Organic Chemistry Organic Chemistry the study of compounds


























- Slides: 57

Organic Chemistry

Organic Chemistry – the study of compounds containing carbon • Organic chemistry was thought to be the study of products made from organisms • Chemists obtain organic compounds in two principal ways: 1. isolation from nature 2. synthesis in the laboratory. • Compounds made in the laboratory are identical in both chemical and physical properties to those in nature.

Why Organic Chemistry? • Organic compounds are found everywhere around us (foods, flavors, fragrances, medicines, toiletries, cosmetics, plastic, paints, our body, and etc. ). • 85% of all known compounds are organic compounds. • Chemists have discovered or synthesized more than 10 million organic compounds.

Typical properties of organic compounds 1. They contain carbon atom. 2. They have covalent bonds. 3. They have low boiling point and melting point. 4. They are flammable (almost all burn). 5. They are soluble in nonpolar compounds. 6. They can be gas, liquid or solid. 7. Can form large molecules.

It is important to know: • Carbon: normally forms 4 covalent bonds and has no unshared pairs of electrons. • Hydrogen: forms 1 covalent bond and no unshared pairs of electrons. • Nitrogen: normally forms 3 covalent bonds and has one unshared pair of electrons. • Oxygen: normally forms 2 covalent bonds and has two unshared pairs of electrons. • Halogen: normally forms 1 covalent bond and has three unshared pairs of electrons.

Hydrocarbons are the large family of organic compounds and they contain only carbon and hydrogen atoms. Hydrocarbons are divided into the two groups: 1. Saturated hydrocarbon: a hydrocarbon that contains only carbon-carbon single bonds (alkanes) 2. Unsaturated hydrocarbon: a hydrocarbon that contains one or more carbon-carbon double bonds, triple bonds, or benzene rings.

Alkanes • Alkanes are saturated hydrocarbon (they have only carbon-carbon single bonds). • The molecular formula of this group is Cn. H 2 n+2 (n is number of carbon atoms). Chemical properties of alkanes: in general, they have low reactivity.

Physical properties of alkanes 1. 2. 3. 4. They are nonpolar. They are insoluble in water. They have a lower density than water. They have low boiling point and melting point. 5. They can be gas (with 1 to 4 carbon atoms), liquid (with 5 to 17 carbon atoms) or solid (with 18 or more carbon atoms).

• The boiling point and the melting point of alkanes increase by increasing the number of carbon atoms. • The boiling point and the melting point of alkanes decrease by increasing the number of branches.

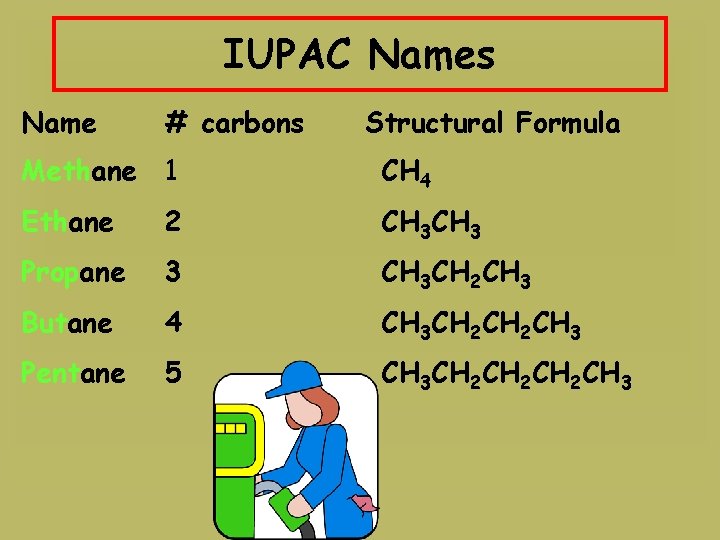
IUPAC Names Name # carbons Structural Formula Methane 1 CH 4 Ethane 2 CH 3 Propane 3 CH 3 CH 2 CH 3 Butane 4 CH 3 CH 2 CH 3 Pentane 5 CH 3 CH 2 CH 2 CH 3

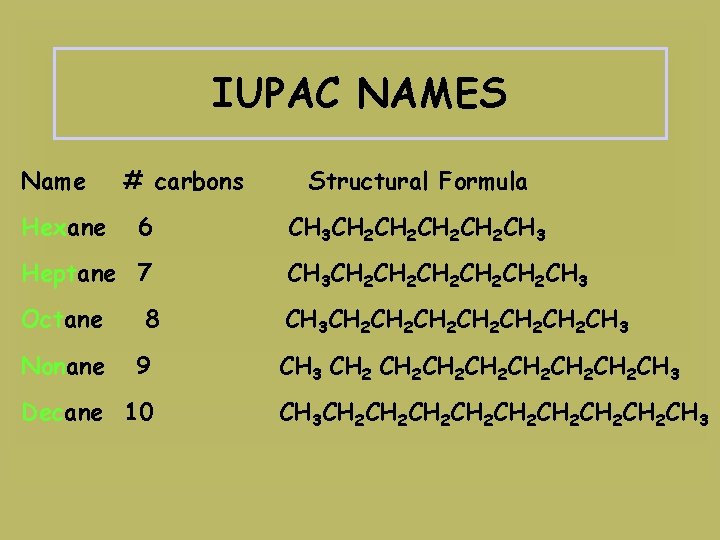
IUPAC NAMES Name Hexane # carbons 6 Structural Formula CH 3 CH 2 CH 2 CH 3 Heptane 7 CH 3 CH 2 CH 2 CH 2 CH 3 Octane CH 3 CH 2 CH 2 CH 2 CH 3 Nonane 8 9 Decane 10 CH 3 CH 2 CH 2 CH 3 CH 3 CH 2 CH 2 CH 3

Mnemonic for first four prefixes First four prefixes • • Meth. Eth. Prop. But- Monkeys Eat Peeled Bananas

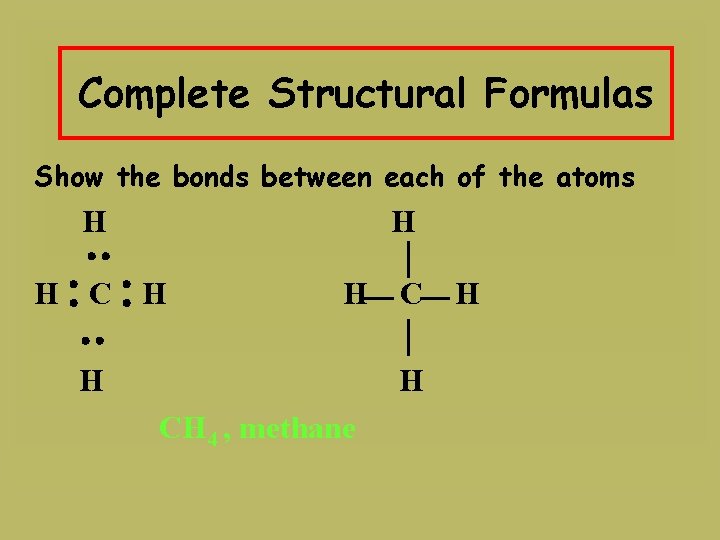
Complete Structural Formulas Show the bonds between each of the atoms H H H CH 4 , methane H

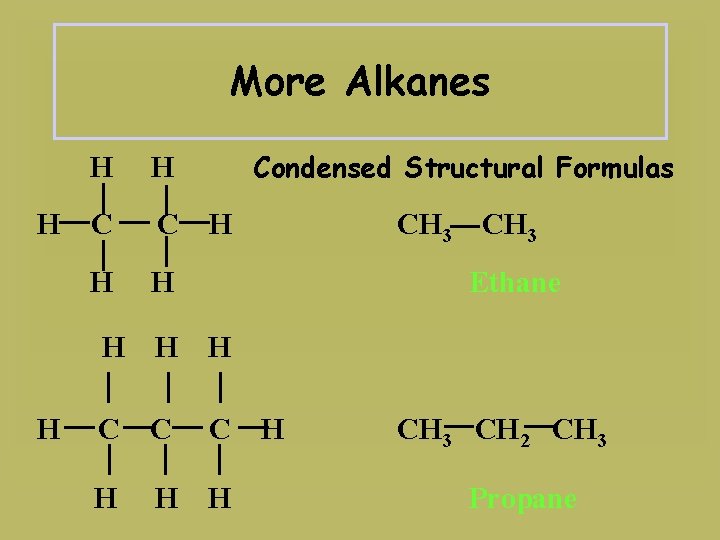
More Alkanes H H C C H H Condensed Structural Formulas H CH 3 Ethane H H H C C C H H CH 3 CH 2 CH 3 Propane

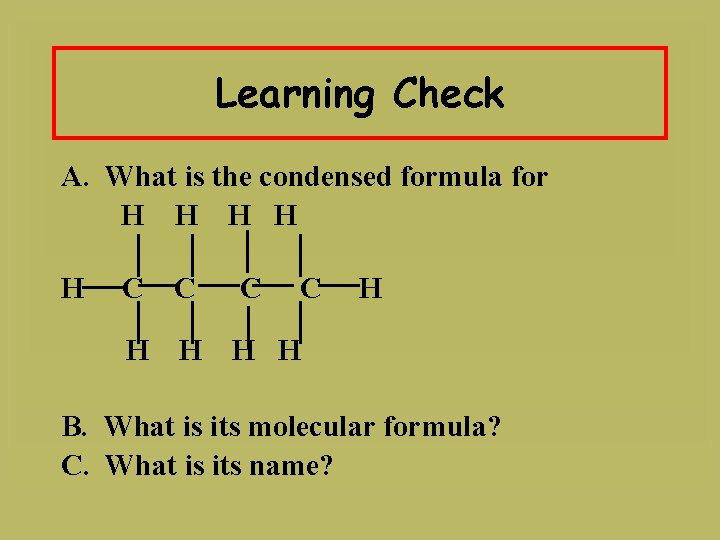
Learning Check A. What is the condensed formula for H H H C C H H H B. What is its molecular formula? C. What is its name?

Naming the unbranched alkanes The IUPAC name consists of two parts: 1. A prefix that shows the number of carbon atoms (meth-, prop-, but-, pent-, hex-, hept-, oct-, non- and dec-). 2. The suffix “-ane”. CH 4 Methane C 2 H 6 Ethane C 3 H 8 Propane C 4 H 10 Butane


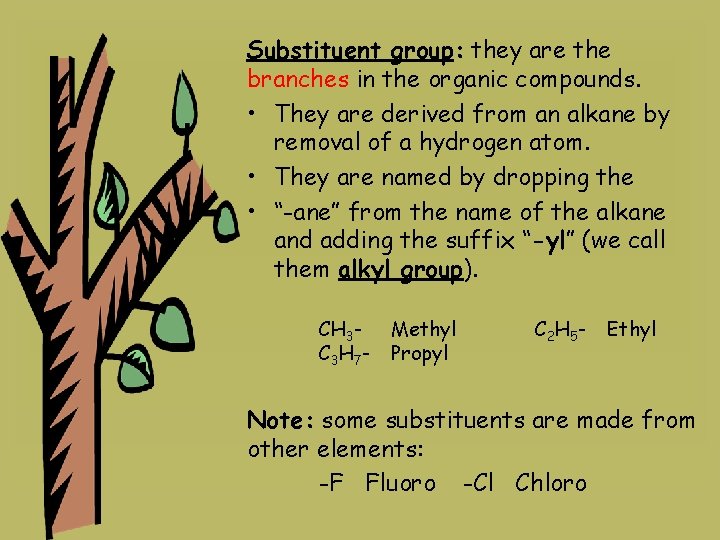
Substituent group: they are the branches in the organic compounds. • They are derived from an alkane by removal of a hydrogen atom. • They are named by dropping the • “-ane” from the name of the alkane and adding the suffix “-yl” (we call them alkyl group). CH 3 C 3 H 7 - Methyl Propyl C 2 H 5 - Ethyl Note: some substituents are made from other elements: -F Fluoro -Cl Chloro

Naming branched alkanes 1. Write the alkane name of the longest continuous chain of carbon atoms. 2. Number carbon atoms starting from the end nearest substituent. 3. Give the location and name of each substituent (alphabetical order) as a prefix to the name of the main chain.

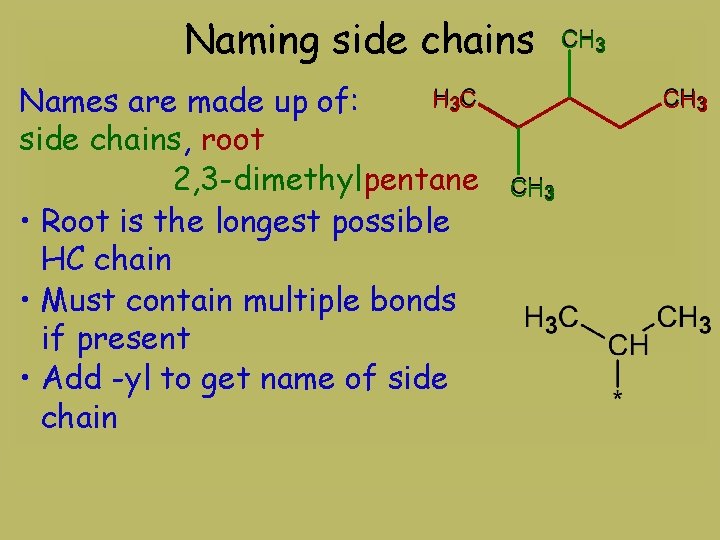
Naming side chains H 3 C Names are made up of: side chains, root 2, 3 -dimethylpentane • Root is the longest possible HC chain • Must contain multiple bonds if present • Add -yl to get name of side chain CH 3

Naming side chains 3 -methylhexane 4 -ethyl-2, 3 -dimethylheptane 5 -ethyl-2, 4, 6 -trimethyloctane

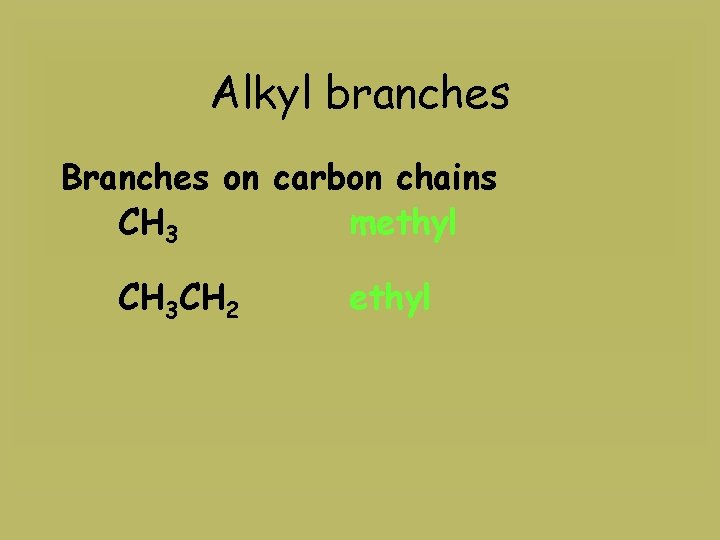
Alkyl branches Branches on carbon chains CH 3 methyl CH 3 CH 2 ethyl

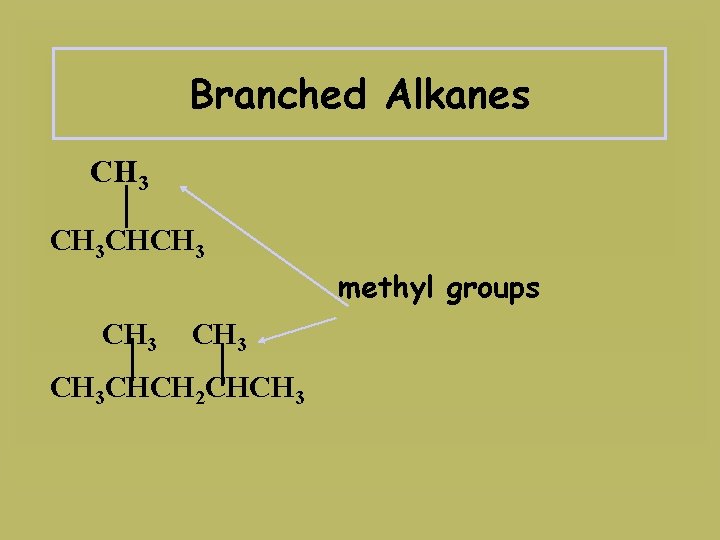
Branched Alkanes CH 3 CHCH 3 methyl groups CH 3 CHCH 2 CHCH 3

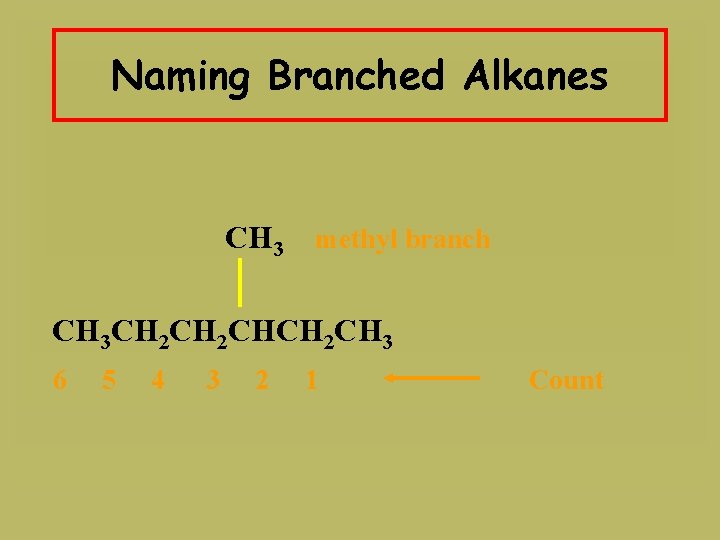
Naming Branched Alkanes CH 3 methyl branch CH 3 CH 2 CHCH 2 CH 3 6 5 4 3 2 1 Count

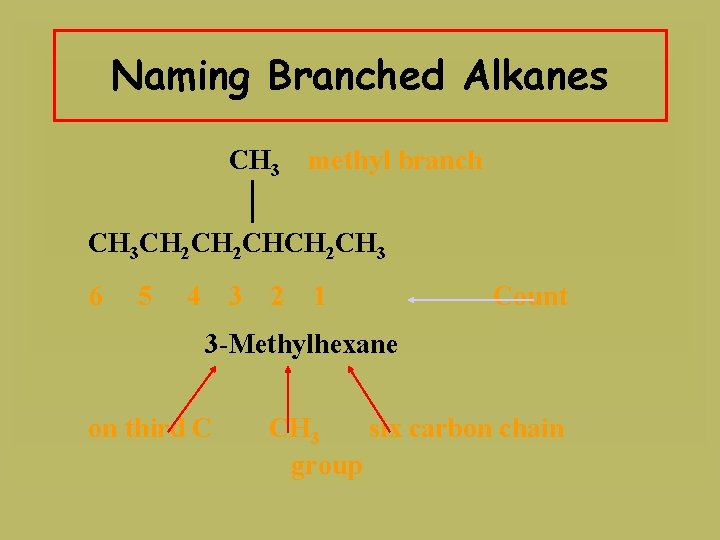
Naming Branched Alkanes CH 3 methyl branch CH 3 CH 2 CHCH 2 CH 3 6 5 4 3 2 1 Count 3 -Methylhexane on third C CH 3 six carbon chain group

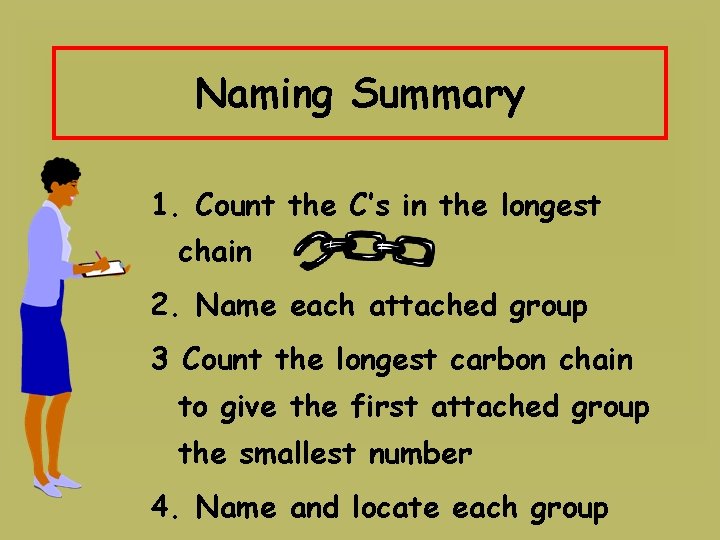
Naming Summary 1. Count the C’s in the longest chain 2. Name each attached group 3 Count the longest carbon chain to give the first attached group the smallest number 4. Name and locate each group

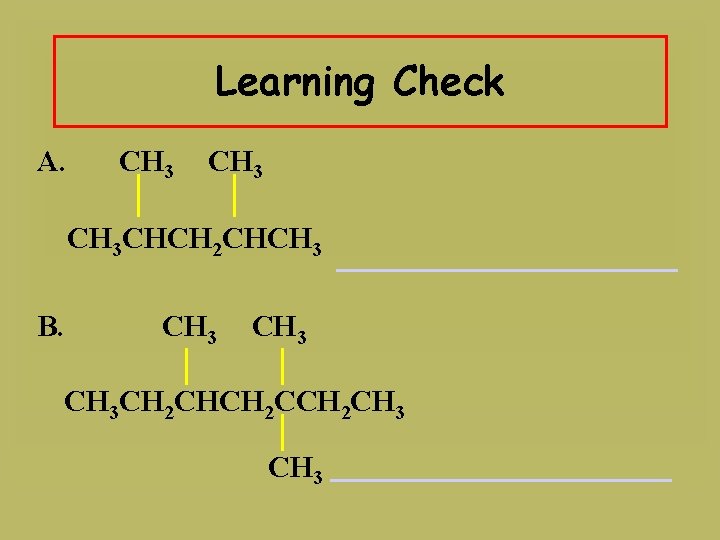
Learning Check A. CH 3 CHCH 2 CHCH 3 B. CH 3 CH 2 CHCH 2 CH 3

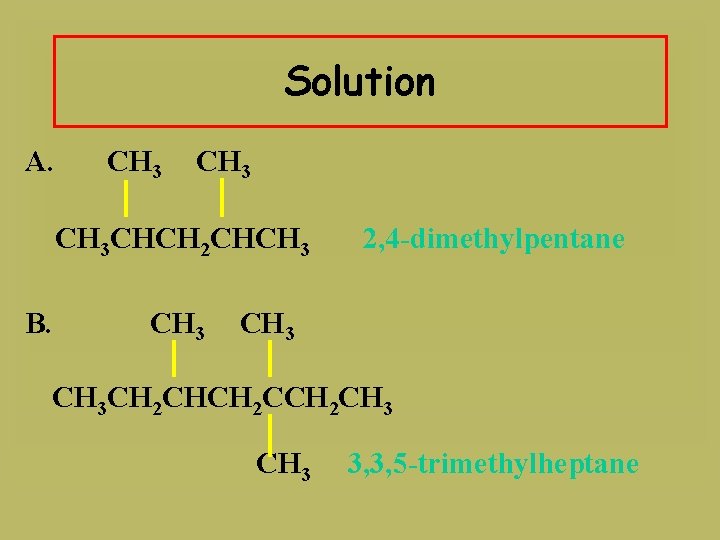
Solution A. CH 3 CHCH 2 CHCH 3 B. CH 3 2, 4 -dimethylpentane CH 3 CH 2 CHCH 2 CH 3 3, 3, 5 -trimethylheptane

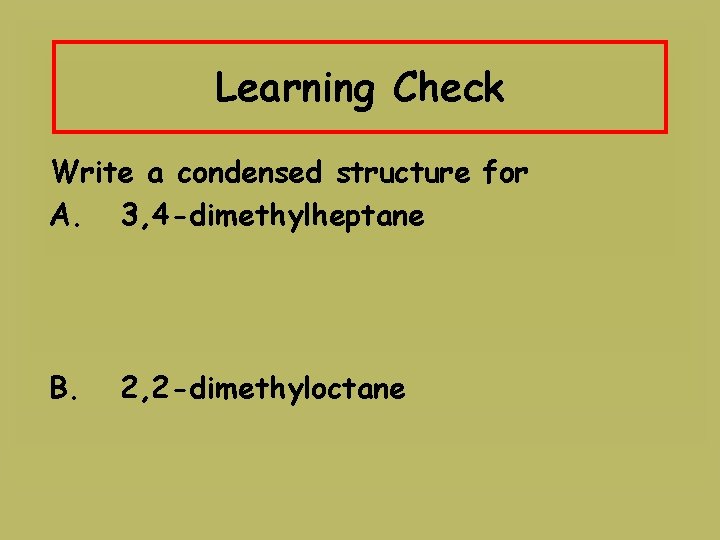
Learning Check Write a condensed structure for A. 3, 4 -dimethylheptane B. 2, 2 -dimethyloctane

Solution A. 3, 4 -dimethylheptane CH 3 CH 2 CHCHCH 2 CH 3 B. 2, 2 -dimethyloctane CH 3 CCH 2 CH 2 CH 2 CH 3

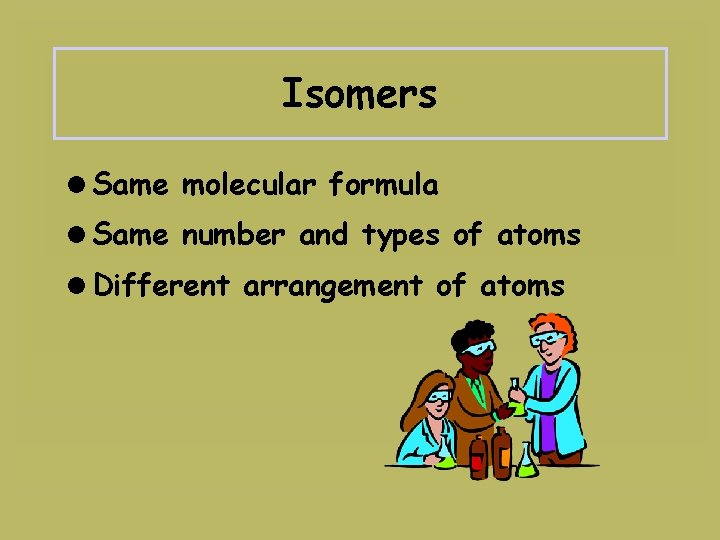
Isomers =Same molecular formula =Same number and types of atoms =Different arrangement of atoms

Isomers: compounds with the same molecular formula but a different connectivity of their atoms. Cycloalkane: a saturated hydrogen that contains carbon atoms bonded to form a ring. A hydrocarbon that contains carbon atoms joined to form a ring is called a cyclic hydrocarbon.

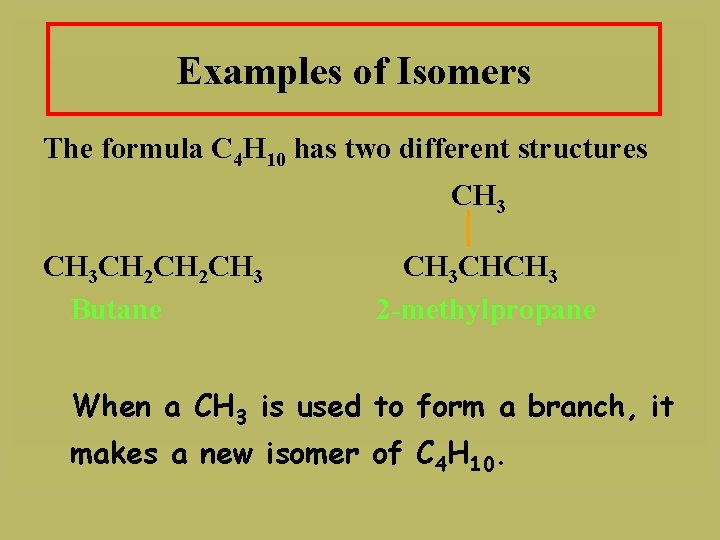
Examples of Isomers The formula C 4 H 10 has two different structures CH 3 CH 2 CH 3 Butane CH 3 CHCH 3 2 -methylpropane When a CH 3 is used to form a branch, it makes a new isomer of C 4 H 10.

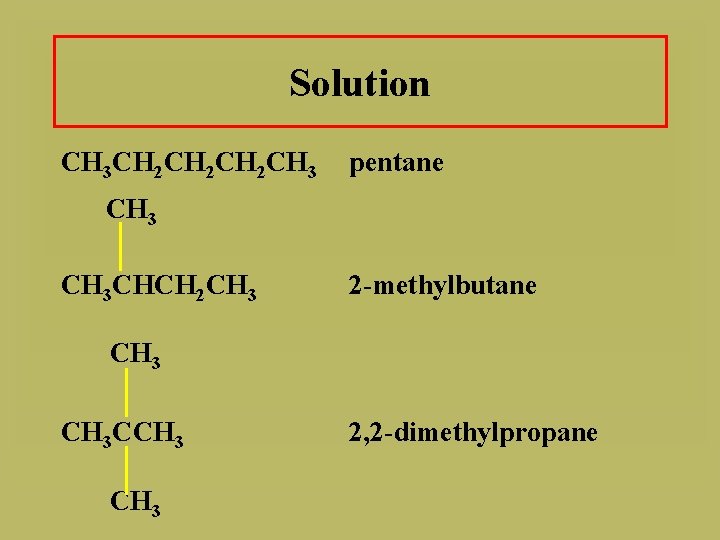
Learning Check Write 3 isomers of C 5 H 12 and name each.

Solution CH 3 CH 2 CH 2 CH 3 pentane CH 3 CHCH 2 CH 3 2 -methylbutane CH 3 CCH 3 2, 2 -dimethylpropane

Alkene • An unsaturated hydrocarbon that contains a carbon-carbon double bond. • The molecular formula of this group is Cn. H 2 n (n is number of carbon atoms). • They have less hydrogen atoms than alkanes. C 2 H 4 CH 2=CH 4 C 3 H 6 CH 2=CH-CH 3

Numbering carbons Q- draw pentene A- Where’s the bond? We number C atoms 1 -pentene • Thus, naming compounds with multiple bonds is more complex than previously indicated • Always give double bond the lowest number • Q - Name these 2 -butene C 2 H 4 Ethene 3 -nonyne

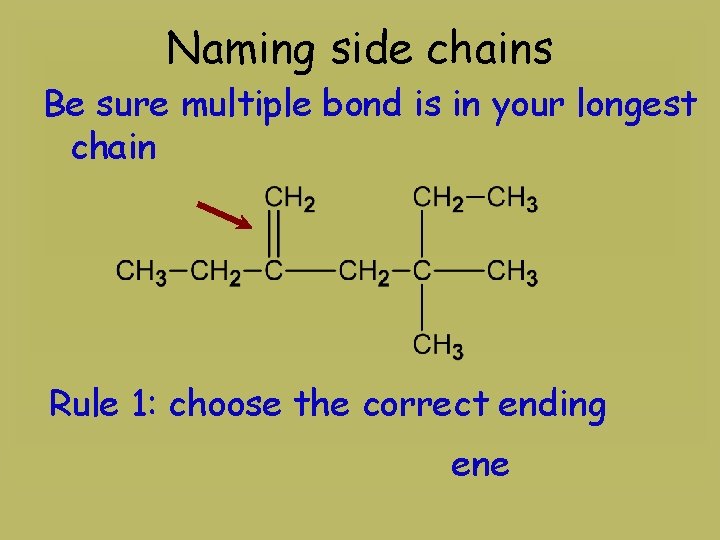
Naming side chains Be sure multiple bond is in your longest chain Rule 1: choose the correct ending ene

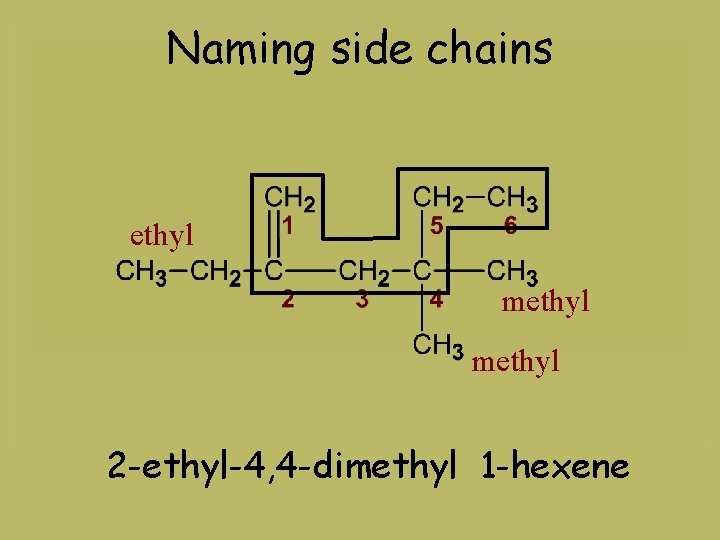
Naming side chains ethyl methyl 2 -ethyl-4, 4 -dimethyl 1 -hexene

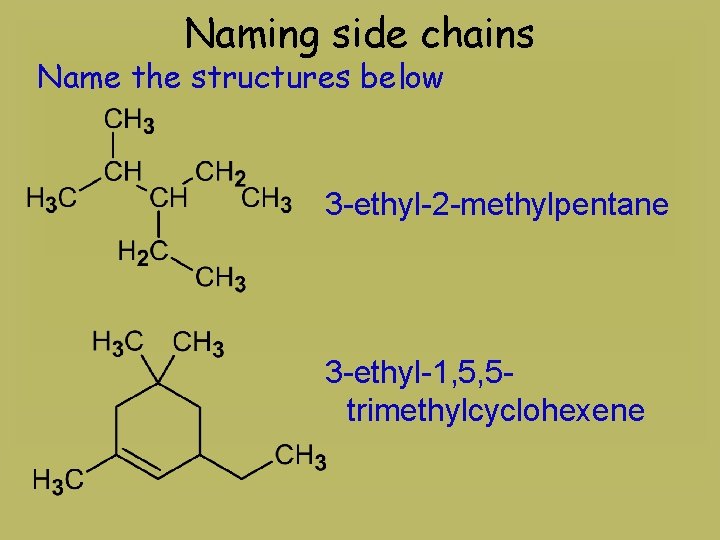
Naming side chains Name the structures below 3 -ethyl-2 -methylpentane 3 -ethyl-1, 5, 5 trimethylcyclohexene

Alkynes • An unsaturated hydrocarbon that contains a carbon-carbon triple bond. • The molecular formula of this group is Cn. H 2 n-2 (n is number of carbon atoms). • They have less hydrogen atoms than alkanes and alkenes.

Naming unbranched alkenes and alkynes Use the IUPAC names for alkanes. • For alkenes, we replace the suffix “-ane” of alkanes by “-ene”. • For alkynes, we replace the suffix “-ane” of alkanes by “-yne”.


Naming branched alkenes and alkynes 1. Name the longest carbon chain with a double or triple bond. 2. Number the carbon chain starting from the end nearest a double or triple bond. 3. Give the location and name of each substituent (alphabetical order) as a prefix to the name.

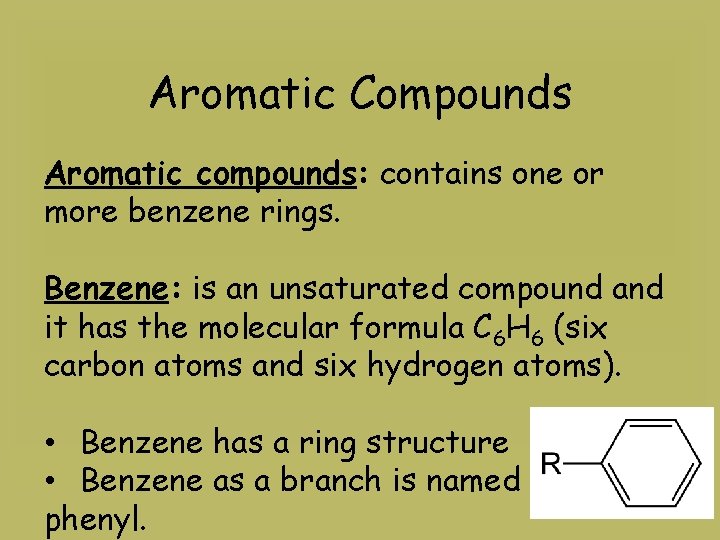
Aromatic Compounds Aromatic compounds: contains one or more benzene rings. Benzene: is an unsaturated compound and it has the molecular formula C 6 H 6 (six carbon atoms and six hydrogen atoms). • Benzene has a ring structure • Benzene as a branch is named phenyl.

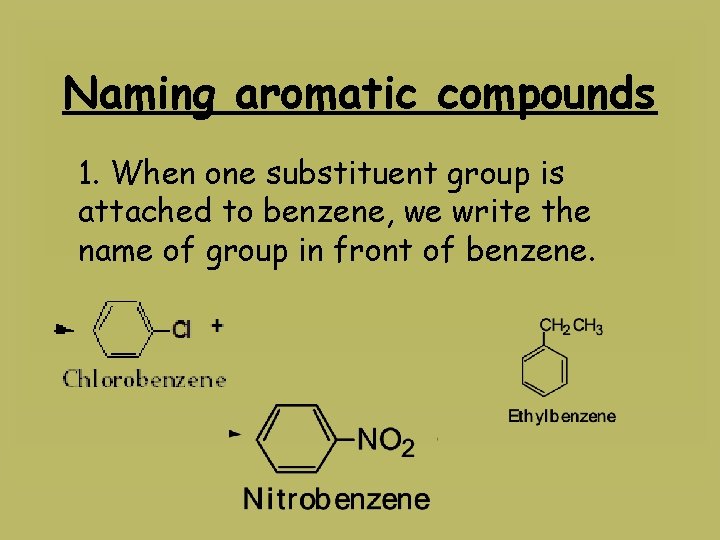
Naming aromatic compounds 1. When one substituent group is attached to benzene, we write the name of group in front of benzene.

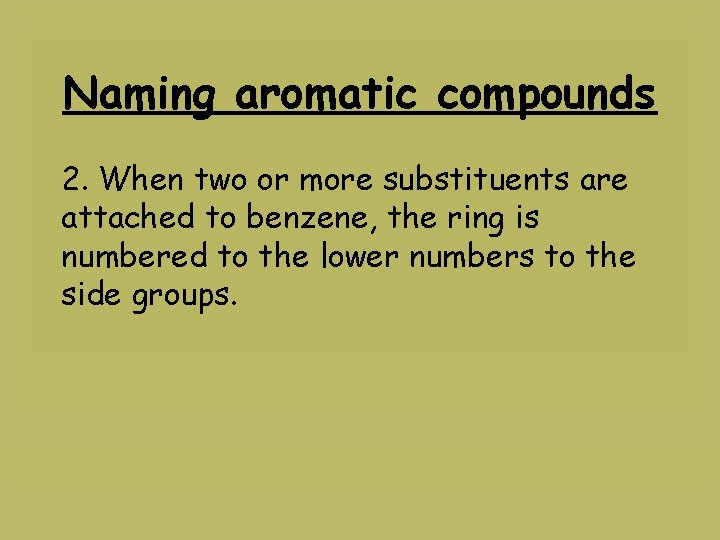
Naming aromatic compounds 2. When two or more substituents are attached to benzene, the ring is numbered to the lower numbers to the side groups.

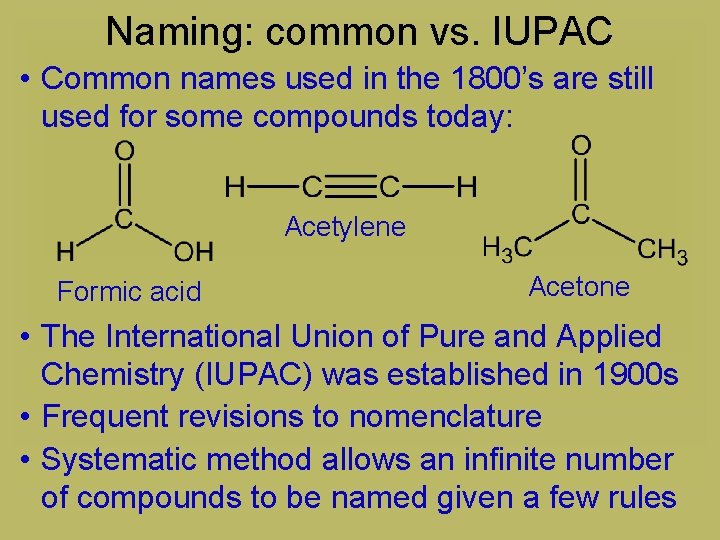
Naming: common vs. IUPAC • Common names used in the 1800’s are still used for some compounds today: Acetylene Formic acid Acetone • The International Union of Pure and Applied Chemistry (IUPAC) was established in 1900 s • Frequent revisions to nomenclature • Systematic method allows an infinite number of compounds to be named given a few rules

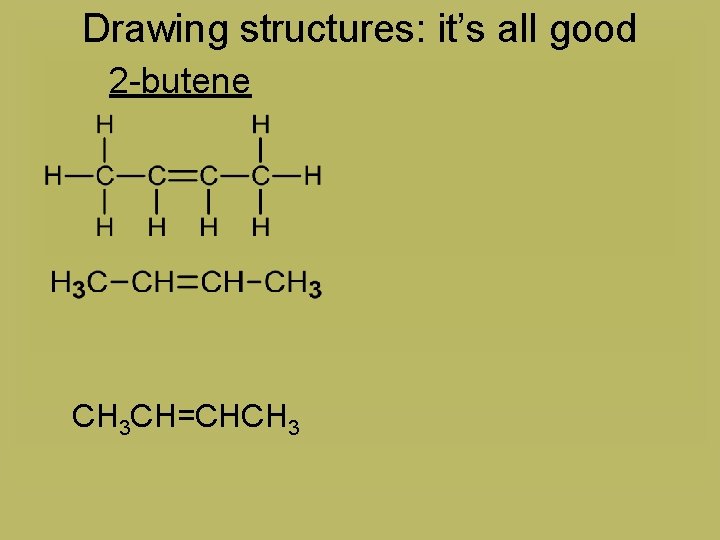
Drawing structures: it’s all good 2 -butene CH 3 CH=CHCH 3

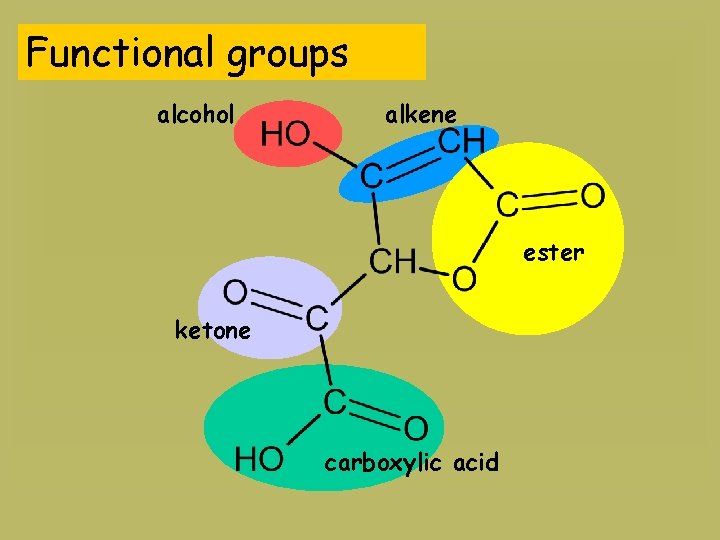
Functional groups alcohol alkene ester ketone carboxylic acid

carboxylic acid ether ester aldehyde amine nitrile


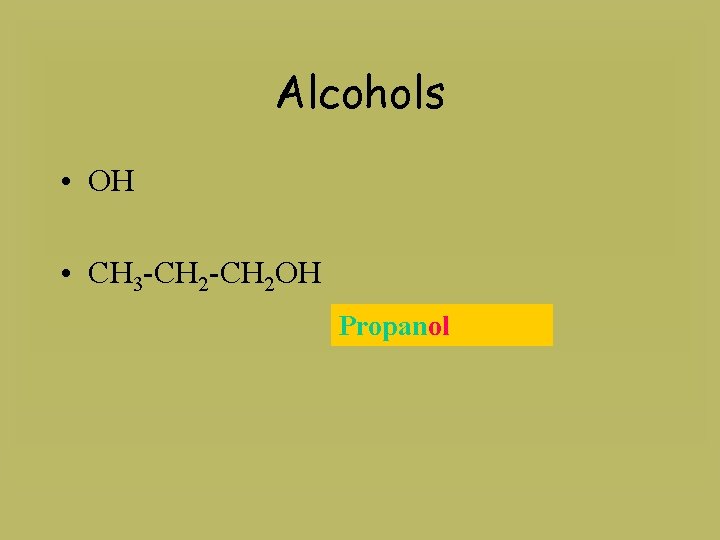
Alcohols • OH • CH 3 -CH 2 OH Propanol

Functional Groups CH 3 CH 2 C O H O CH 3 CH 2 CH 3 aldehyde butanal ketone 3 -pentanone Diethyl ketone

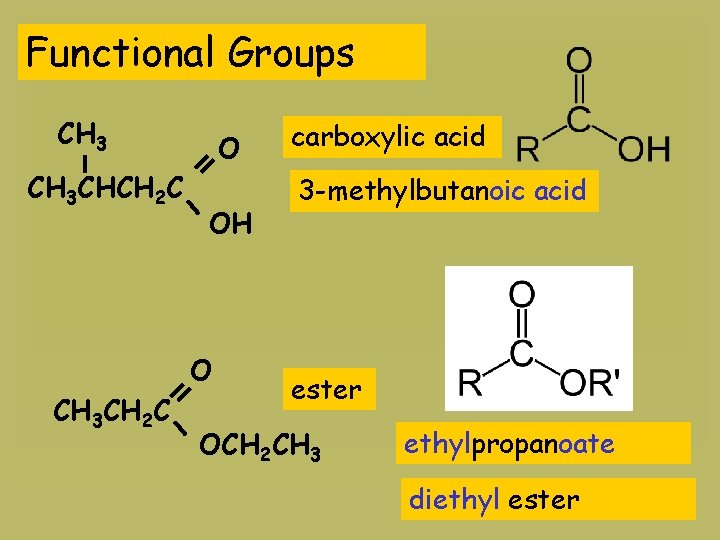
Functional Groups CH 3 CHCH 2 C O OH O CH 3 CH 2 C carboxylic acid 3 -methylbutanoic acid ester OCH 2 CH 3 ethylpropanoate diethyl ester

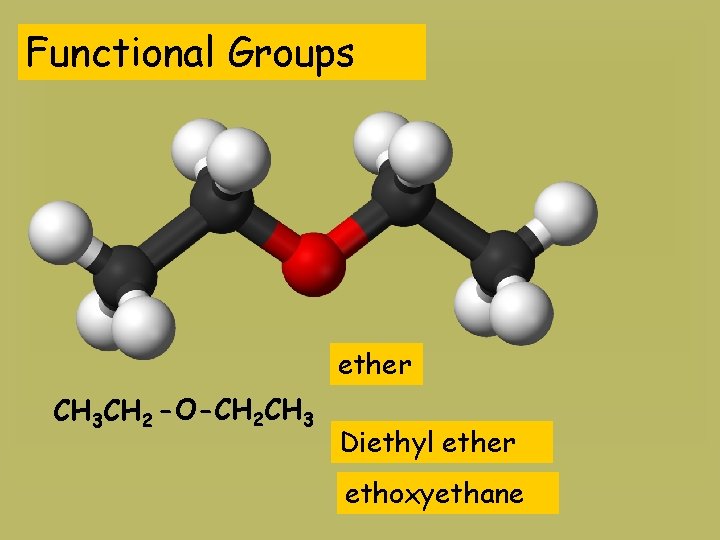
Functional Groups ether CH 3 CH 2 -O-CH 2 CH 3 Diethyl ether ethoxyethane

Polymerization • Polymers are huge molecules made by combining small molecules called monomers • Example: Compounds with double bonds add on to each other, end-to-end forming cross-linked chains CH 2 =CH 2 + CH 2 =CH 2 -CH 2 -CH 2 -
 Priority order of functional group ncert
Priority order of functional group ncert Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Ionic metallic and covalent bonds venn diagram
Ionic metallic and covalent bonds venn diagram Water soluble vitamin absorption
Water soluble vitamin absorption Vitamins classification chart
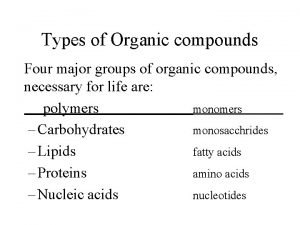
Vitamins classification chart Four types of organic compound
Four types of organic compound Decomposition of organic matter equation
Decomposition of organic matter equation Charring test of organic and inorganic compounds
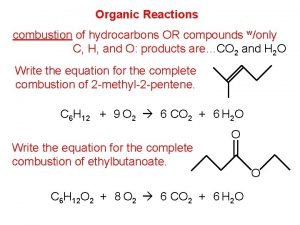
Charring test of organic and inorganic compounds Burning of organic compounds
Burning of organic compounds Is hagfish slime a lipid
Is hagfish slime a lipid All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain:
Organic compounds must contain: Importance of organic compounds
Importance of organic compounds Organic vs inorganic compounds
Organic vs inorganic compounds Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic vs inorganic compounds
Organic vs inorganic compounds What is the classification of organic compounds
What is the classification of organic compounds Group of carbon
Group of carbon Principle of distillation
Principle of distillation Maltose and cellobiose difference
Maltose and cellobiose difference Organic compounds grade 10 life science
Organic compounds grade 10 life science Organic halogen compounds
Organic halogen compounds Vitamins are organic compounds
Vitamins are organic compounds Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________. Families of organic compounds
Families of organic compounds Quantitative analysis of organic compounds ppt
Quantitative analysis of organic compounds ppt Type of organic compounds
Type of organic compounds Sextext of electrons
Sextext of electrons How are organic compounds classified
How are organic compounds classified Celiac beri beri
Celiac beri beri What is a nucleotide
What is a nucleotide Number of organic compounds
Number of organic compounds Organic versus inorganic compounds
Organic versus inorganic compounds Are unsaturated fats solid at room temperature
Are unsaturated fats solid at room temperature Rules for naming ionic compounds
Rules for naming ionic compounds Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Met et prop but pent hex hept oct
Met et prop but pent hex hept oct Solution
Solution Solution
Solution Cycloalkanes
Cycloalkanes Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry C10h22oh
C10h22oh Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s David klein organic chemistry
David klein organic chemistry Is alkane an organic compound
Is alkane an organic compound Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry lab report sample
Organic chemistry lab report sample Alkane organic chemistry
Alkane organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Organic chemistry wade
Organic chemistry wade