Integrated Computational Materials Engineering Education Lecture on Molecular

![The Link Equilibrium Statistical Mechanics A(N, T, V) vs. A[{r. N(t)}, {p. N(t)}] Macroscopic The Link Equilibrium Statistical Mechanics A(N, T, V) vs. A[{r. N(t)}, {p. N(t)}] Macroscopic](https://slidetodoc.com/presentation_image_h2/52ad031b1a514c1685f107a0f29e6f1b/image-5.jpg)








- Slides: 38

Integrated Computational Materials Engineering Education Lecture on Molecular Dynamics An Introduction Liang Qi Department of Materials Science and Engineering University of Michigan Ann Arbor, MI, 48109 MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Acknowledgements • Portions of these lectures are based on material put together by Mark Asta (UC Berkeley), Daryl Chrzan (UC Berkeley), and Jeff Grossman (MIT) as part of a TMS short course that the three of us taught at the 2010 annual meeting. • The Division of Materials Research at the National Science Foundation is acknowledged for generously providing financial support MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

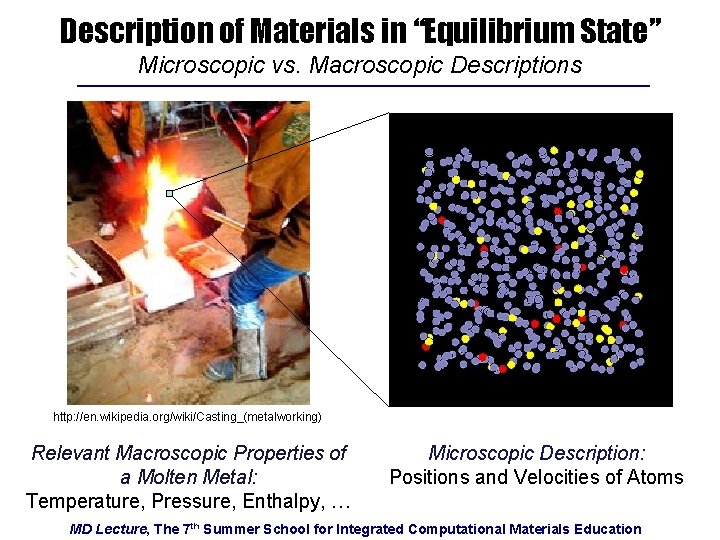
Description of Materials in “Equilibrium State” Microscopic vs. Macroscopic Descriptions http: //en. wikipedia. org/wiki/Casting_(metalworking) Relevant Macroscopic Properties of a Molten Metal: Temperature, Pressure, Enthalpy, … Microscopic Description: Positions and Velocities of Atoms MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

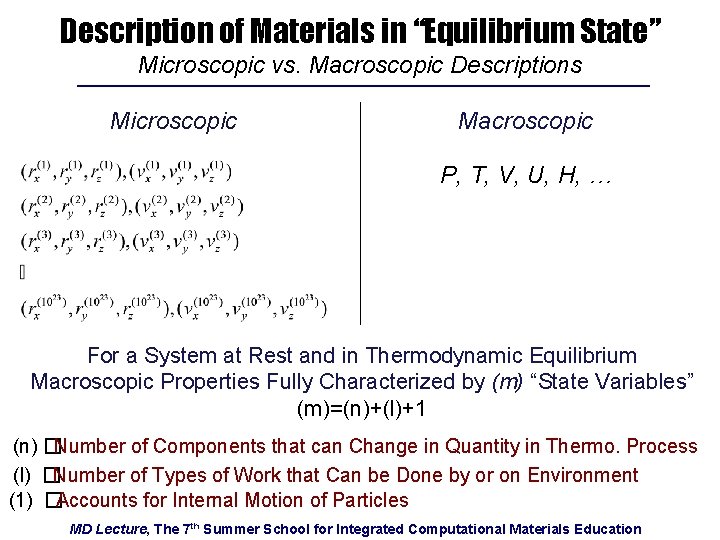
Description of Materials in “Equilibrium State” Microscopic vs. Macroscopic Descriptions Microscopic Macroscopic P, T, V, U, H, … For a System at Rest and in Thermodynamic Equilibrium Macroscopic Properties Fully Characterized by (m) “State Variables” (m)=(n)+(l)+1 (n) �Number of Components that can Change in Quantity in Thermo. Process (l) �Number of Types of Work that Can be Done by or on Environment (1) �Accounts for Internal Motion of Particles MD Lecture, The 7 th Summer School for Integrated Computational Materials Education
![The Link Equilibrium Statistical Mechanics AN T V vs Ar Nt p Nt Macroscopic The Link Equilibrium Statistical Mechanics A(N, T, V) vs. A[{r. N(t)}, {p. N(t)}] Macroscopic](https://slidetodoc.com/presentation_image_h2/52ad031b1a514c1685f107a0f29e6f1b/image-5.jpg)
The Link Equilibrium Statistical Mechanics A(N, T, V) vs. A[{r. N(t)}, {p. N(t)}] Macroscopic “Thermodynamic” value of Property A (e. g. , Energy Pressure, Order Parameter, …) Microscopic {r. N(t)} -> Positions of all Atoms {p. N(t)} -> Momenta of all Atoms MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

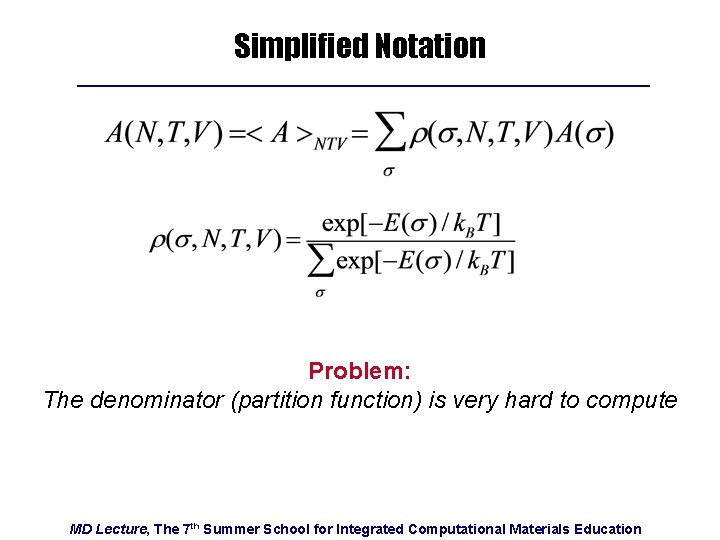
Simplified Notation Problem: The denominator (partition function) is very hard to compute MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

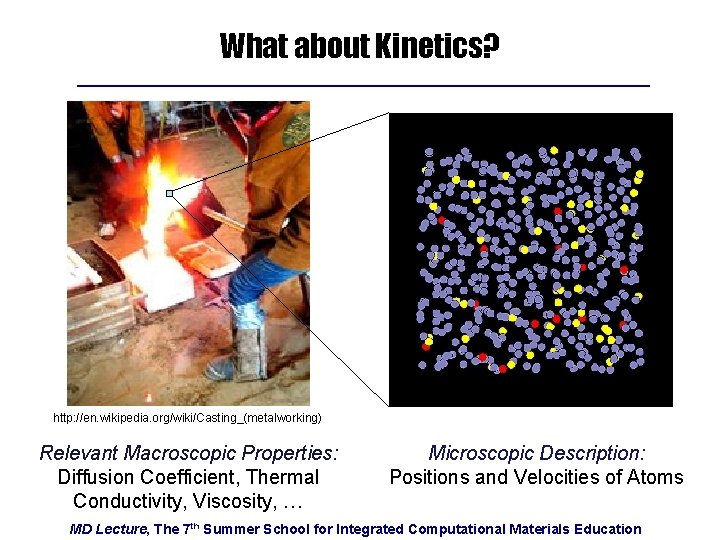
What about Kinetics? http: //en. wikipedia. org/wiki/Casting_(metalworking) Relevant Macroscopic Properties: Diffusion Coefficient, Thermal Conductivity, Viscosity, … Microscopic Description: Positions and Velocities of Atoms MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

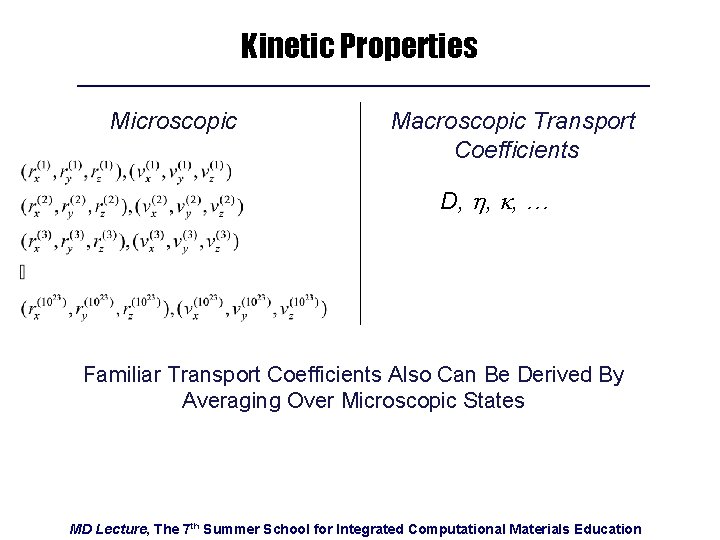
Kinetic Properties Microscopic Macroscopic Transport Coefficients D, h, k, … Familiar Transport Coefficients Also Can Be Derived By Averaging Over Microscopic States MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

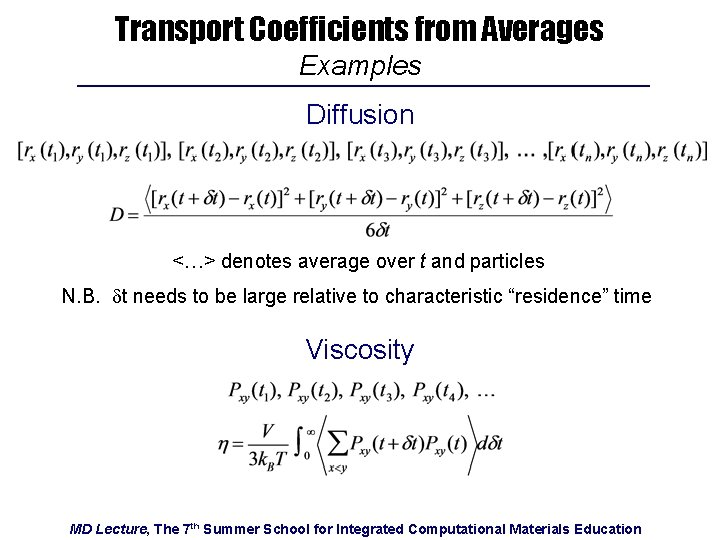
Transport Coefficients from Averages Examples Diffusion <…> denotes average over t and particles N. B. dt needs to be large relative to characteristic “residence” time Viscosity MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

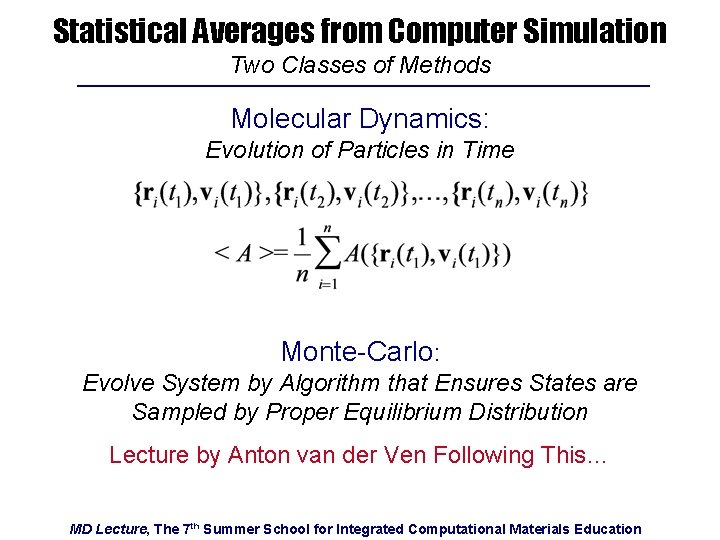
Statistical Averages from Computer Simulation Two Classes of Methods Molecular Dynamics: Evolution of Particles in Time Monte-Carlo: Evolve System by Algorithm that Ensures States are Sampled by Proper Equilibrium Distribution Lecture by Anton van der Ven Following This… MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Outline • Nuts and bolts – Equations of motion and their numerical integration – How to obtain intermolecular forces • Some examples – Melting point – Liquid densities and diffusion coefficients – Interface mobilities • Teaching/Learning MD – Available codes – Some examples to work through MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Integration of Newton’s Equations of Motion Verlet Algorithm Newton’s Equations for Ions … … Verlet Algorithm: Some Variants: Velocity Verlet and Leapfrog MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

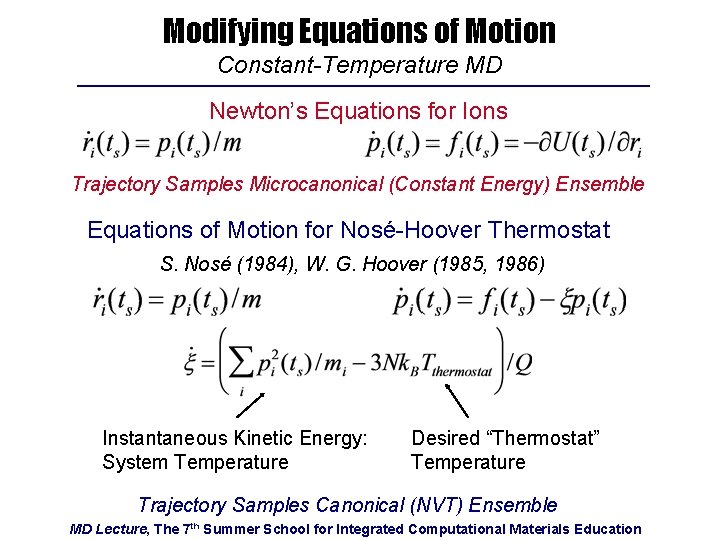
Modifying Equations of Motion Constant-Temperature MD Newton’s Equations for Ions Trajectory Samples Microcanonical (Constant Energy) Ensemble Equations of Motion for Nosé-Hoover Thermostat S. Nosé (1984), W. G. Hoover (1985, 1986) Instantaneous Kinetic Energy: System Temperature Desired “Thermostat” Temperature Trajectory Samples Canonical (NVT) Ensemble MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

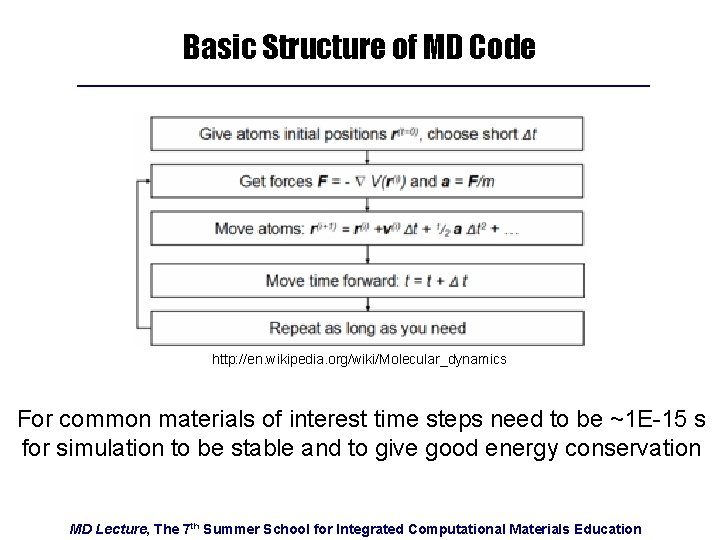
Basic Structure of MD Code http: //en. wikipedia. org/wiki/Molecular_dynamics For common materials of interest time steps need to be ~1 E-15 s for simulation to be stable and to give good energy conservation MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Intermolecular Forces Algorithm requires knowledge of forces on atoms at each time step Two main approaches: 1. Forces from DFT − − − Accurate chemistry Computationally demanding Simulations limited to ~500 atoms, ~10 -100 ps in time 2. Classical interatomic potentials − − − Classical descriptions of intermolecular forces in terms of bond distances and bond angles More challenging to get chemistry right across many environments But, enables much longer and larger systems (millions of atoms and microseconds relatively routine) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Classical Interatomic Potentials • Large-scale atomistic simulations require simplified models of interatomic interactions – Energies and forces typically expressed only in terms of ion positions – In some cases electronic degrees of freedom also represented (e. g. , through charges & polarizabilities) • Ingredients – Bond stretching/breaking, bond bending – May include additional “non-bonded” terms from electrostatic & van der Waals interactions – To increase transferability, often necessary to incorporate environmental dependencies leading to many-body potential formalisms • Some background references http: //cmm. info. nih. gov/intro_simulation/node 15. html – M. W. Finnis, Interatomic Forces in Condensed Matter, (Oxford University Press, 2003) – Y. Mishin, M. Asta and J. Li, Acta Mater. 58, 1117 -1151 (2010) – R Drautz, XW Zhou, DA Murdick, B Gillespie, HNG Wadley and DG Pettifor, “Modelling Electrons and Atoms for Materials Science, ” Prog Mater Sci 52, 196 (2007) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

“Pair Functionals” for Metallic Bonding Non-Linear Function of Local Environment-Independent Pair-Potential Environment Characterized by Pair-Wise Sum over Radial Functions Modifications of Pair-Functional Form to Handle Angular Contributions Modified Embedded Atom Method: MI Baskes, Phys. Rev. Lett 59, 2666 (1987) Angularly Dependent Potentials: Y Mishin, MJ Mehl, DA Papaconstantopoulos, Acta Mater. 53, 4029 (2005) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Embedded-Atom/Effective-Medium-Theory From Jacobsen, Norskov and Puska (1987) Embedded-Atom Method M. S. Daw and M. I. Baskes, 1983, 1984 Effective Medium Theory J. K. Norskov, M. Manninen and co-workers, 1980 -1987 Energy to Embed Atom i in Charge Density ri Due to Neighboring Atoms MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Effective Pair-Potential Formalism (Carlsson, 1985; Foiles, 1985; Finnis and Sinclair, 1984, 1986) Effective Pair Potential MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

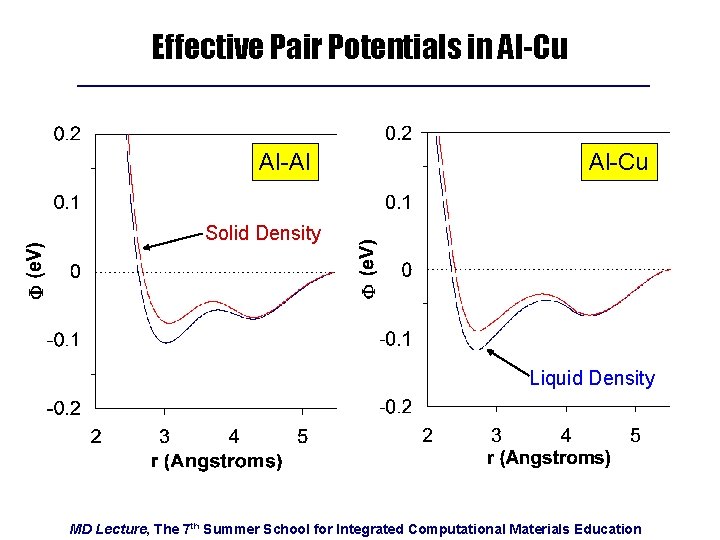
Effective Pair Potentials in Al-Cu Al-Al Al-Cu Solid Density Liquid Density MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

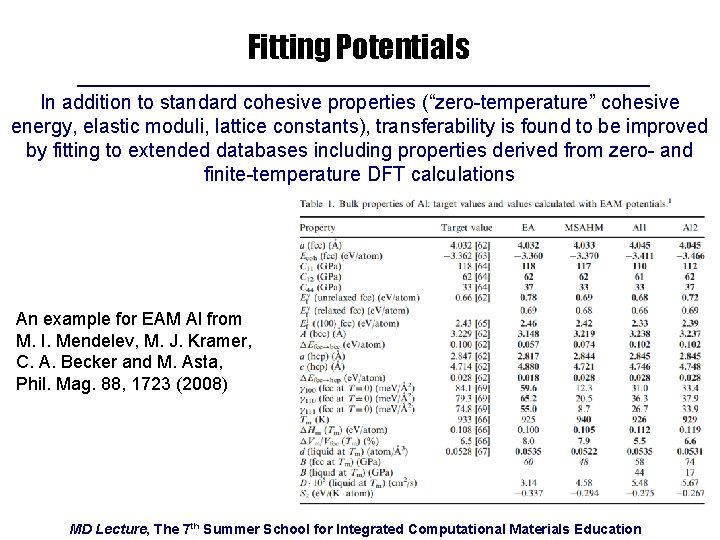
Fitting Potentials In addition to standard cohesive properties (“zero-temperature” cohesive energy, elastic moduli, lattice constants), transferability is found to be improved by fitting to extended databases including properties derived from zero- and finite-temperature DFT calculations An example for EAM Al from M. I. Mendelev, M. J. Kramer, C. A. Becker and M. Asta, Phil. Mag. 88, 1723 (2008) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

On-Line Resources http: //www. ctcms. nist. gov/potentials/ See also Knowledgebase of Interatomic Models Project: https: //openkim. org/ MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Outline • Nuts and bolts – Equations of motion and their numerical integration – How to obtain intermolecular forces • Some examples – Melting point – Liquid densities and diffusion coefficients – Interface mobilities • Teaching/Learning MD – Available codes – Some examples to work through MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

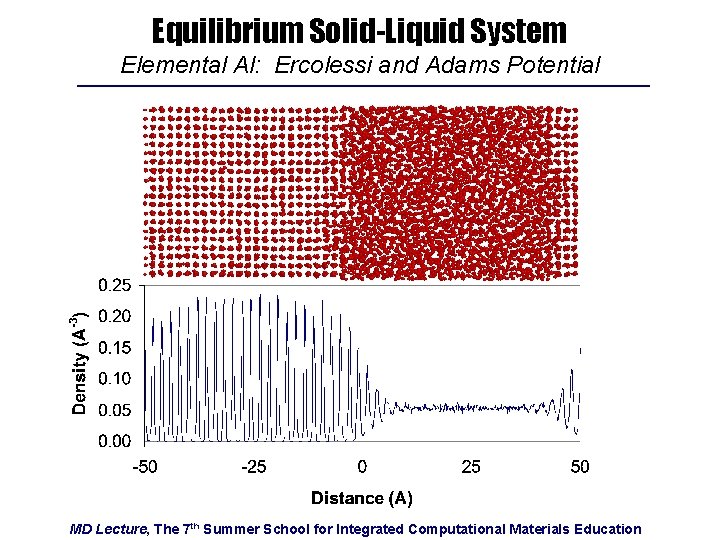
Equilibrium Solid-Liquid System Elemental Al: Ercolessi and Adams Potential MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

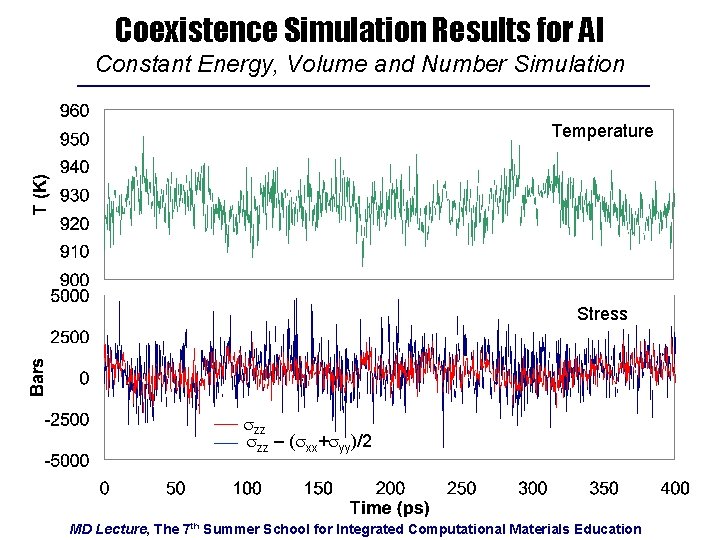
Coexistence Simulation Results for Al Constant Energy, Volume and Number Simulation Temperature Stress szz – (sxx+syy)/2 MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Analysis MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

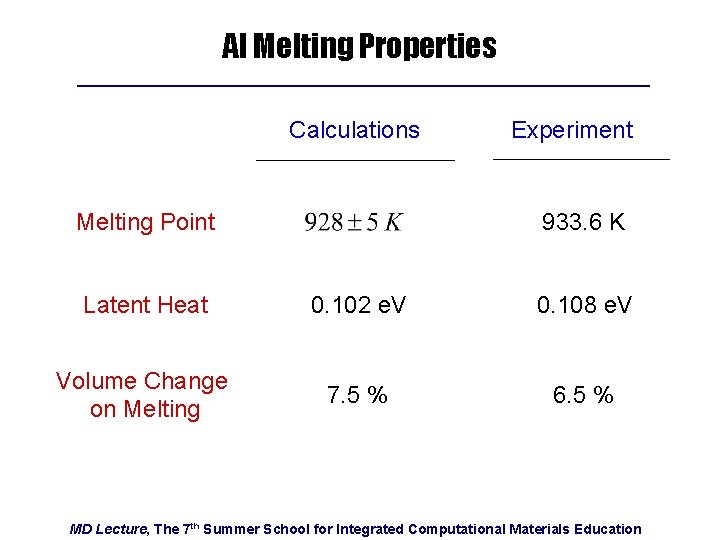
Al Melting Properties Calculations Melting Point Experiment 933. 6 K Latent Heat 0. 102 e. V 0. 108 e. V Volume Change on Melting 7. 5 % 6. 5 % MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

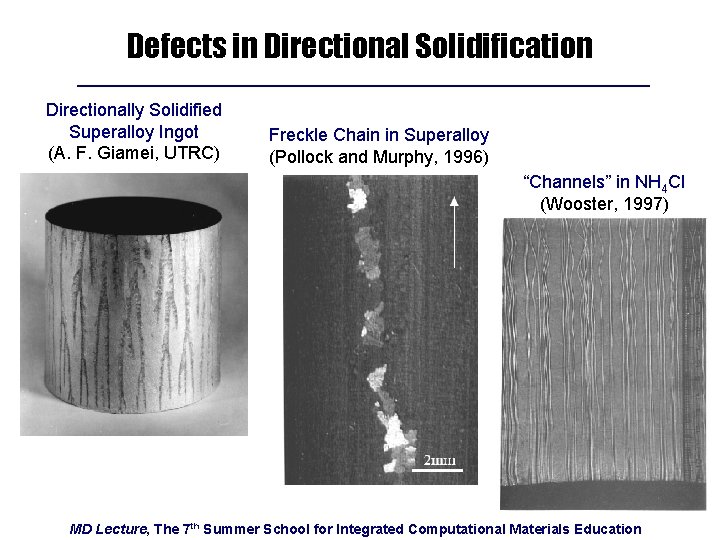
Defects in Directional Solidification Directionally Solidified Superalloy Ingot (A. F. Giamei, UTRC) Freckle Chain in Superalloy (Pollock and Murphy, 1996) “Channels” in NH 4 Cl (Wooster, 1997) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

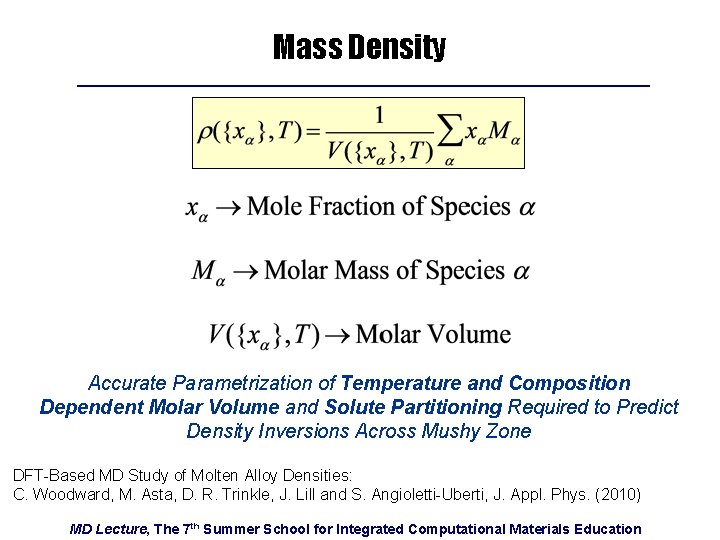
Mass Density Accurate Parametrization of Temperature and Composition Dependent Molar Volume and Solute Partitioning Required to Predict Density Inversions Across Mushy Zone DFT-Based MD Study of Molten Alloy Densities: C. Woodward, M. Asta, D. R. Trinkle, J. Lill and S. Angioletti-Uberti, J. Appl. Phys. (2010) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

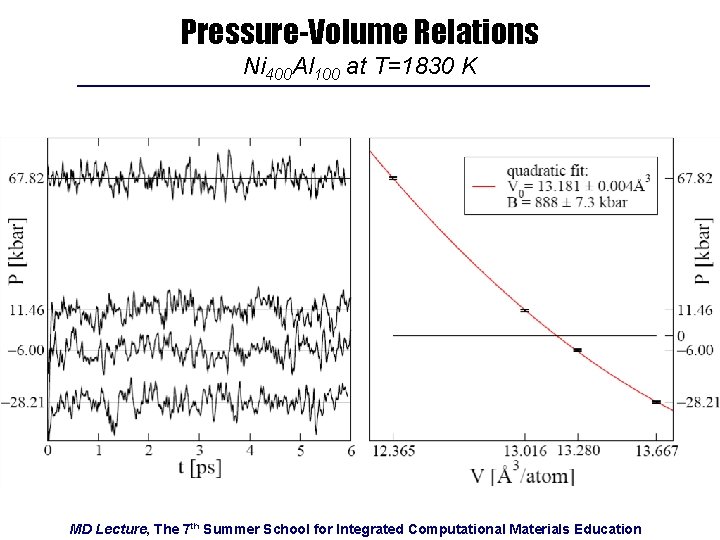
Pressure-Volume Relations Ni 400 Al 100 at T=1830 K MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

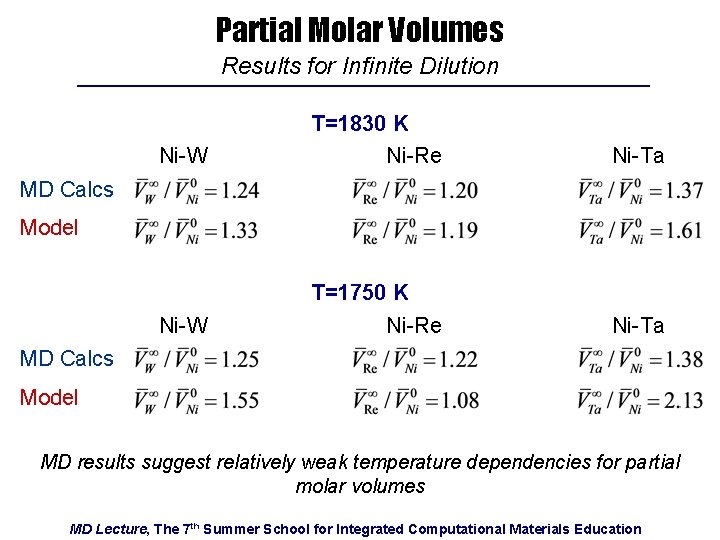
Partial Molar Volumes Results for Infinite Dilution Ni-W T=1830 K Ni-Re Ni-Ta Ni-W T=1750 K Ni-Re Ni-Ta MD Calcs Model MD results suggest relatively weak temperature dependencies for partial molar volumes MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

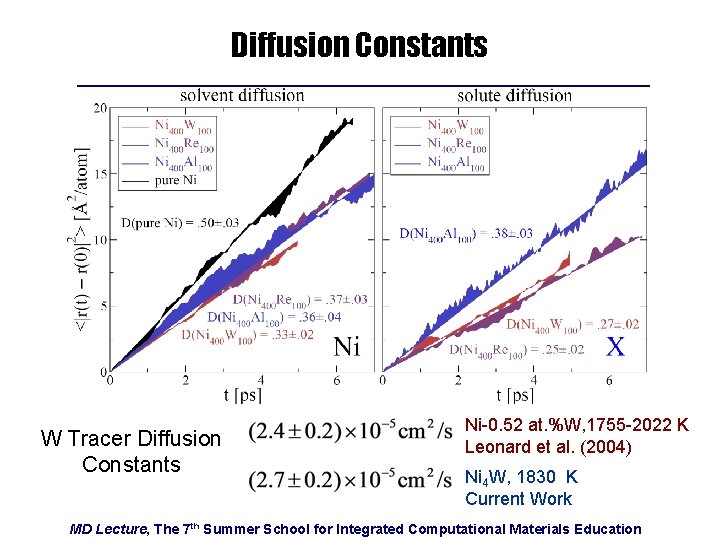
Diffusion Constants W Tracer Diffusion Constants Ni-0. 52 at. %W, 1755 -2022 K Leonard et al. (2004) Ni 4 W, 1830 K Current Work MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

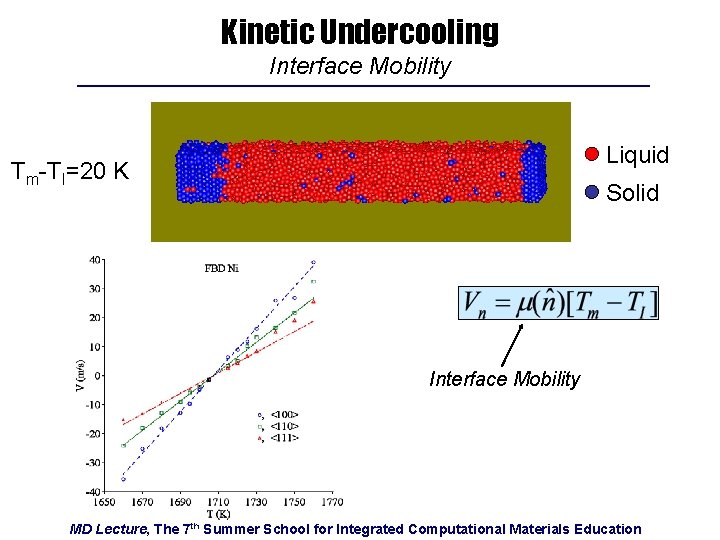
Kinetic Undercooling Interface Mobility Liquid Tm-TI=20 K Solid Interface Mobility MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Outline • Nuts and bolts – Equations of motion and their numerical integration – How to obtain intermolecular forces • Some examples – Melting point – Liquid densities and diffusion coefficients – Interface mobilities • Teaching/Learning MD – Available codes – Some examples to work through MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Some Codes for Classical MD • LAMMPS (http: //lammps. sandia. gov/) – – Free to download Distributed by Steve Plimpton at Sandia Labs Interfaces with potentials available on NIST repository Highly scalable on parallel computers, many options for potentials, dynamics, on-the-fly analysis • GULP (http: //projects. ivec. org/gulp/) – Free to academics, commercially available through accelrys for commercial and government use – Widely used for ionic potentials (ceramics) – Includes utilities to fit potentials • nanohub. org – Several different packages to run several different classes of examples. One example: nano-Materials Simulation Toolkit MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

Teaching/Learning MD Objectives • Textbooks for an appreciation of “what is under the hood” − D. Frenkel and B. Smit, Understanding Molecular Simulations From Algorithms to Applications − M. P. Allen and D. J. Tildesley, Computer Simulations of Liquids • Using a standard package − LAMMPS or GULP can be installed or work with nanohub • Numerical issues in calculations − Effect of time steps − System size effects − Statistical analysis of data to derive a useful materials property (with uncertainty estimates) MD Lecture, The 7 th Summer School for Integrated Computational Materials Education

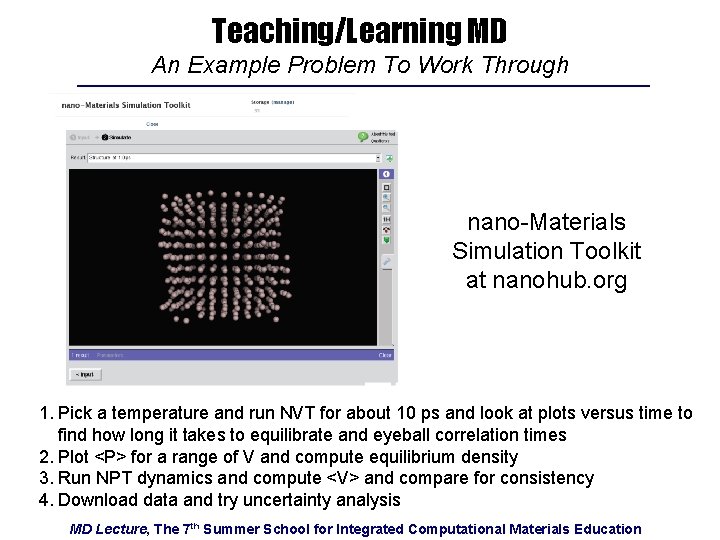
Teaching/Learning MD An Example Problem To Work Through nano-Materials Simulation Toolkit at nanohub. org 1. Pick a temperature and run NVT for about 10 ps and look at plots versus time to find how long it takes to equilibrate and eyeball correlation times 2. Plot <P> for a range of V and compute equilibrium density 3. Run NPT dynamics and compute <V> and compare for consistency 4. Download data and try uncertainty analysis MD Lecture, The 7 th Summer School for Integrated Computational Materials Education