells. a)[100] and [110] b)](https://slidetodoc.com/presentation_image_h2/3b9b73b6587db8ab1d8b928f7b386c56/image-25.jpg)

- Slides: 55

PGT 120 Engineering Materials Lecture 3: Crystal Structure

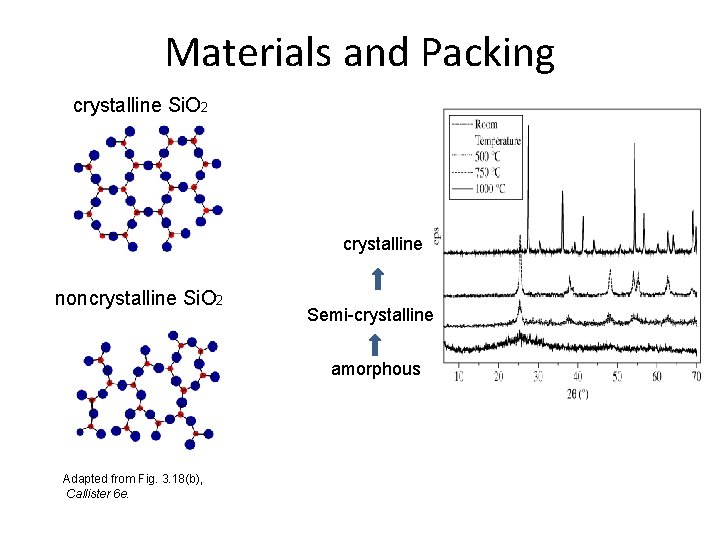
Materials and Packing Materials – Atoms/ions arrangement • Crystalline material - Material whose atoms and ions arranged in a pattern (long-range order) • amorphous or noncrystalline - Material whose atoms and ions that are not arranged in a long range, periodic, and repeated manner (shortrange order )

Materials and Packing Crystalline materials. . . • atoms pack in periodic • eg: -metals -many ceramics -some polymers Noncrystalline materials. . . • atoms have no periodic packing • occurs for: -complex structures -rapid cooling "Amorphous" = Noncrystalline -glass 3

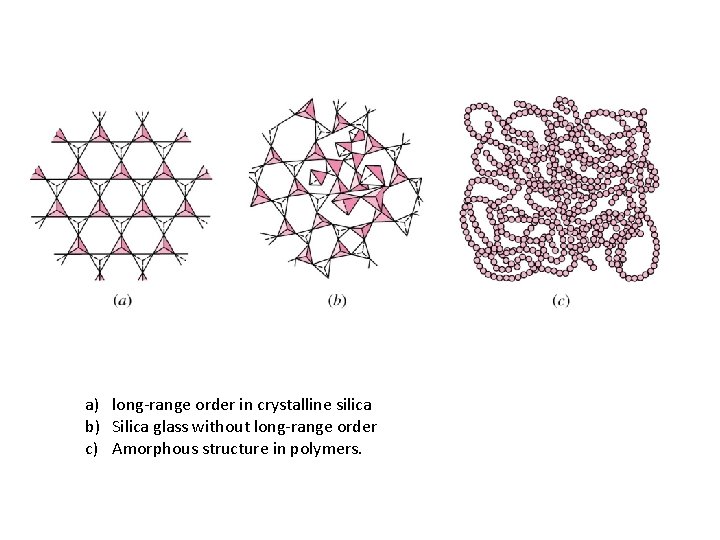
Materials and Packing crystalline Si. O 2 crystalline noncrystalline Si. O 2 Semi-crystalline amorphous Adapted from Fig. 3. 18(b), Callister 6 e.

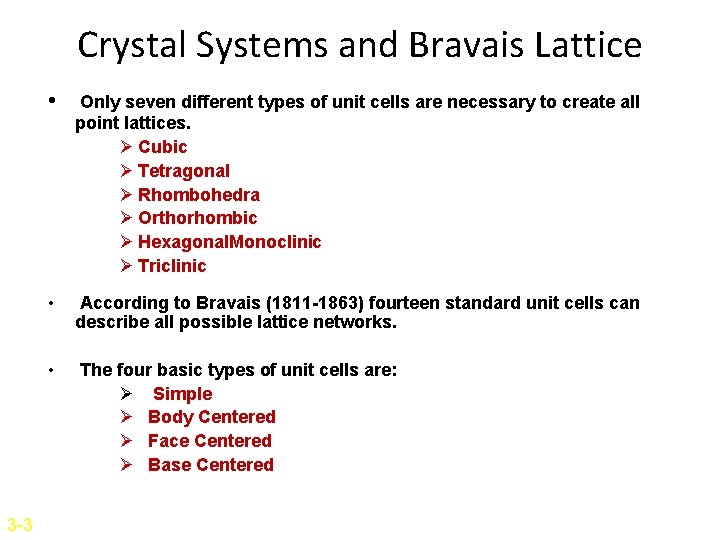
Crystal Systems and Bravais Lattice 3 -3 • Only seven different types of unit cells are necessary to create all point lattices. Ø Cubic Ø Tetragonal Ø Rhombohedra Ø Orthorhombic Ø Hexagonal. Monoclinic Ø Triclinic • According to Bravais (1811 -1863) fourteen standard unit cells can describe all possible lattice networks. • The four basic types of unit cells are: Ø Simple Ø Body Centered Ø Face Centered Ø Base Centered

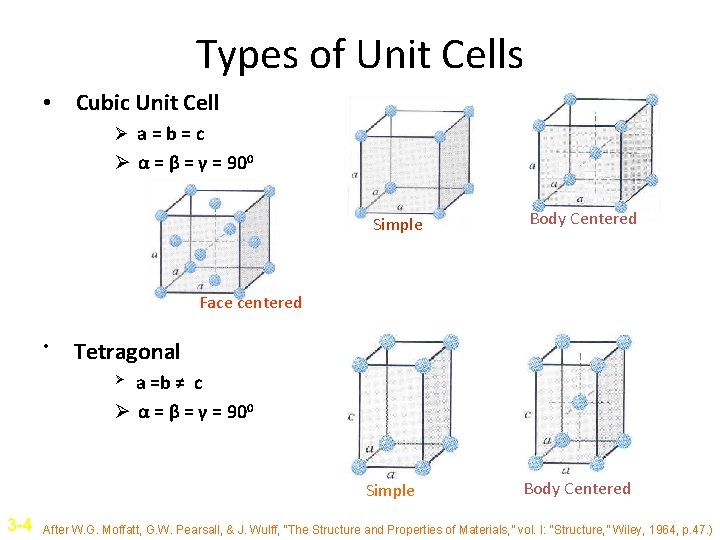
Types of Unit Cells • Cubic Unit Cell Ø a=b=c Ø α = β = γ = 900 Simple Body Centered Face centered • Tetragonal a =b ≠ c Ø α = β = γ = 900 Ø Simple 3 -4 Body Centered After W. G. Moffatt, G. W. Pearsall, & J. Wulff, “The Structure and Properties of Materials, ” vol. I: “Structure, ” Wiley, 1964, p. 47. )

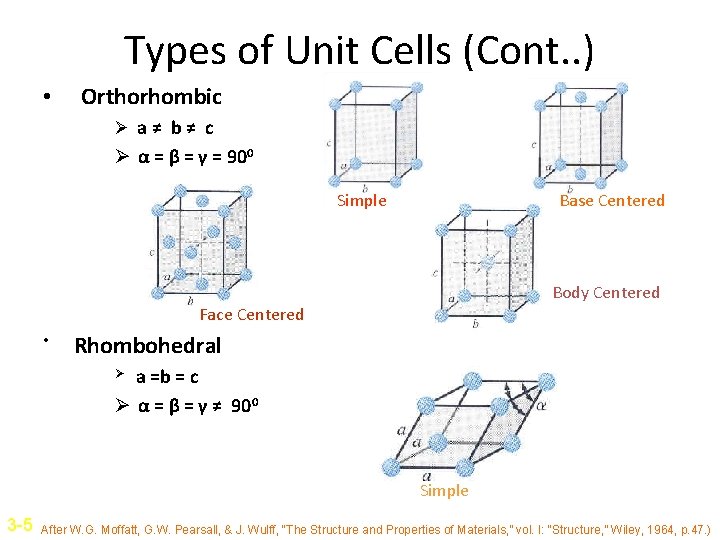
Types of Unit Cells (Cont. . ) • Orthorhombic Ø a≠ b≠ c Ø α = β = γ = 900 Simple Base Centered Body Centered Face Centered • Rhombohedral a =b = c Ø α = β = γ ≠ 900 Ø Simple 3 -5 After W. G. Moffatt, G. W. Pearsall, & J. Wulff, “The Structure and Properties of Materials, ” vol. I: “Structure, ” Wiley, 1964, p. 47. )

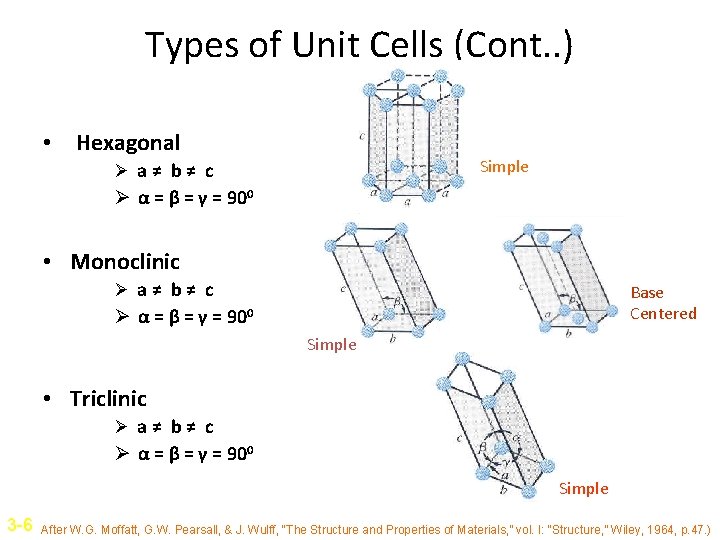
Types of Unit Cells (Cont. . ) • Hexagonal Simple Ø a≠ b≠ c Ø α = β = γ = 900 • Monoclinic Ø a≠ b≠ c Base Centered Ø α = β = γ = 900 Simple • Triclinic Ø a≠ b≠ c Ø α = β = γ = 900 Simple 3 -6 After W. G. Moffatt, G. W. Pearsall, & J. Wulff, “The Structure and Properties of Materials, ” vol. I: “Structure, ” Wiley, 1964, p. 47. )

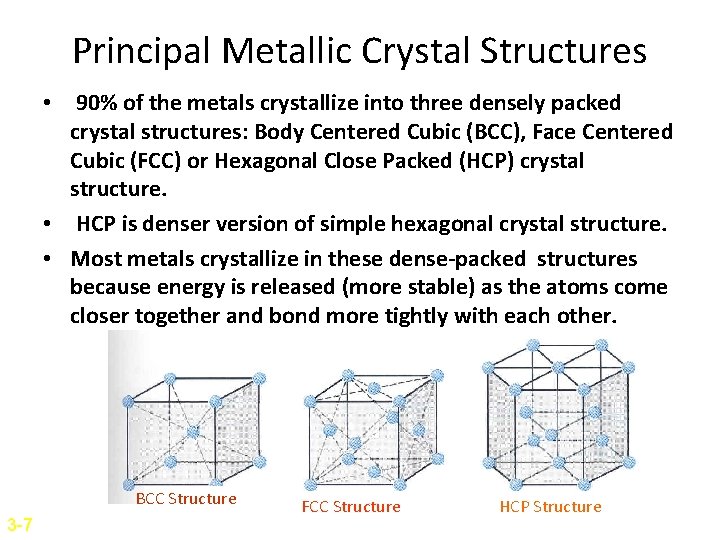
Principal Metallic Crystal Structures • 90% of the metals crystallize into three densely packed crystal structures: Body Centered Cubic (BCC), Face Centered Cubic (FCC) or Hexagonal Close Packed (HCP) crystal structure. • HCP is denser version of simple hexagonal crystal structure. • Most metals crystallize in these dense-packed structures because energy is released (more stable) as the atoms come closer together and bond more tightly with each other. BCC Structure 3 -7 FCC Structure HCP Structure

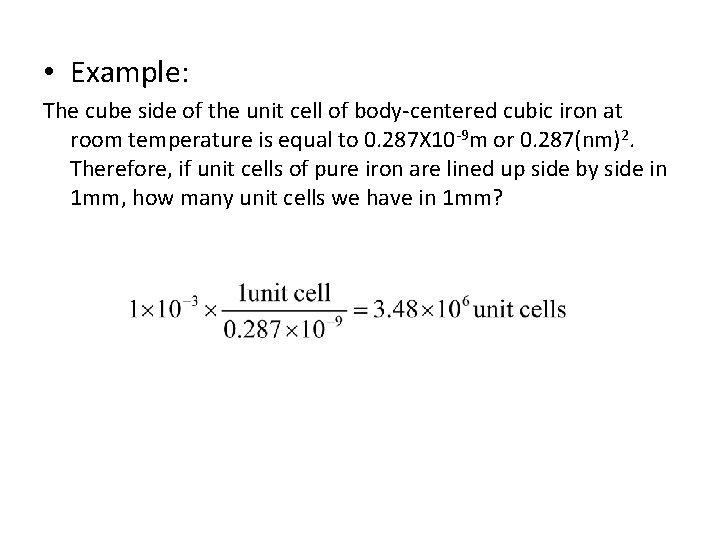
• Example: The cube side of the unit cell of body-centered cubic iron at room temperature is equal to 0. 287 X 10 -9 m or 0. 287(nm)2. Therefore, if unit cells of pure iron are lined up side by side in 1 mm, how many unit cells we have in 1 mm?

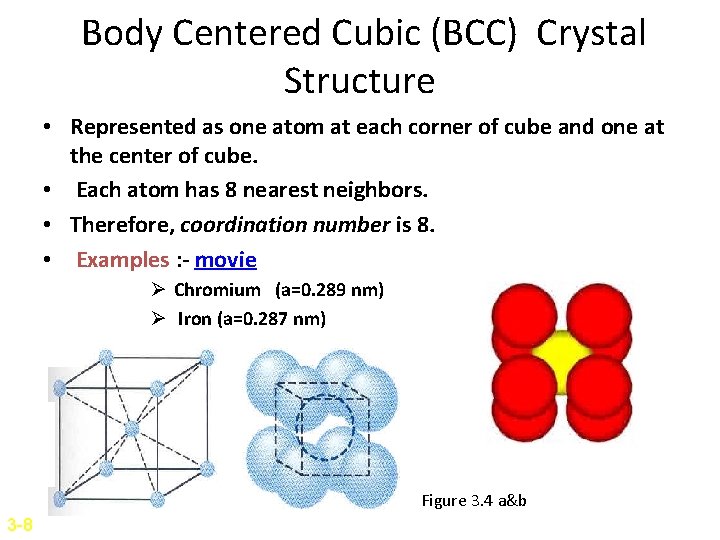
Body Centered Cubic (BCC) Crystal Structure • Represented as one atom at each corner of cube and one at the center of cube. • Each atom has 8 nearest neighbors. • Therefore, coordination number is 8. • Examples : - movie Ø Chromium (a=0. 289 nm) Ø Iron (a=0. 287 nm) Ø Sodium (a=0. 429 nm) Figure 3. 4 a&b 3 -8

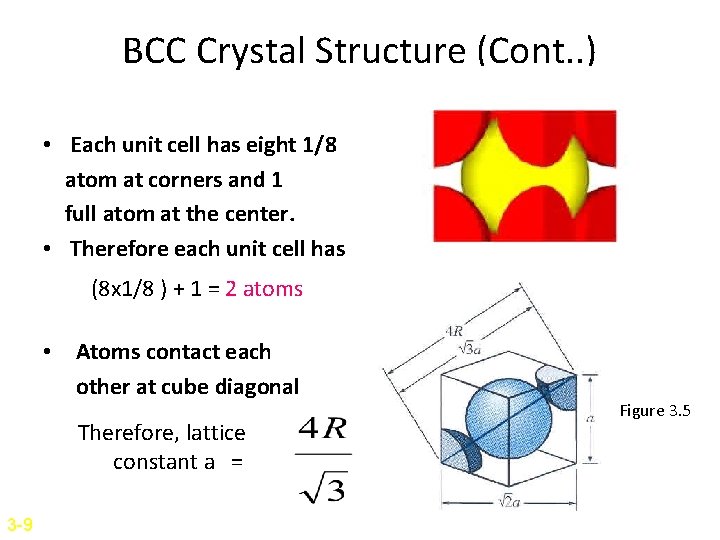
BCC Crystal Structure (Cont. . ) • Each unit cell has eight 1/8 atom at corners and 1 full atom at the center. • Therefore each unit cell has (8 x 1/8 ) + 1 = 2 atoms • Atoms contact each other at cube diagonal Therefore, lattice constant a = 3 -9 Figure 3. 5

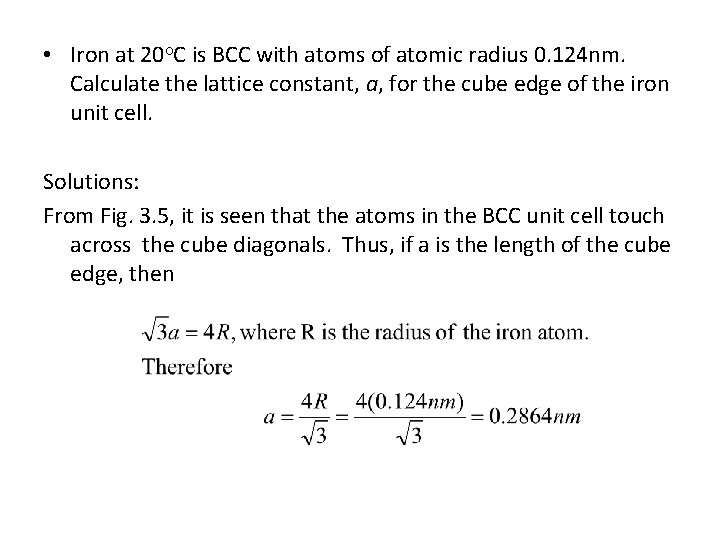
• Iron at 20 C is BCC with atoms of atomic radius 0. 124 nm. Calculate the lattice constant, a, for the cube edge of the iron unit cell. Solutions: From Fig. 3. 5, it is seen that the atoms in the BCC unit cell touch across the cube diagonals. Thus, if a is the length of the cube edge, then

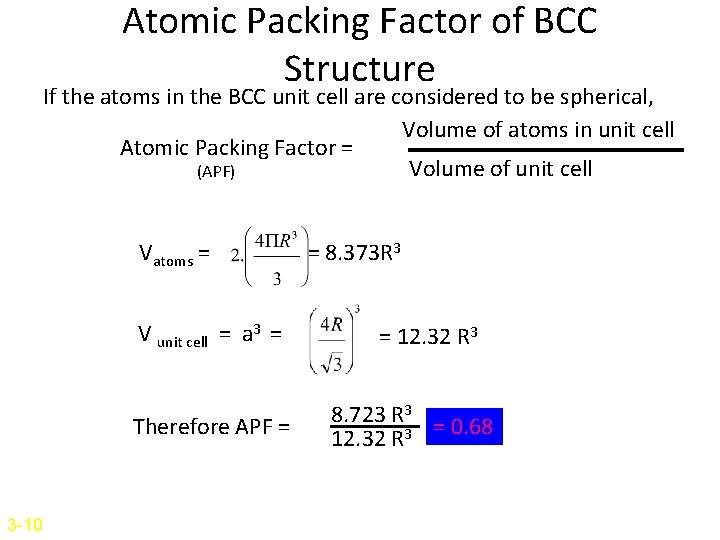
Atomic Packing Factor of BCC Structure If the atoms in the BCC unit cell are considered to be spherical, Volume of atoms in unit cell Atomic Packing Factor = Volume of unit cell (APF) Vatoms = V unit cell = a 3 = Therefore APF = 3 -10 = 8. 373 R 3 = 12. 32 R 3 8. 723 R 3 = 0. 68 12. 32 R 3

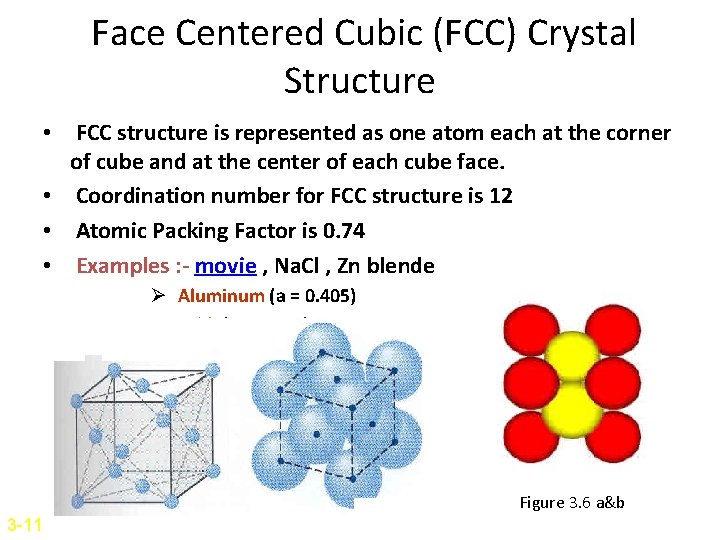
Face Centered Cubic (FCC) Crystal Structure • FCC structure is represented as one atom each at the corner of cube and at the center of each cube face. • Coordination number for FCC structure is 12 • Atomic Packing Factor is 0. 74 • Examples : - movie , Na. Cl , Zn blende Ø Aluminum (a = 0. 405) Ø Gold (a = 0. 408) Figure 3. 6 a&b 3 -11

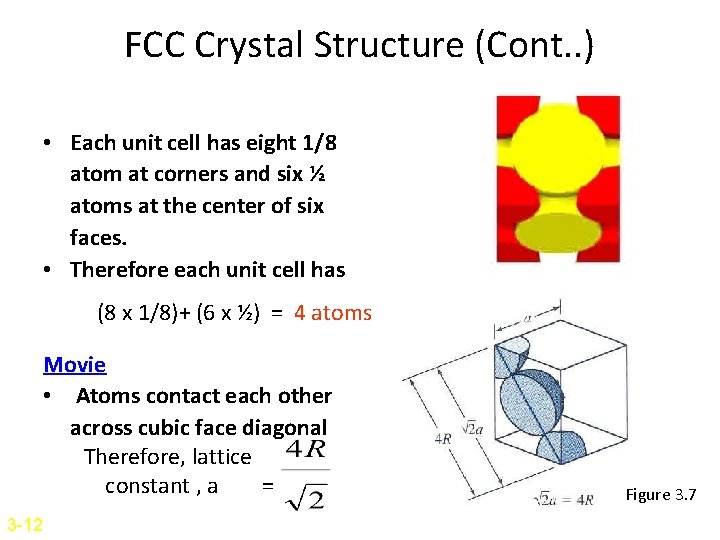
FCC Crystal Structure (Cont. . ) • Each unit cell has eight 1/8 atom at corners and six ½ atoms at the center of six faces. • Therefore each unit cell has (8 x 1/8)+ (6 x ½) = 4 atoms Movie • Atoms contact each other across cubic face diagonal Therefore, lattice constant , a = 3 -12 Figure 3. 7

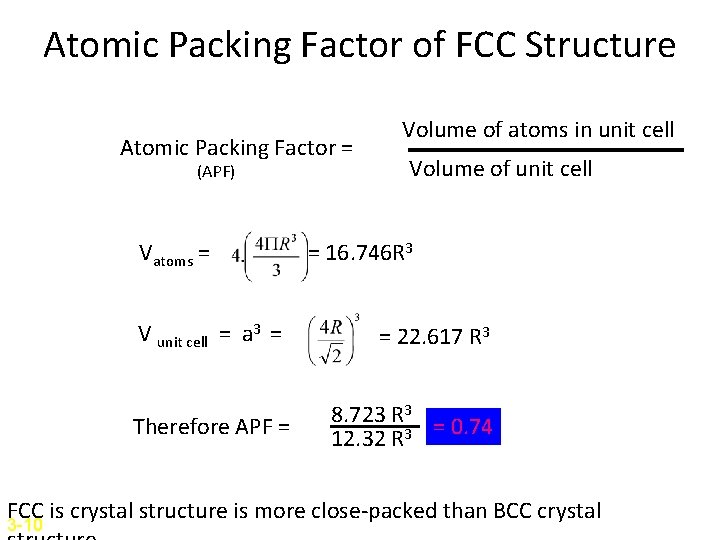
Atomic Packing Factor of FCC Structure Atomic Packing Factor = (APF) Vatoms = V unit cell = a 3 = Therefore APF = Volume of atoms in unit cell Volume of unit cell = 16. 746 R 3 = 22. 617 R 3 8. 723 R 3 = 0. 74 12. 32 R 3 FCC is crystal structure is more close-packed than BCC crystal 3 -10

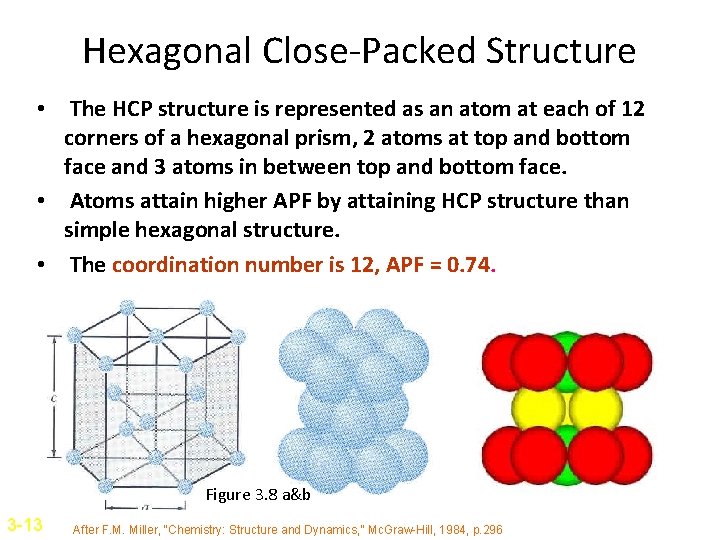
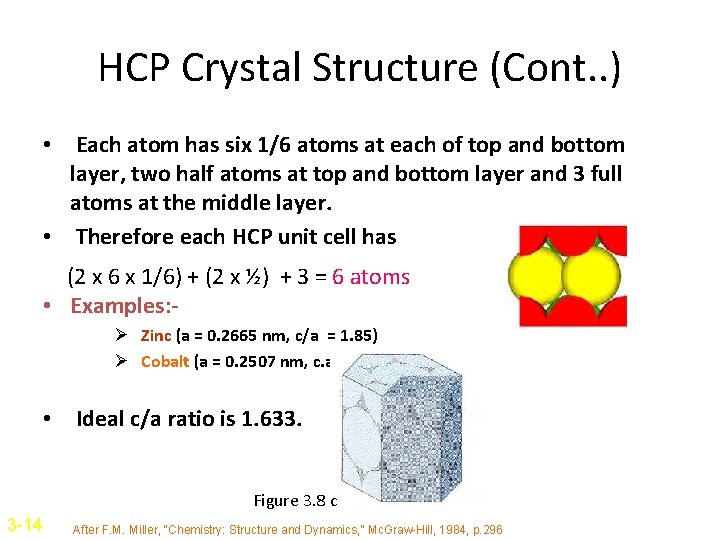
Hexagonal Close-Packed Structure • The HCP structure is represented as an atom at each of 12 corners of a hexagonal prism, 2 atoms at top and bottom face and 3 atoms in between top and bottom face. • Atoms attain higher APF by attaining HCP structure than simple hexagonal structure. • The coordination number is 12, APF = 0. 74. Figure 3. 8 a&b 3 -13 After F. M. Miller, “Chemistry: Structure and Dynamics, ” Mc. Graw-Hill, 1984, p. 296

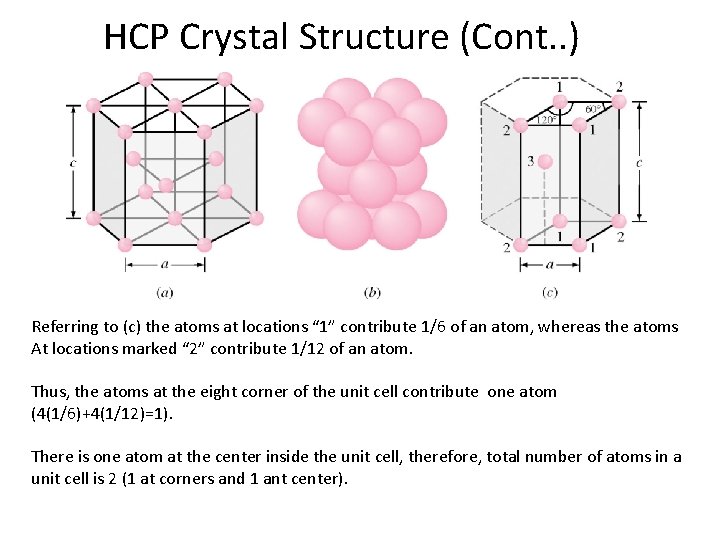
HCP Crystal Structure (Cont. . ) • Each atom has six 1/6 atoms at each of top and bottom layer, two half atoms at top and bottom layer and 3 full atoms at the middle layer. • Therefore each HCP unit cell has (2 x 6 x 1/6) + (2 x ½) + 3 = 6 atoms • Examples: Ø Zinc (a = 0. 2665 nm, c/a = 1. 85) Ø Cobalt (a = 0. 2507 nm, c. a = 1. 62) • Ideal c/a ratio is 1. 633. Figure 3. 8 c 3 -14 After F. M. Miller, “Chemistry: Structure and Dynamics, ” Mc. Graw-Hill, 1984, p. 296

HCP Crystal Structure (Cont. . ) Referring to (c) the atoms at locations “ 1” contribute 1/6 of an atom, whereas the atoms At locations marked “ 2” contribute 1/12 of an atom. Thus, the atoms at the eight corner of the unit cell contribute one atom (4(1/6)+4(1/12)=1). There is one atom at the center inside the unit cell, therefore, total number of atoms in a unit cell is 2 (1 at corners and 1 ant center).

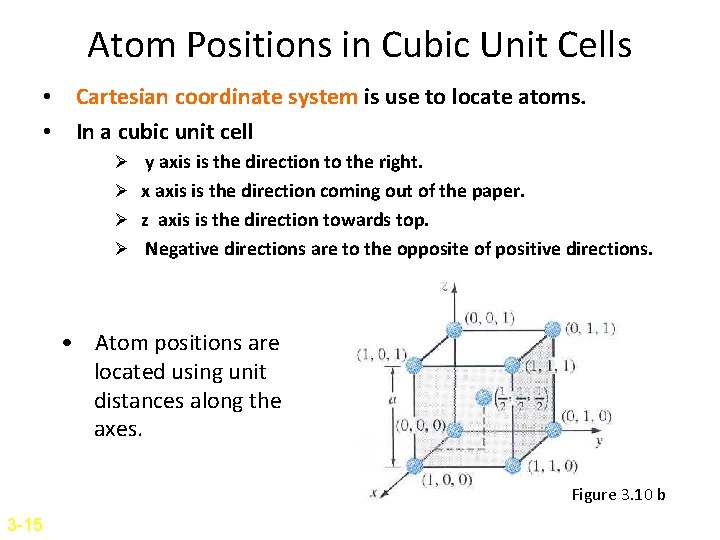
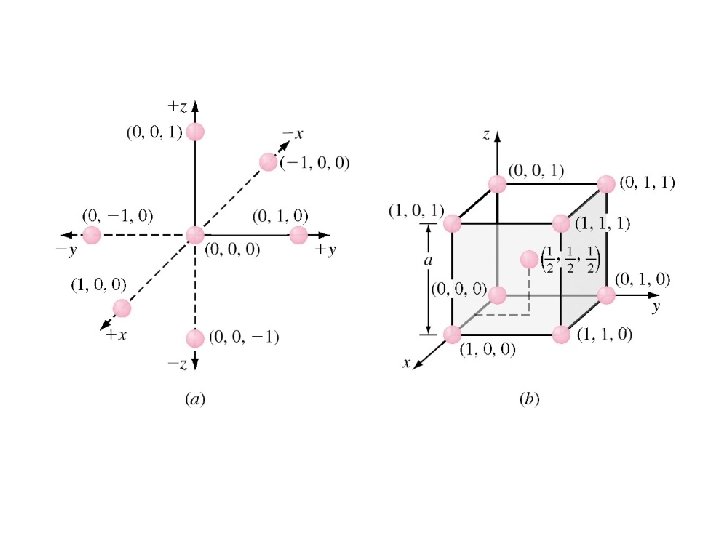
Atom Positions in Cubic Unit Cells • Cartesian coordinate system is use to locate atoms. • In a cubic unit cell Ø y axis is the direction to the right. Ø x axis is the direction coming out of the paper. Ø z axis is the direction towards top. Ø Negative directions are to the opposite of positive directions. • Atom positions are located using unit distances along the axes. Figure 3. 10 b 3 -15


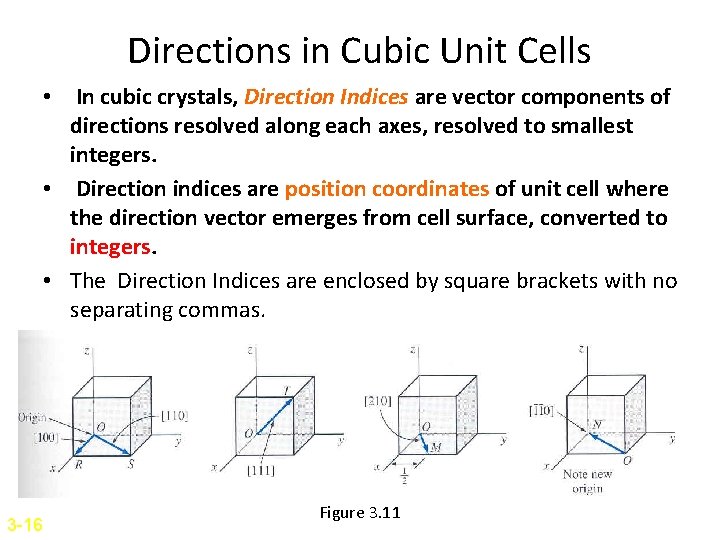
Directions in Cubic Unit Cells • In cubic crystals, Direction Indices are vector components of directions resolved along each axes, resolved to smallest integers. • Direction indices are position coordinates of unit cell where the direction vector emerges from cell surface, converted to integers. • The Direction Indices are enclosed by square brackets with no separating commas. 3 -16 Figure 3. 11

Two important things to remember for direction indices…… • Note that all parallel direction vectors have the same direction indices. • Directions are said to be crystallographical equivalent if the atom spacing along each direction is the same. Example: [100], [010], [001], [100]=<100> Equivalent directions are called indices of family or form.
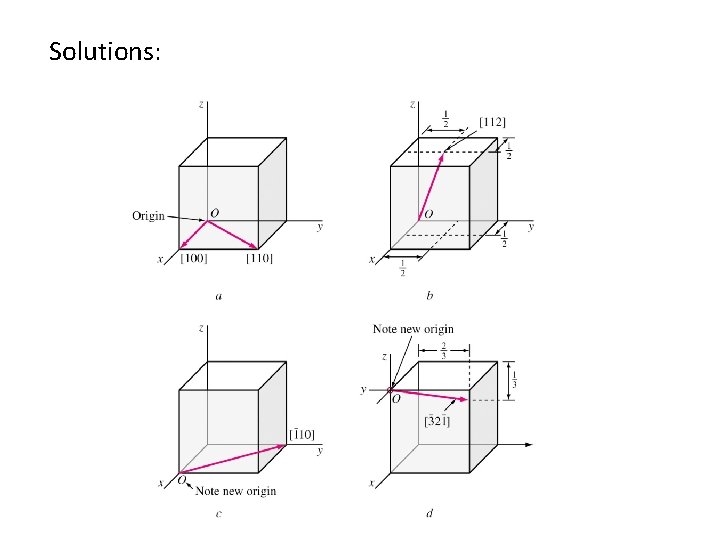
![Example Draw the following direction vectors in cubic unit cells a100 and 110 b Example: Draw the following direction vectors in cubic unit cells. a)[100] and [110] b)](https://slidetodoc.com/presentation_image_h2/3b9b73b6587db8ab1d8b928f7b386c56/image-25.jpg)
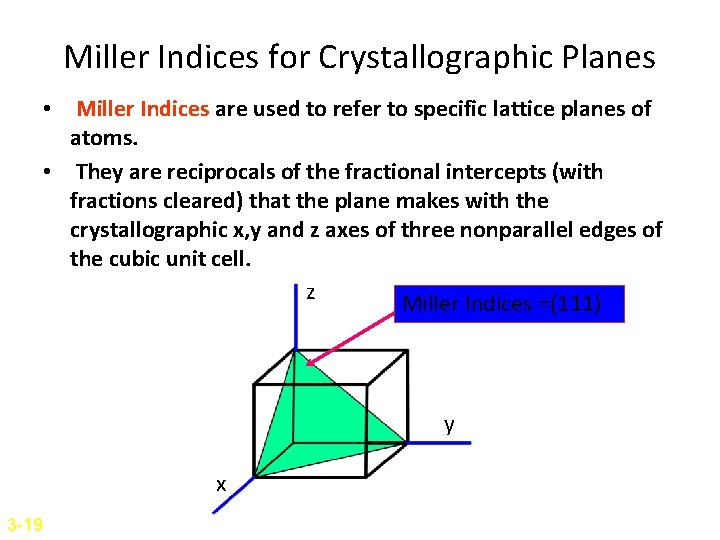
Example: Draw the following direction vectors in cubic unit cells. a)[100] and [110] b) [112] c)[110] d) [321]

Solutions:

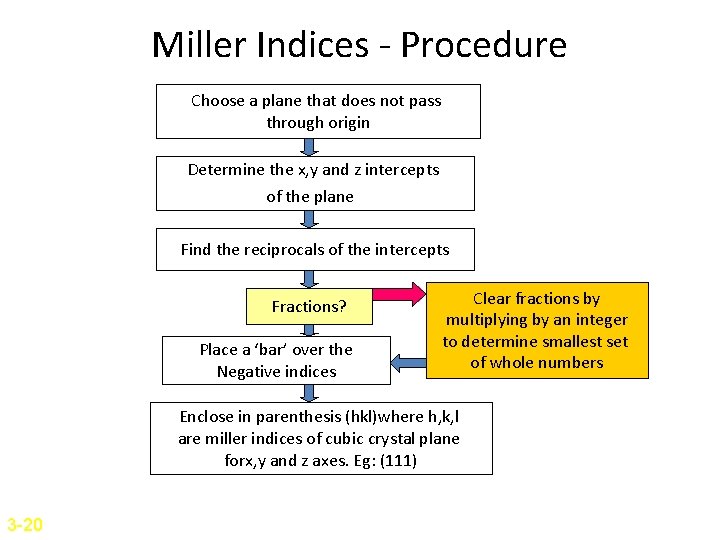
Miller Indices for Crystallographic Planes • Miller Indices are used to refer to specific lattice planes of atoms. • They are reciprocals of the fractional intercepts (with fractions cleared) that the plane makes with the crystallographic x, y and z axes of three nonparallel edges of the cubic unit cell. z Miller Indices =(111) y x 3 -19

Miller Indices - Procedure Choose a plane that does not pass through origin Determine the x, y and z intercepts of the plane Find the reciprocals of the intercepts Fractions? Place a ‘bar’ over the Negative indices Clear fractions by multiplying by an integer to determine smallest set of whole numbers Enclose in parenthesis (hkl)where h, k, l are miller indices of cubic crystal plane forx, y and z axes. Eg: (111) 3 -20

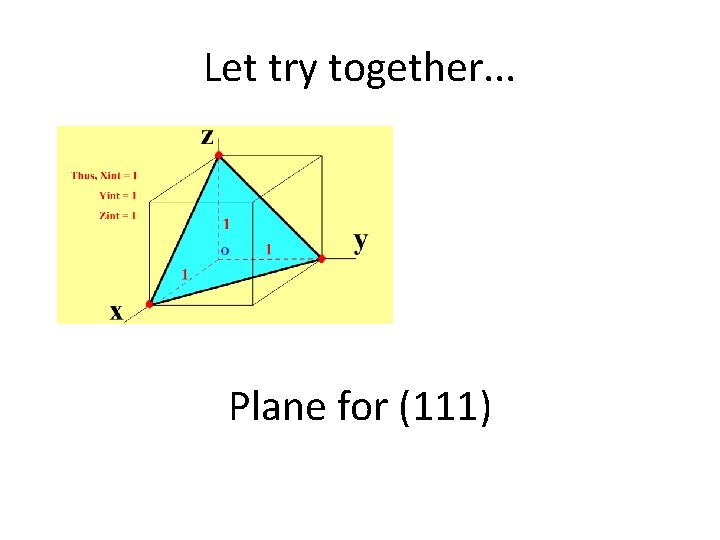
Let try together. . . Plane for (111)

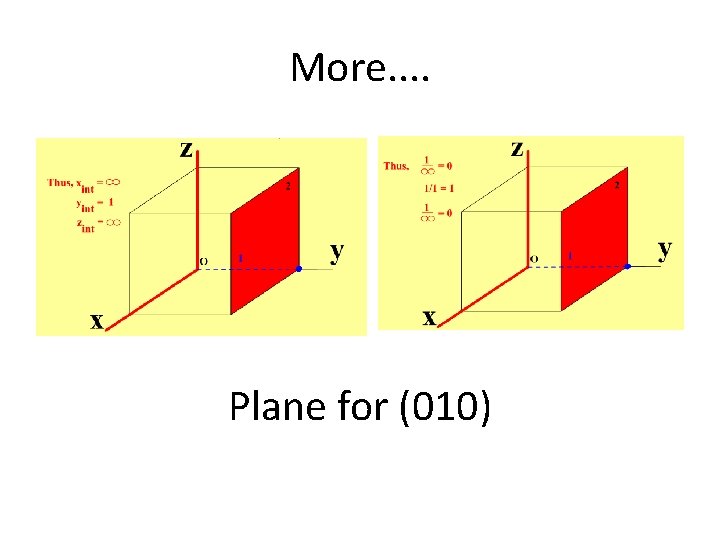
More. . Plane for (010)

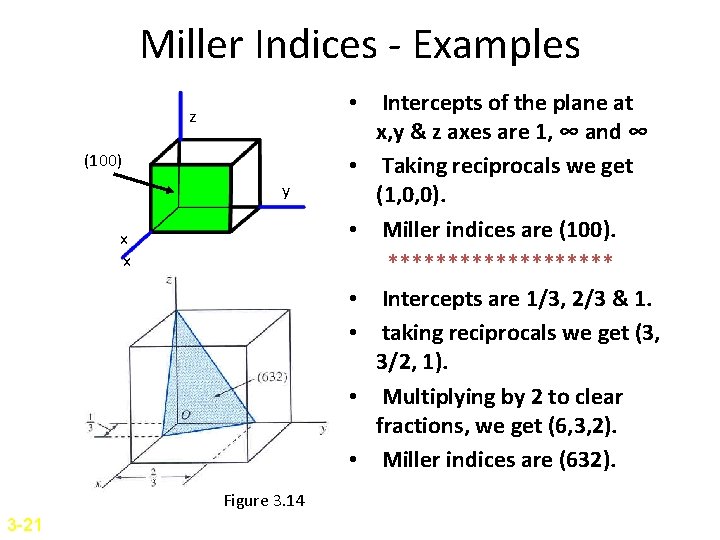
Miller Indices - Examples z (100) y x x Figure 3. 14 3 -21 • Intercepts of the plane at x, y & z axes are 1, ∞ and ∞ • Taking reciprocals we get (1, 0, 0). • Miller indices are (100). ********** • Intercepts are 1/3, 2/3 & 1. • taking reciprocals we get (3, 3/2, 1). • Multiplying by 2 to clear fractions, we get (6, 3, 2). • Miller indices are (632).

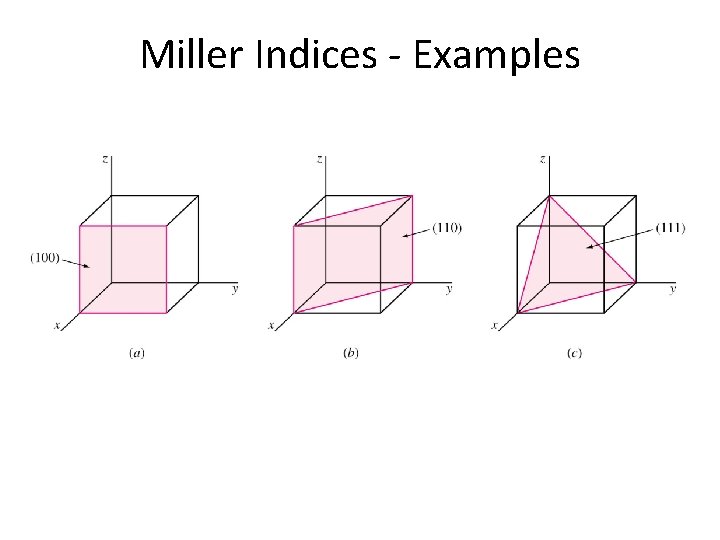
Miller Indices - Examples

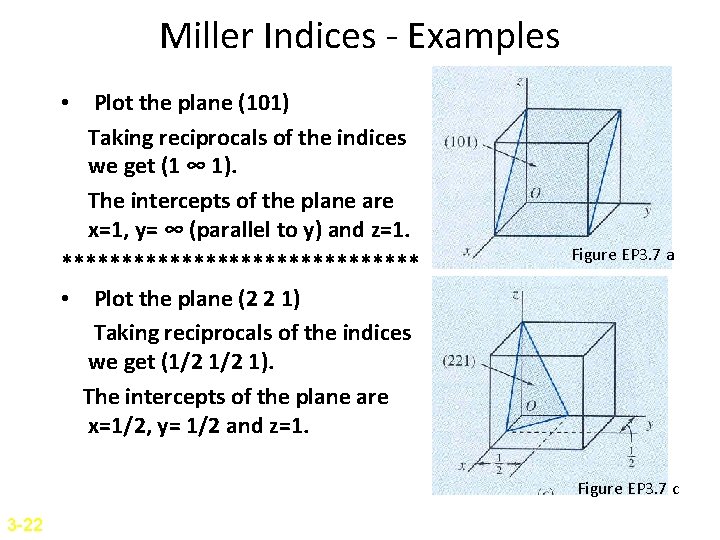
Miller Indices - Examples • Plot the plane (101) Taking reciprocals of the indices we get (1 ∞ 1). The intercepts of the plane are x=1, y= ∞ (parallel to y) and z=1. *************** • Plot the plane (2 2 1) Taking reciprocals of the indices we get (1/2 1). The intercepts of the plane are x=1/2, y= 1/2 and z=1. Figure EP 3. 7 a Figure EP 3. 7 c 3 -22

Remember…. • All equal spaced parallel planes are indicated by the same Miller indices. • If sets of equivalent lattice planes are related by the symmetry of the crystal system, they are called planes of a family or form. • Example: Miller indices (100), (010), (001) are designated as a family by notation {100}.

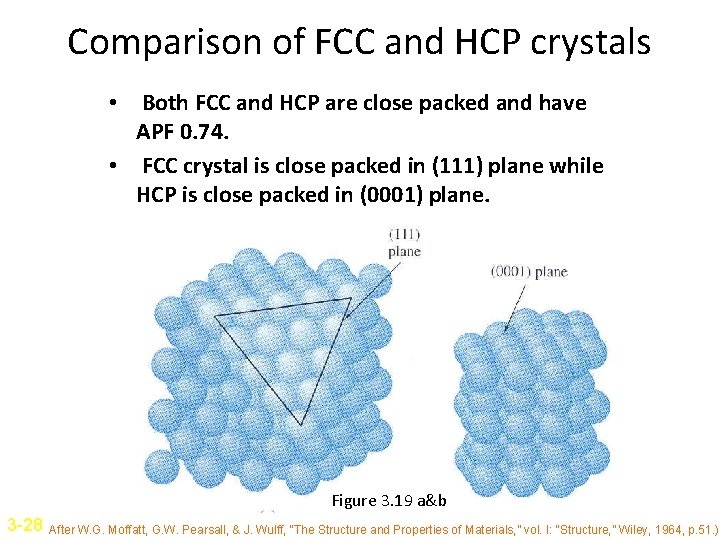
Comparison of FCC and HCP crystals • Both FCC and HCP are close packed and have APF 0. 74. • FCC crystal is close packed in (111) plane while HCP is close packed in (0001) plane. Figure 3. 19 a&b 3 -28 After W. G. Moffatt, G. W. Pearsall, & J. Wulff, “The Structure and Properties of Materials, ” vol. I: “Structure, ” Wiley, 1964, p. 51. )

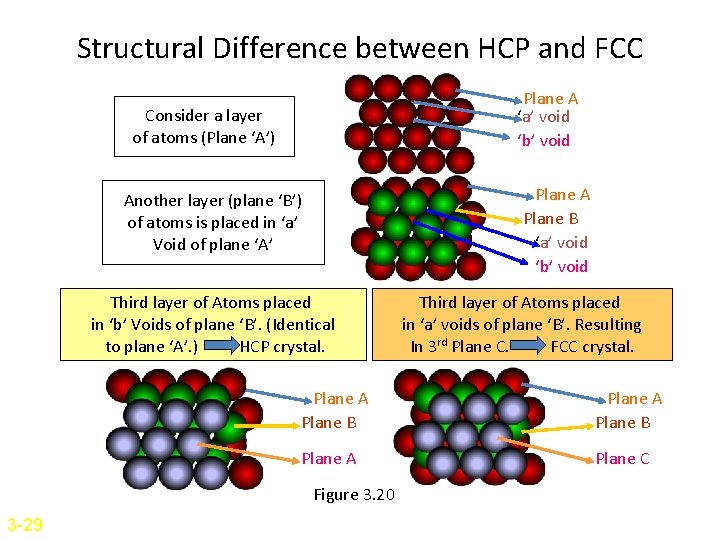
Structural Difference between HCP and FCC Plane A ‘a’ void ‘b’ void Consider a layer of atoms (Plane ‘A’) Plane A Plane B ‘a’ void ‘b’ void Another layer (plane ‘B’) of atoms is placed in ‘a’ Void of plane ‘A’ Third layer of Atoms placed in ‘b’ Voids of plane ‘B’. (Identical to plane ‘A’. ) HCP crystal. Plane A Plane B Plane A Plane C Figure 3. 20 3 -29 Third layer of Atoms placed in ‘a’ voids of plane ‘B’. Resulting In 3 rd Plane C. FCC crystal.

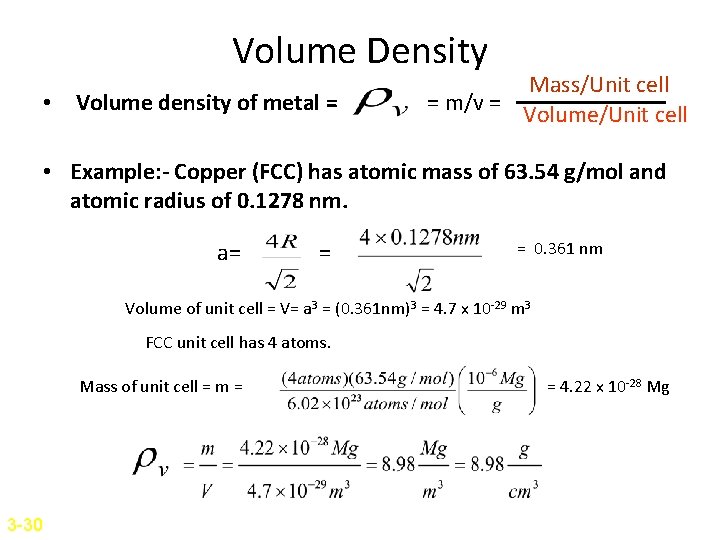
Volume Density • Volume density of metal = Mass/Unit cell = m/v = Volume/Unit cell • Example: - Copper (FCC) has atomic mass of 63. 54 g/mol and atomic radius of 0. 1278 nm. a= = = 0. 361 nm Volume of unit cell = V= a 3 = (0. 361 nm)3 = 4. 7 x 10 -29 m 3 FCC unit cell has 4 atoms. Mass of unit cell = m = 3 -30 = 4. 22 x 10 -28 Mg

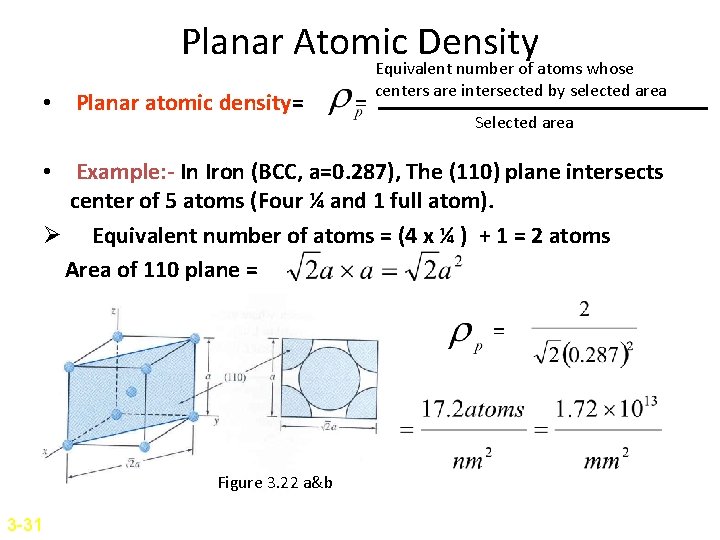
Planar Atomic Density • Planar atomic density= = Equivalent number of atoms whose centers are intersected by selected area Selected area • Example: - In Iron (BCC, a=0. 287), The (110) plane intersects center of 5 atoms (Four ¼ and 1 full atom). Ø Equivalent number of atoms = (4 x ¼ ) + 1 = 2 atoms Area of 110 plane = = Figure 3. 22 a&b 3 -31

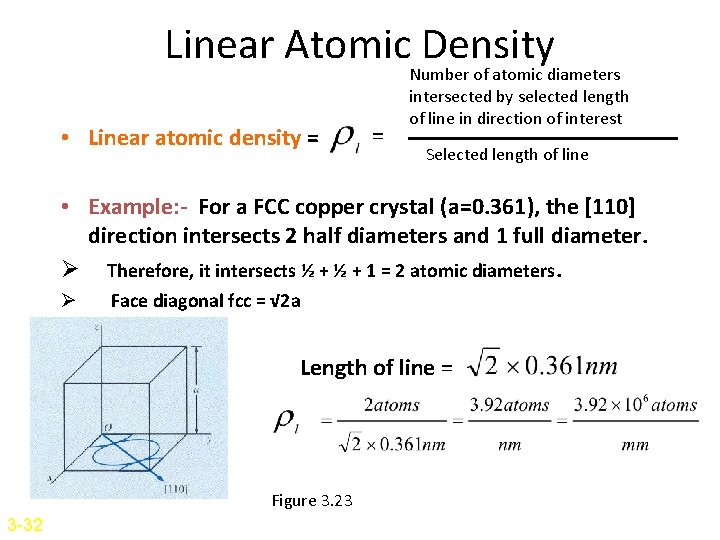
Linear Atomic Density • Linear atomic density = = Number of atomic diameters intersected by selected length of line in direction of interest Selected length of line • Example: - For a FCC copper crystal (a=0. 361), the [110] direction intersects 2 half diameters and 1 full diameter. Ø Therefore, it intersects ½ + 1 = 2 atomic diameters. Ø Face diagonal fcc = √ 2 a Length of line = Figure 3. 23 3 -32

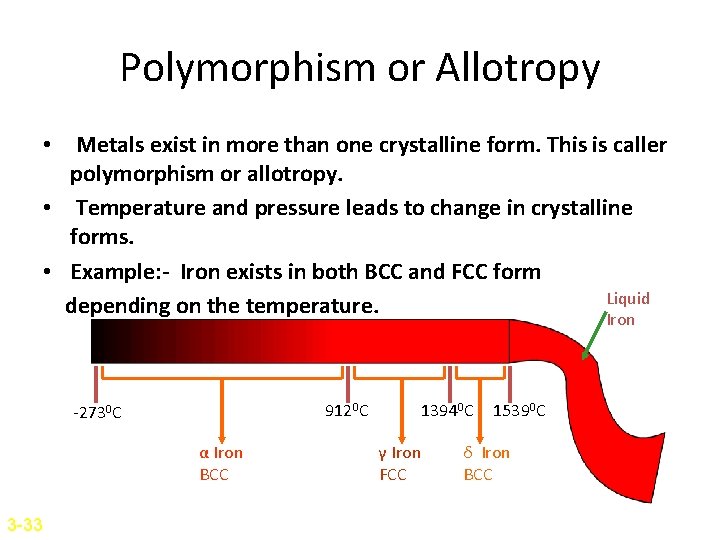
Polymorphism or Allotropy • Metals exist in more than one crystalline form. This is caller polymorphism or allotropy. • Temperature and pressure leads to change in crystalline forms. • Example: - Iron exists in both BCC and FCC form Liquid depending on the temperature. Iron 9120 C -2730 C α Iron BCC 3 -33 13940 C γ Iron FCC 15390 C δ Iron BCC

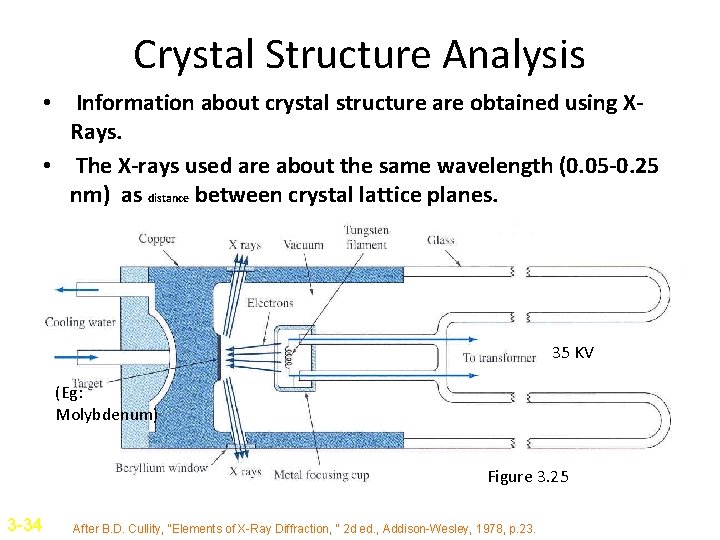
Crystal Structure Analysis • Information about crystal structure are obtained using XRays. • The X-rays used are about the same wavelength (0. 05 -0. 25 nm) as distance between crystal lattice planes. 35 KV (Eg: Molybdenum) Figure 3. 25 3 -34 After B. D. Cullity, “Elements of X-Ray Diffraction, “ 2 d ed. , Addison-Wesley, 1978, p. 23.

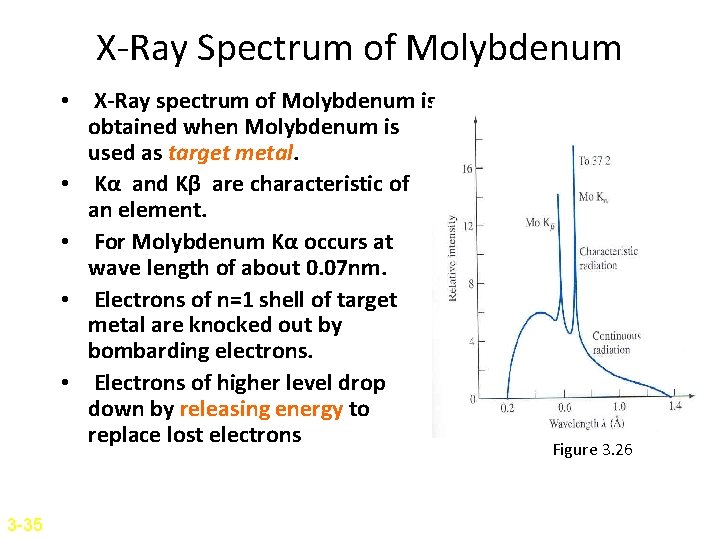
X-Ray Spectrum of Molybdenum • X-Ray spectrum of Molybdenum is obtained when Molybdenum is used as target metal. • Kα and Kβ are characteristic of an element. • For Molybdenum Kα occurs at wave length of about 0. 07 nm. • Electrons of n=1 shell of target metal are knocked out by bombarding electrons. • Electrons of higher level drop down by releasing energy to replace lost electrons 3 -35 Figure 3. 26

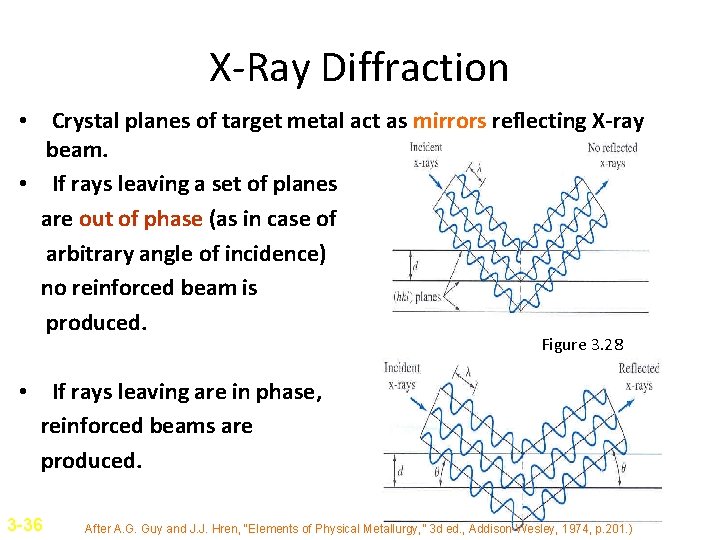
X-Ray Diffraction • Crystal planes of target metal act as mirrors reflecting X-ray beam. • If rays leaving a set of planes are out of phase (as in case of arbitrary angle of incidence) no reinforced beam is produced. Figure 3. 28 • If rays leaving are in phase, reinforced beams are produced. 3 -36 After A. G. Guy and J. J. Hren, “Elements of Physical Metallurgy, ” 3 d ed. , Addison-Wesley, 1974, p. 201. )

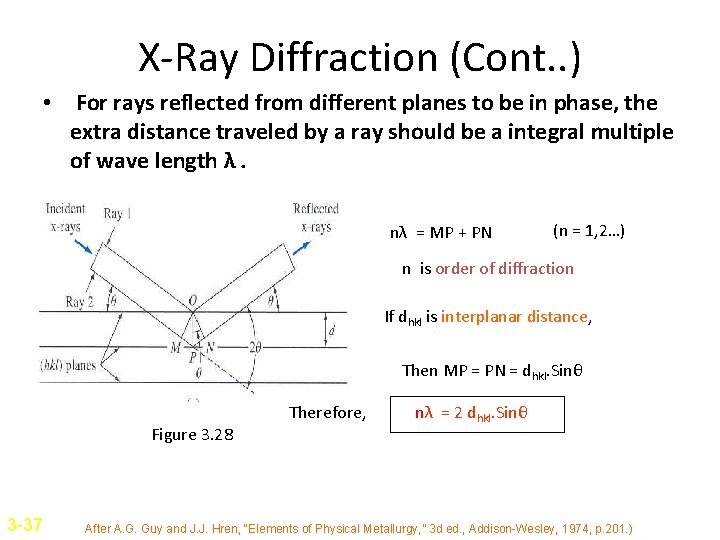
X-Ray Diffraction (Cont. . ) • For rays reflected from different planes to be in phase, the extra distance traveled by a ray should be a integral multiple of wave length λ. nλ = MP + PN (n = 1, 2…) n is order of diffraction If dhkl is interplanar distance, Then MP = PN = dhkl. Sinθ Therefore, Figure 3. 28 3 -37 nλ = 2 dhkl. Sinθ After A. G. Guy and J. J. Hren, “Elements of Physical Metallurgy, ” 3 d ed. , Addison-Wesley, 1974, p. 201. )

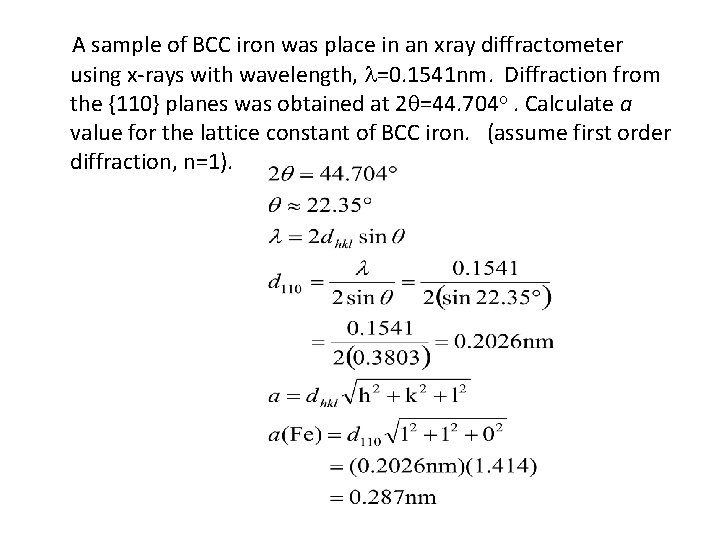
A sample of BCC iron was place in an xray diffractometer using x-rays with wavelength, =0. 1541 nm. Diffraction from the {110} planes was obtained at 2 =44. 704 . Calculate a value for the lattice constant of BCC iron. (assume first order diffraction, n=1).

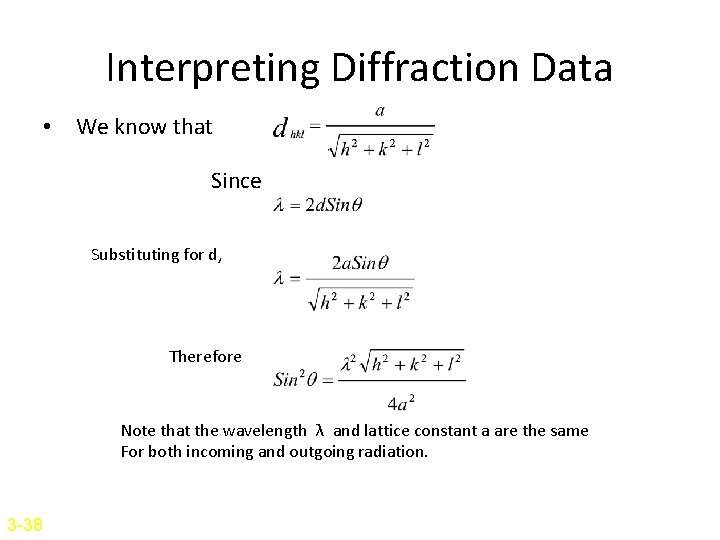
Interpreting Diffraction Data • We know that Since Substituting for d, Therefore Note that the wavelength λ and lattice constant a are the same For both incoming and outgoing radiation. 3 -38

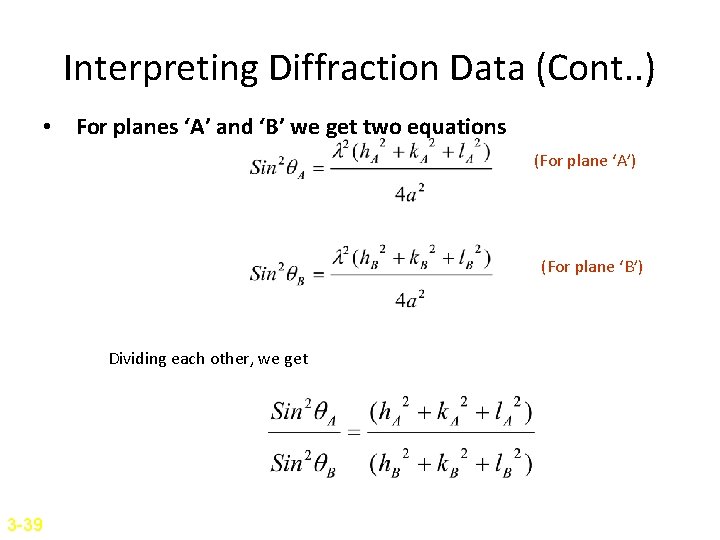
Interpreting Diffraction Data (Cont. . ) • For planes ‘A’ and ‘B’ we get two equations (For plane ‘A’) (For plane ‘B’) Dividing each other, we get 3 -39

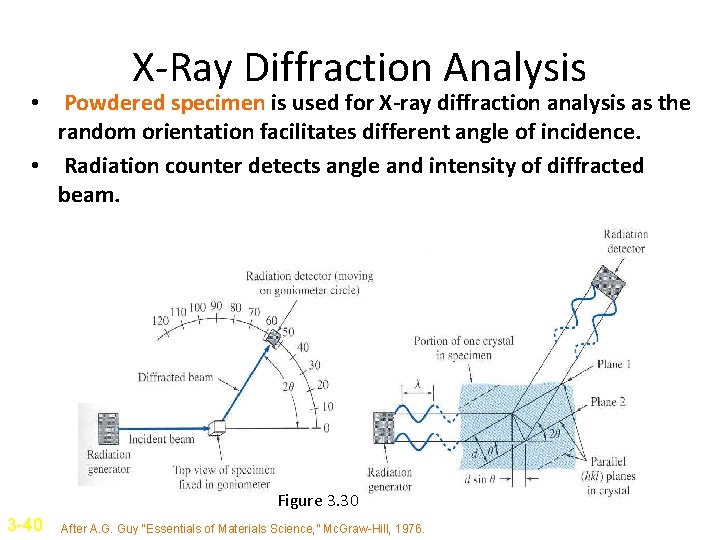
X-Ray Diffraction Analysis • Powdered specimen is used for X-ray diffraction analysis as the random orientation facilitates different angle of incidence. • Radiation counter detects angle and intensity of diffracted beam. Figure 3. 30 3 -40 After A. G. Guy “Essentials of Materials Science, ” Mc. Graw-Hill, 1976.

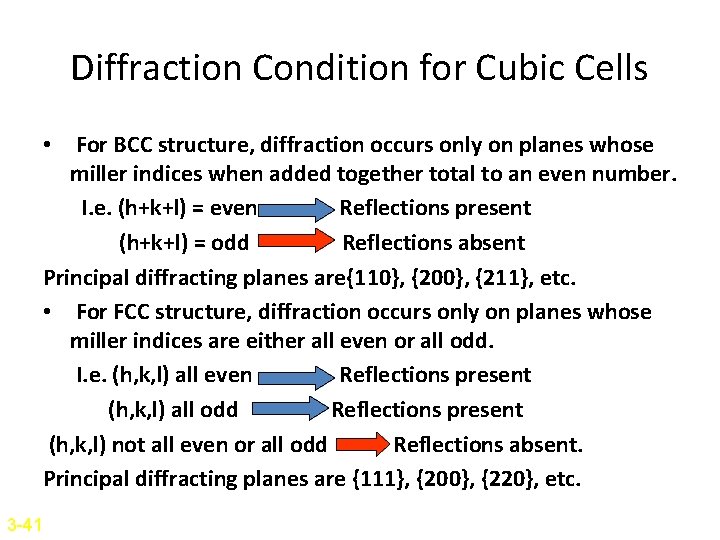
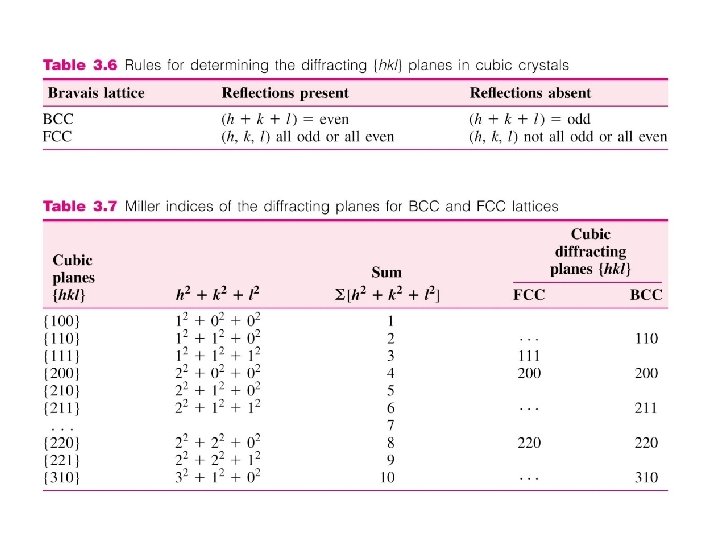
Diffraction Condition for Cubic Cells • For BCC structure, diffraction occurs only on planes whose miller indices when added together total to an even number. I. e. (h+k+l) = even Reflections present (h+k+l) = odd Reflections absent Principal diffracting planes are{110}, {200}, {211}, etc. • For FCC structure, diffraction occurs only on planes whose miller indices are either all even or all odd. I. e. (h, k, l) all even Reflections present (h, k, l) all odd Reflections present (h, k, l) not all even or all odd Reflections absent. Principal diffracting planes are {111}, {200}, {220}, etc. 3 -41


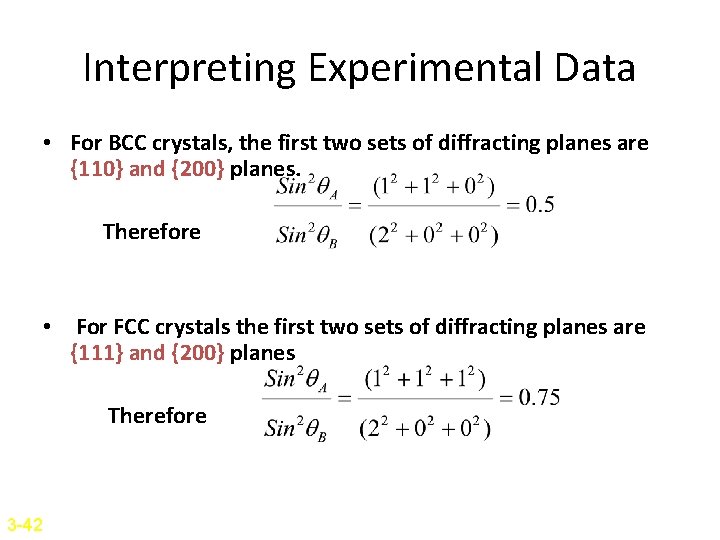
Interpreting Experimental Data • For BCC crystals, the first two sets of diffracting planes are {110} and {200} planes. Therefore • For FCC crystals the first two sets of diffracting planes are {111} and {200} planes Therefore 3 -42

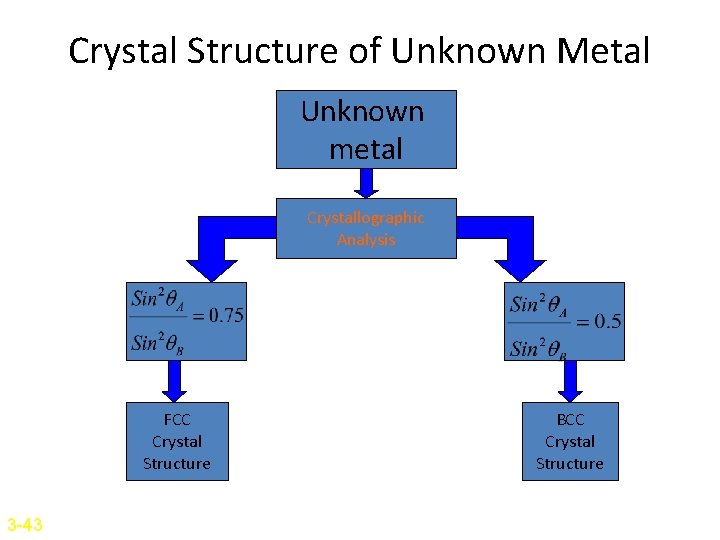
Crystal Structure of Unknown Metal Unknown metal Crystallographic Analysis FCC Crystal Structure 3 -43 BCC Crystal Structure

Amorphous Materials • Random spatial positions of atoms • Polymers: Secondary bonds do not allow formation of parallel and tightly packed chains during solidification. E. g. polyvinylchloride Ø Polymers can be semicrystalline. E. g. polyethylene • Glass is a ceramic made up of Si. O 4 4 - tetrahedron subunits – limited mobility. • Rapid cooling of metals (10 8 K/s) can give rise to amorphous structure (metallic glass). • Metallic glass has superior metallic properties.

a) long-range order in crystalline silica b) Silica glass without long-range order c) Amorphous structure in polymers.

Assignment Today. . do it now