Lesson 28 Atomic Nucleus and Radioactivity Eleanor Roosevelt
















































- Slides: 94

Lesson 28 Atomic Nucleus and Radioactivity Eleanor Roosevelt High School Chin-Sung Lin

Atomic Nucleus and Radioactivity

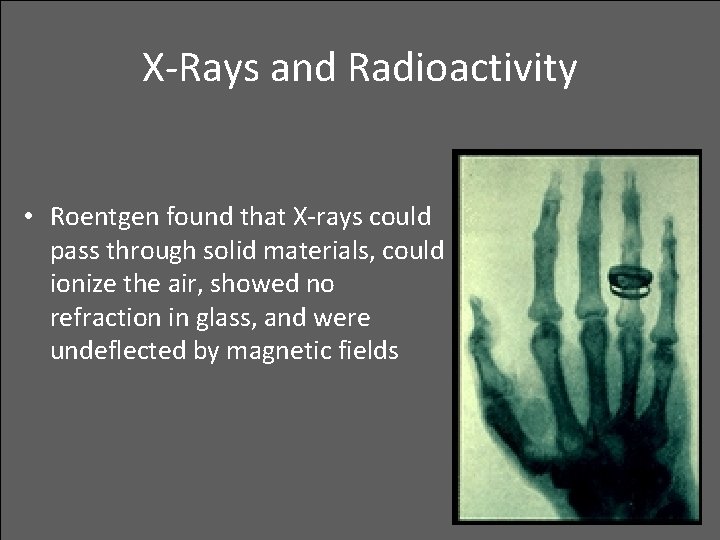
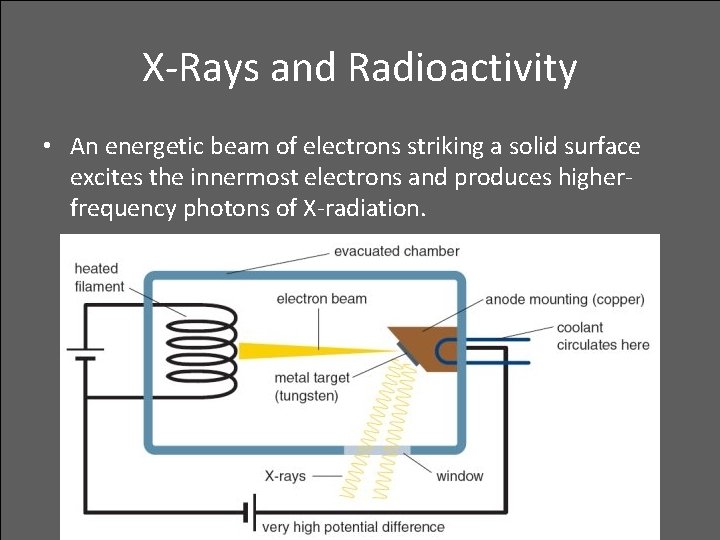
X-Rays and Radioactivity • In 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his lab and discovered X-rays produced by a beam of electrons striking the glass surface of a gas-discharge tube

X-Rays and Radioactivity • Roentgen found that X-rays could pass through solid materials, could ionize the air, showed no refraction in glass, and were undeflected by magnetic fields

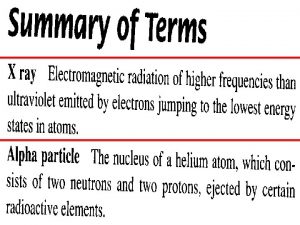
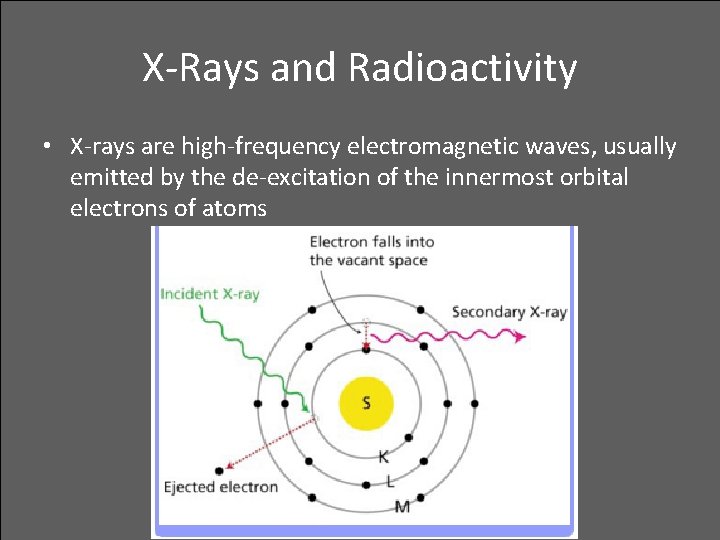
X-Rays and Radioactivity • X-rays are high-frequency electromagnetic waves, usually emitted by the de-excitation of the innermost orbital electrons of atoms

X-Rays and Radioactivity • An energetic beam of electrons striking a solid surface excites the innermost electrons and produces higherfrequency photons of X-radiation.

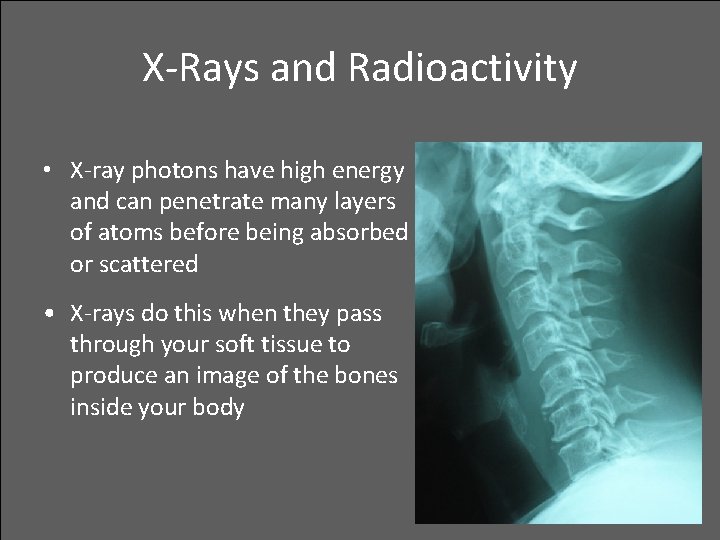
X-Rays and Radioactivity • X-ray photons have high energy and can penetrate many layers of atoms before being absorbed or scattered • X-rays do this when they pass through your soft tissue to produce an image of the bones inside your body

X-Rays and Radioactivity • Radioactivity is the process of nuclear decay (radioactive decay) • Nothing new in the environment; it’s been going on since time zero • It warms Earth’s interior, is in the air we breathe, and is present in all rocks (some in trace amounts) • It is natural

X-Rays and Radioactivity CHECK YOUR NEIGHBOR The radioactive decay of nature’s elements occurs in the A. B. C. D. soil we walk on. air we breathe. interior of Earth. All of the above.

X-Rays and Radioactivity CHECK YOUR ANSWER The radioactive decay of nature’s elements occurs in the A. B. C. D. soil we walk on. air we breathe. interior of Earth. All of the above.

Alpha, Beta, and Gamma Rays

Alpha, Beta, and Gamma Rays

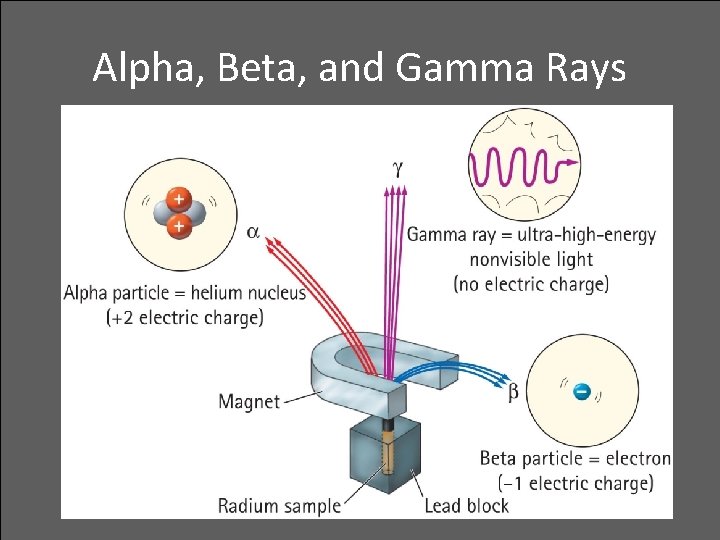
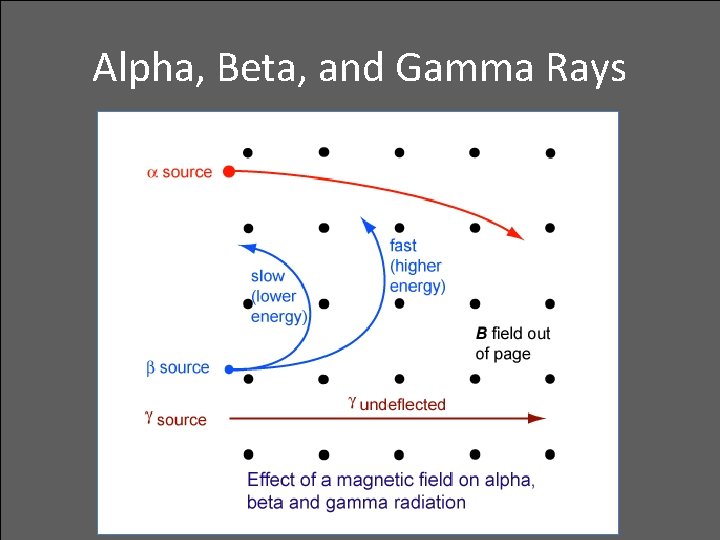
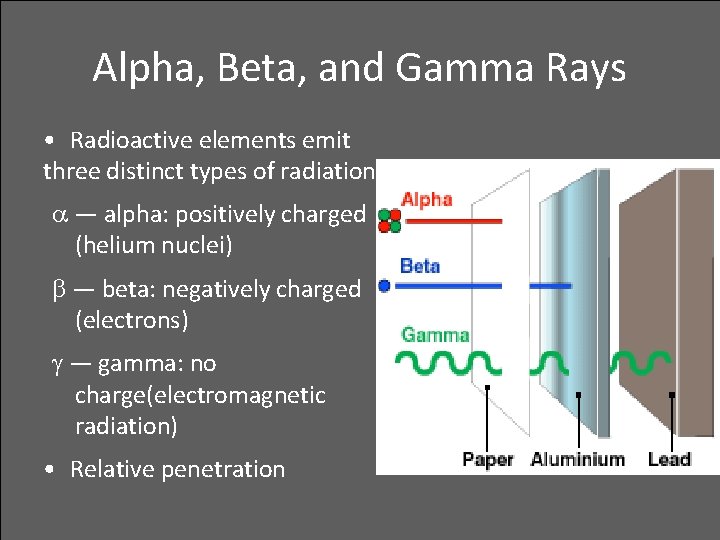
Alpha, Beta, and Gamma Rays • Radioactive elements emit three distinct types of radiation: — alpha: positively charged (helium nuclei) — beta: negatively charged (electrons) — gamma: no charge(electromagnetic radiation) • Relative penetration

Alpha, Beta, and Gamma Rays CHECK YOUR NEIGHBOR The origins of radioactivity go back to A. B. C. D. military activities in the mid-20 th century. the Industrial Revolution two centuries ago. the beginning of human error. before humans emerged on Earth.

Alpha, Beta, and Gamma Rays CHECK YOUR ANSWER The origins of radioactivity go back to A. B. C. D. military activities in the mid-20 th century. the Industrial Revolution two centuries ago. the beginning of human error. before humans emerged on Earth.

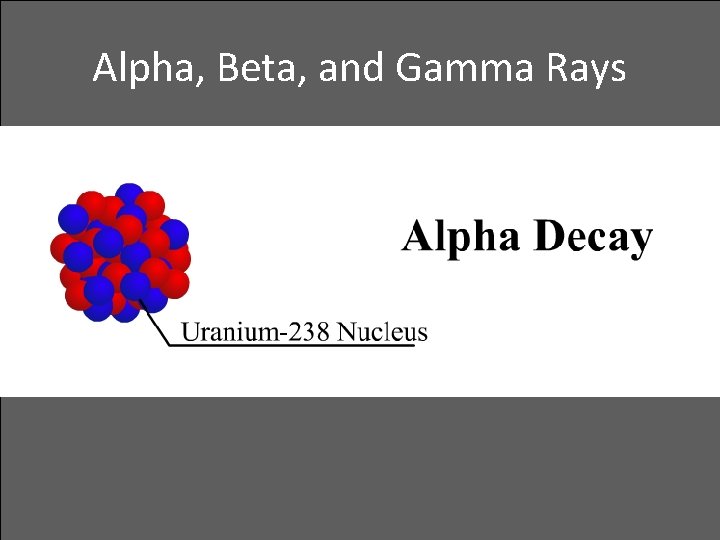
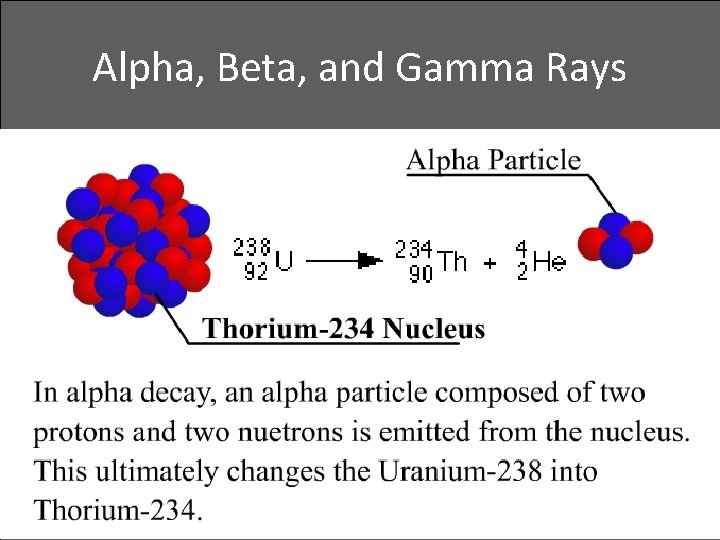
Alpha, Beta, and Gamma Rays • Alpha decay

Alpha, Beta, and Gamma Rays • Alpha decay

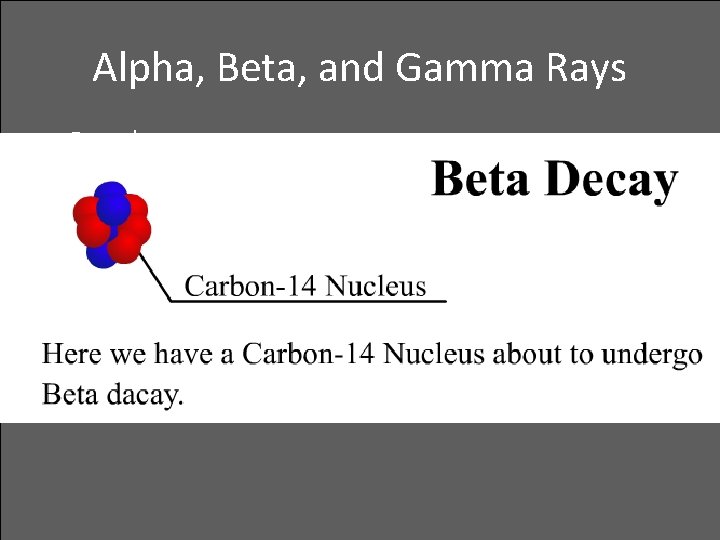
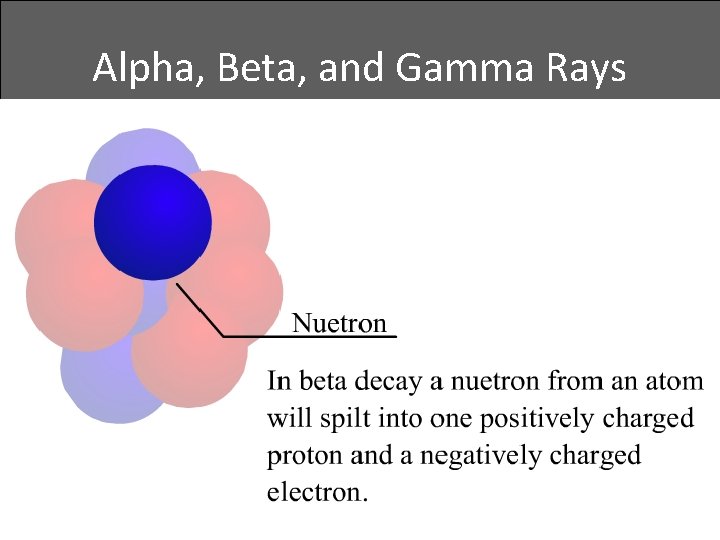
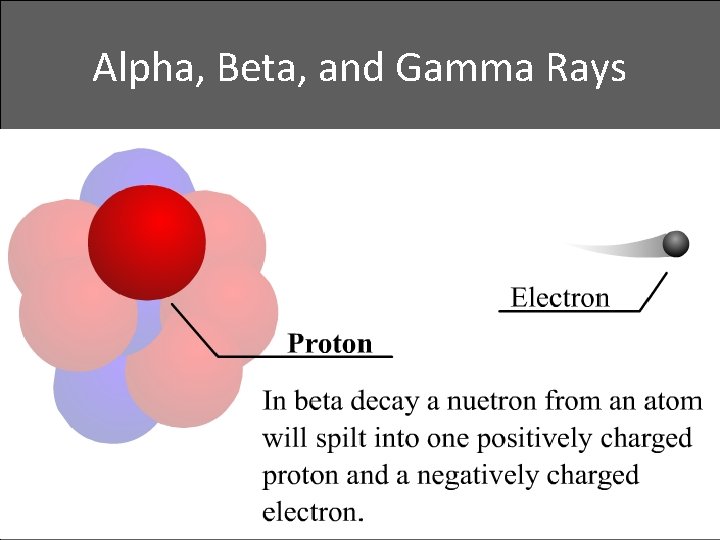
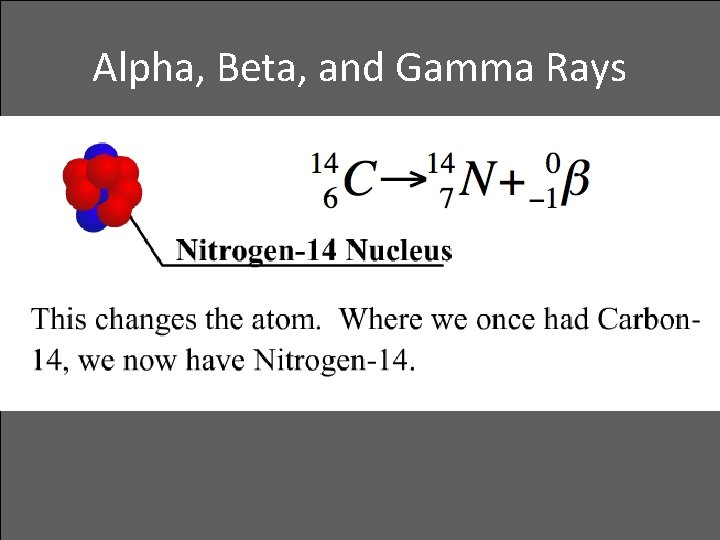
Alpha, Beta, and Gamma Rays • Beta decay

Alpha, Beta, and Gamma Rays • Beta decay

Alpha, Beta, and Gamma Rays • Beta decay

Alpha, Beta, and Gamma Rays • Beta decay

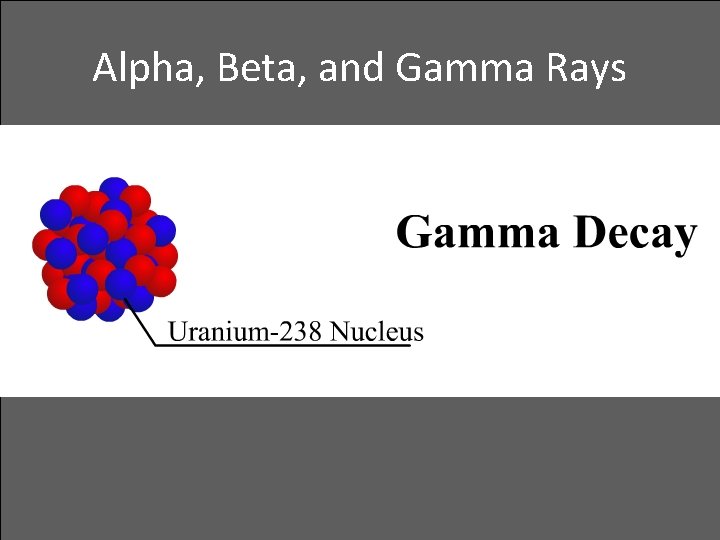
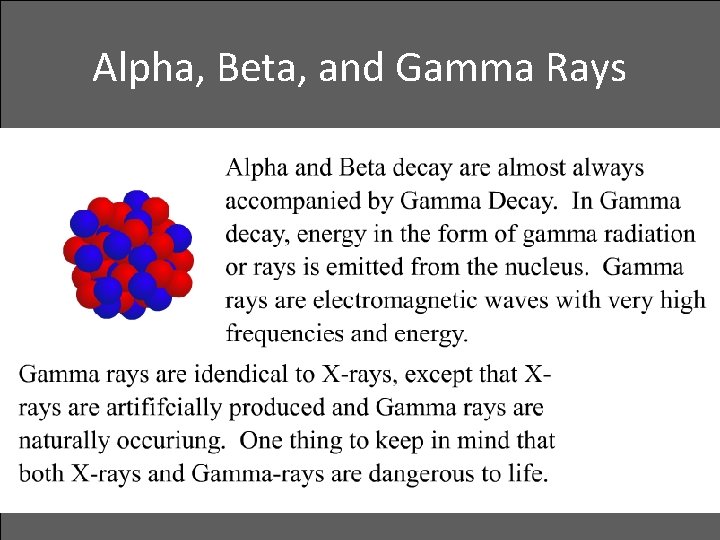
Alpha, Beta, and Gamma Rays • Gamma decay

Alpha, Beta, and Gamma Rays • Gamma decay

Alpha, Beta, and Gamma Rays CHECK YOUR NEIGHBOR Any atom that emits an alpha particle or beta particle A. B. C. D. becomes an atom of a different element, always. may become an atom of a different element. becomes a different isotope of the same element. increases its mass.

Alpha, Beta, and Gamma Rays CHECK YOUR ANSWER Any atom that emits an alpha particle or beta particle A. B. C. D. becomes an atom of a different element, always. may become an atom of a different element. becomes a different isotope of the same element. increases its mass.

Alpha, Beta, and Gamma Rays CHECK YOUR NEIGHBOR Which of these is actually a high-speed electron? A. B. C. Alpha Beta Gamma

Alpha, Beta, and Gamma Rays CHECK YOUR ANSWER Which of these is actually a high-speed electron? A. B. C. Alpha Beta Gamma

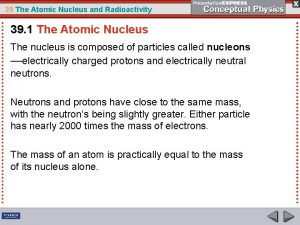
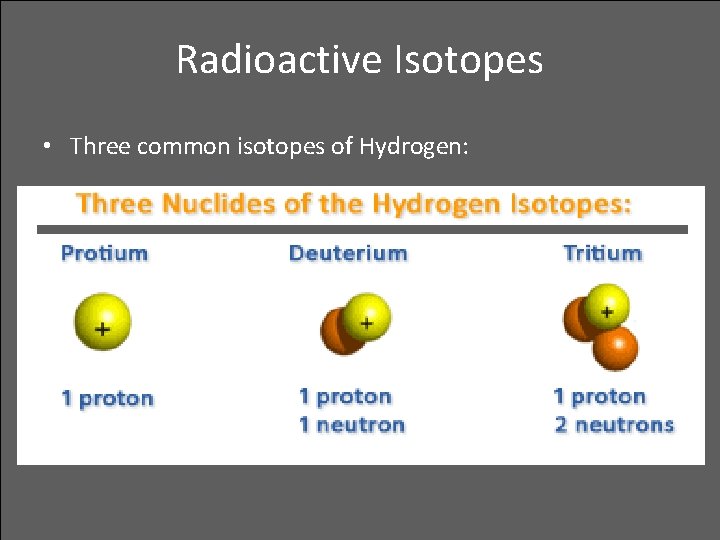
Radioactive Isotopes • The number of protons in an atomic nucleus determine the number of electrons surrounding the nucleus in a neutral atom • When there is a different number of electrons than the nuclear protons, the atom is charged and is called an ion • When there is a same number of protons (atomic numbers) but different number of neutrons (mass numbers), they are called isotopes • When an isotope is radioactive, it is called radioactive isotope

Radioactive Isotopes • Three common isotopes of Hydrogen:

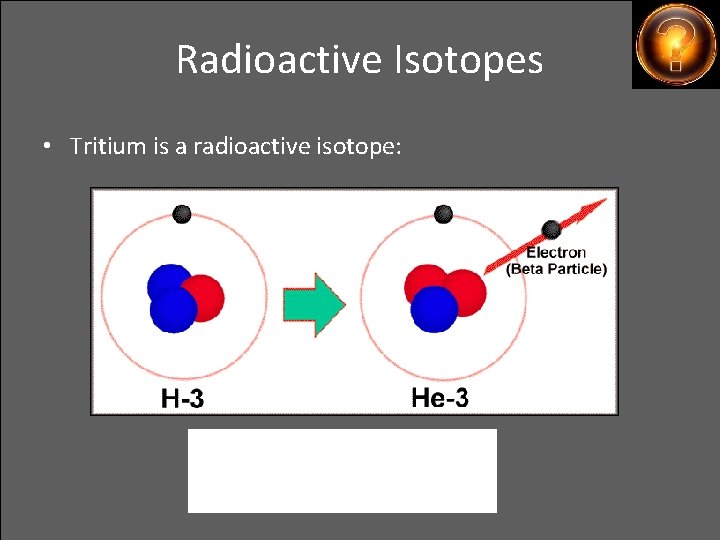
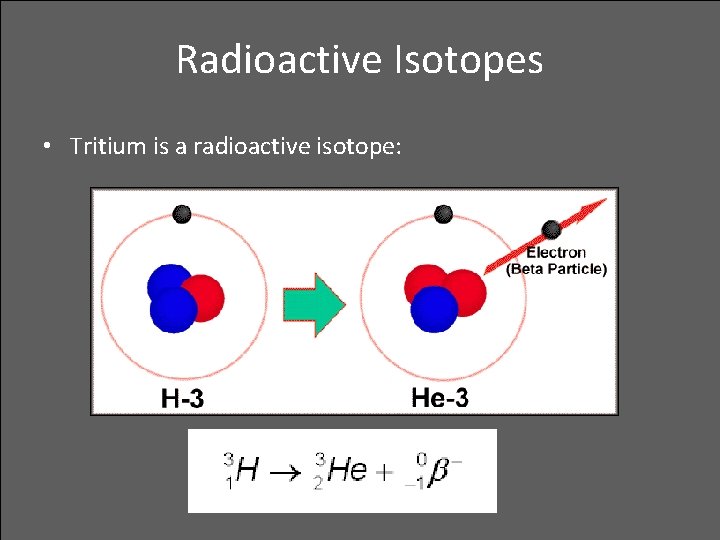
Radioactive Isotopes • Tritium is a radioactive isotope:

Radioactive Isotopes • Tritium is a radioactive isotope:

Exercise: Radioactive Isotopes The nucleus of beryllium-8 (Be-8) undergoes a special kind of radioactive decay: it split into two equal halves. What nuclei are the products of this decay? What is the form of this decay?

Exercise: Radioactive Isotopes The nucleus of beryllium-8 (Be-8) undergoes a special kind of radioactive decay: it split into two equal halves. What nuclei are the products of this decay? What is the form of this decay? 8 Be 4 –> 2 24 He alpha decay

Radioactive Isotopes • The electric force of repulsion between the protons in a heavy nucleus acts over a greater distance than the attractive forces among the neutrons and protons in the nucleus • Each proton is repelled by every other proton in the nucleus, but is attracted only by the nucleons closest to it • In a large nucleus, electric repulsion can exceed nuclear attraction • This instability makes all the heaviest atoms radioactive

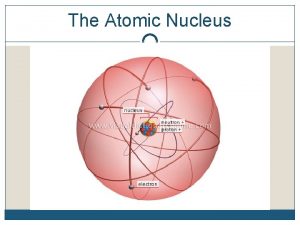
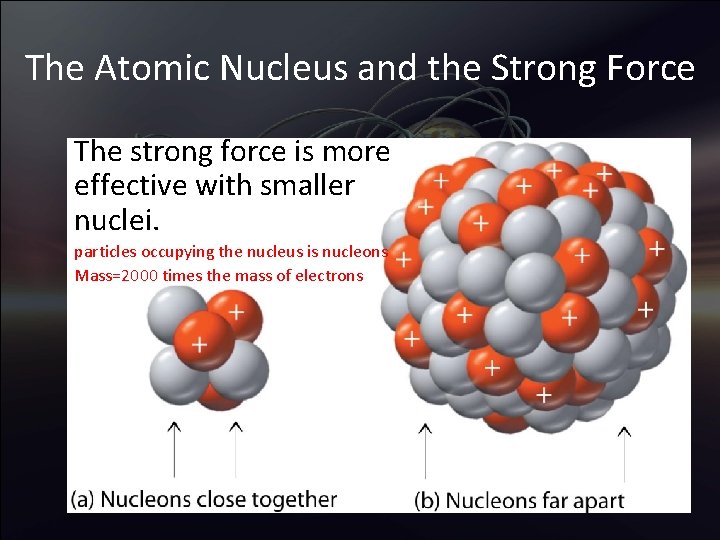
The Atomic Nucleus and the Strong Force The strong force is more effective with smaller nuclei. particles occupying the nucleus is nucleons Mass=2000 times the mass of electrons

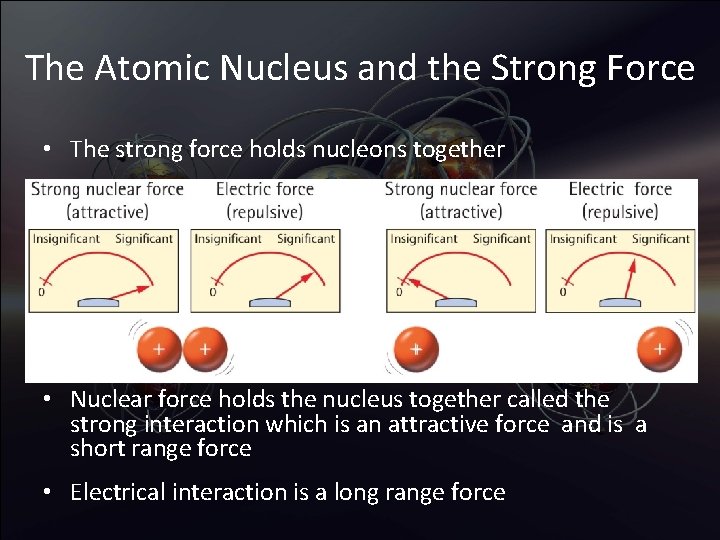
The Atomic Nucleus and the Strong Force • The strong force holds nucleons together • Nuclear force holds the nucleus together called the strong interaction which is an attractive force and is a short range force • Electrical interaction is a long range force

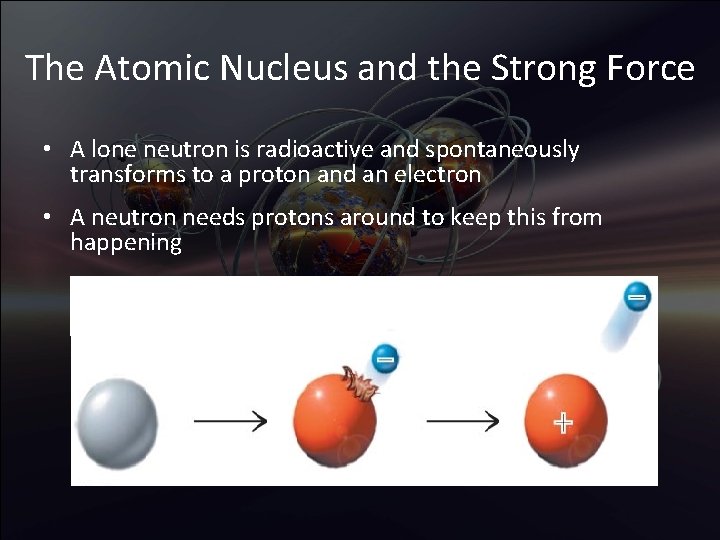
The Atomic Nucleus and the Strong Force • A lone neutron is radioactive and spontaneously transforms to a proton and an electron • A neutron needs protons around to keep this from happening

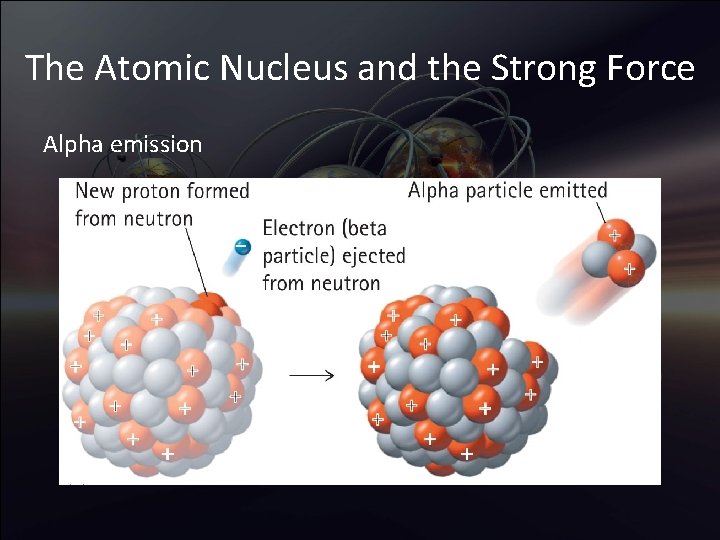
The Atomic Nucleus and the Strong Force Alpha emission

The Atomic Nucleus and the Strong Force CHECK YOUR NEIGHBOR The strong force is a force in the A. B. C. D. atom that holds electrons in orbit. nucleus that holds nucleons together. Both A and B. Neither A nor B.

The Atomic Nucleus and the Strong Force CHECK YOUR ANSWER The strong force is a force in the A. B. C. D. atom that holds electrons in orbit. nucleus that holds nucleons together. Both A and B. Neither A nor B.

The Atomic Nucleus and the Strong Force CHECK YOUR NEIGHBOR In the nucleus of an atom, the strong force is a relatively A. B. C. D. short-range force. long-range force. unstable force. neutralizing force.

The Atomic Nucleus and the Strong Force CHECK YOUR ANSWER In the nucleus of an atom, the strong force is a relatively A. B. C. D. short-range force. long-range force. unstable force. neutralizing force.

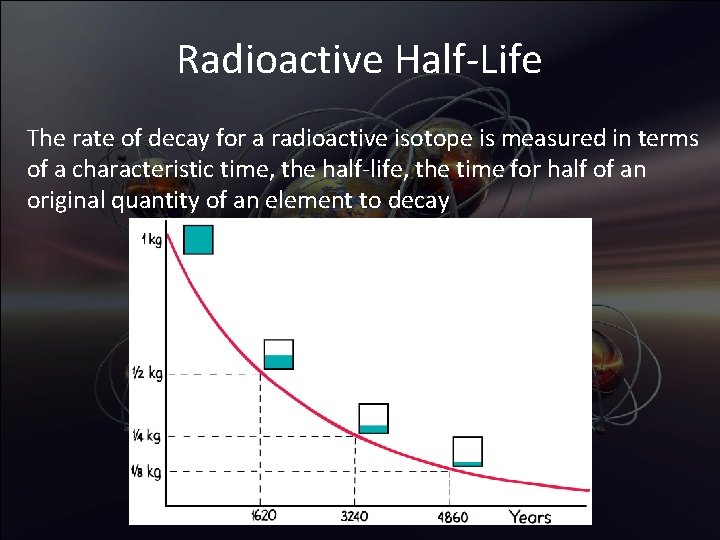
Radioactive Half-Life The rate of decay for a radioactive isotope is measured in terms of a characteristic time, the half-life, the time for half of an original quantity of an element to decay

Radioactive Half-Life CHECK YOUR NEIGHBOR Suppose the number of neutrons in a reactor that is starting up doubles each minute, reaching 1 billion neutrons in 10 minutes. When did the number of neutrons reach half a billion? A. B. C. D. 1 minute 2 minutes 5 minutes 9 minutes

Radioactive Half-Life CHECK YOUR ANSWER Suppose the number of neutrons in a reactor that is starting up doubles each minute, reaching 1 billion neutrons in 10 minutes. When did the number of neutrons reach half a billion? A. B. C. D. 1 minute 2 minutes 5 minutes 9 minutes Explanation: This question would be appropriate with Appendix D, Exponential Growth and Doubling Time. Can you see that working backward, each minute has half the number of neutrons?

Radiation Half-Life CHECK YOUR NEIGHBOR A certain isotope has a half-life of 10 years. This means the amount of that isotope remaining at the end of 10 years will be A. B. C. D. zero. one-quarter. Half. the same.

Radiation Half-Life CHECK YOUR ANSWER A certain isotope has a half-life of 10 years. This means the amount of that isotope remaining at the end of 10 years will be A. B. C. D. zero. one-quarter. Half. the same.

Exercise: Radioactive Half-Life If a sample of radioactive isotope has a half-life of 1 year, how much of the original sample will be left at the end of the second year?

Exercise: Radioactive Half-Life If a sample of radioactive isotope has a half-life of 1 year, how much of the original sample will be left at the end of the second year? (1/2)2 = 1/4

Exercise: Radioactive Half-Life If Fukushima Daiichi nuclear power plant in Japan releases a certain amount of radioactive iodine (I-131), how many percent of the original material will be left at the end of the 50 days? (The half-life of I-131 is 8. 02 day)

Exercise: Radioactive Half-Life If Fukushima Daiichi nuclear power plant in Japan releases a certain amount of radioactive iodine (I-131), how many percent of the original material will be left at the end of the 50 days? (The half-life of I-131 is 8. 02 day) (1/2) (50 d/8. 02 d) = 1. 33%

Exercise: Radioactive Half-Life The half-life of Th-227 is 18. 2 day. How many days are required for 0. 70 of a given sample to decay?

Exercise: Radioactive Half-Life The half-life of Th-227 is 18. 2 day. How many days are required for 0. 70 of a given sample to decay? (1/2) (t/18. 2 days) = (1. 0 – 0. 7) t = 31. 61 days

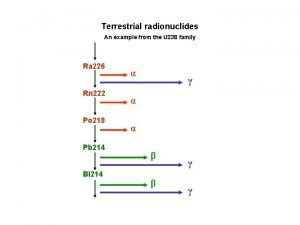
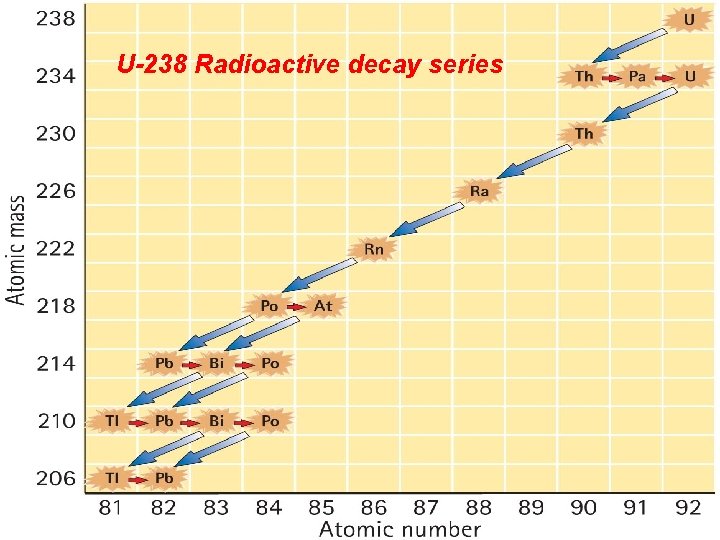
Radioactive Half-Life Radioactive decay series: • Uranium-238 to lead-206 through a series of alpha and beta decays • In 4. 5 billion years, half the uranium presently in Earth will be lead

Alpha, Beta, and Gamma Rays • Irradiation is the process by which an item is exposed to radiation • Food irradiation kills microbes, but doesn’t make the food radioactive

Alpha, Beta, and Gamma Rays CHECK YOUR NEIGHBOR Which of these is the nucleus of the helium atom? A. B. C. D. Alpha Beta Gamma All are different forms of helium.

Alpha, Beta, and Gamma Rays CHECK YOUR ANSWERS Which of these is the nucleus of the helium atom? A. B. C. D. Alpha Beta Gamma All are different forms of helium Explanation: Contrary to the failures of alchemists of old to change elements from one to another, this was going on all around them—unnoticed

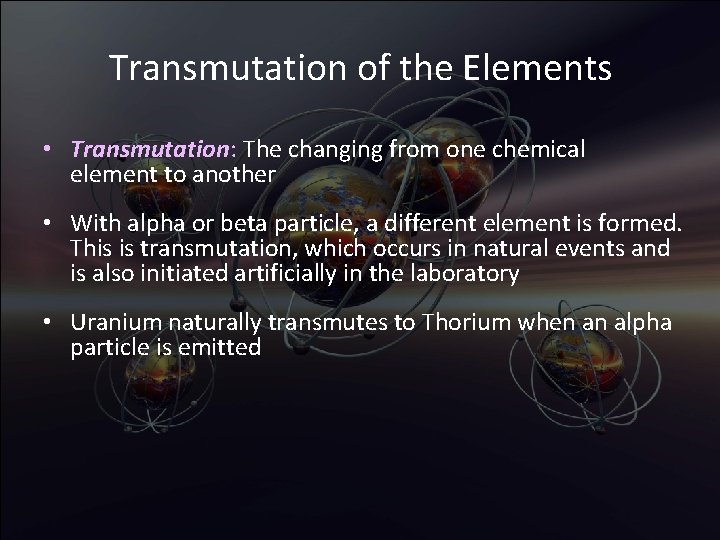
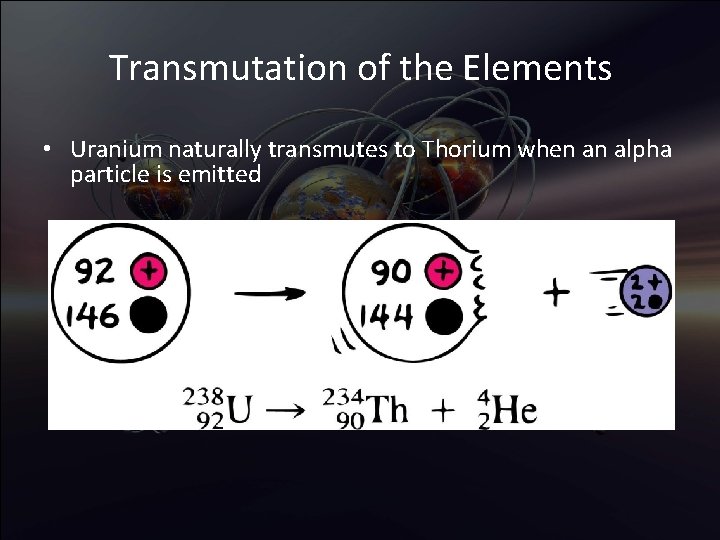
Transmutation of the Elements • Transmutation: The changing from one chemical element to another • With alpha or beta particle, a different element is formed. This is transmutation, which occurs in natural events and is also initiated artificially in the laboratory • Uranium naturally transmutes to Thorium when an alpha particle is emitted

Transmutation of the Elements • Uranium naturally transmutes to Thorium when an alpha particle is emitted

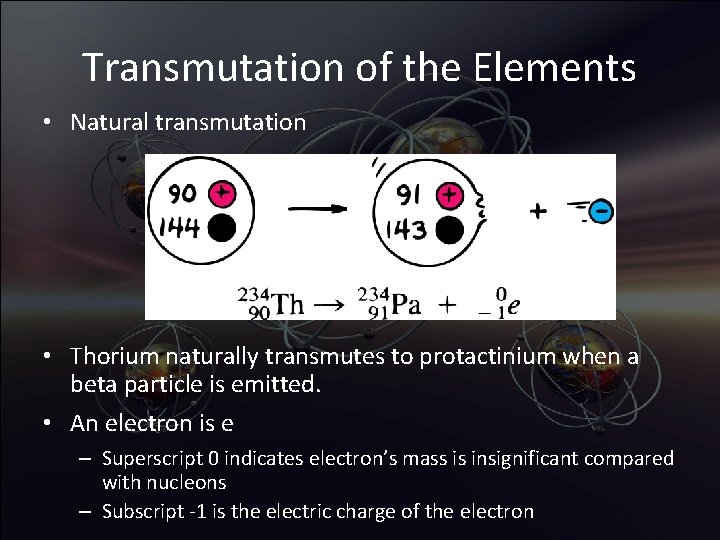
Transmutation of the Elements • Natural transmutation • Thorium naturally transmutes to protactinium when a beta particle is emitted. • An electron is e – Superscript 0 indicates electron’s mass is insignificant compared with nucleons – Subscript -1 is the electric charge of the electron

Radioactive Half-Life U-238 Radioactive decay series

Transmutation of the Elements CHECK YOUR NEIGHBOR When an element ejects an alpha particle, the atomic number of the resulting element A. B. C. D. reduces by 2. reduces by 4. increases by 2. increases by 4.

Transmutation of the Elements CHECK YOUR ANSWER When an element ejects an alpha particle, the atomic number of the resulting element A. B. C. D. reduces by 2. reduces by 4. increases by 2. increases by 4. Explanation: An alpha particle (a helium nucleus) has atomic number 2. Ejection of an alpha particle means a loss of 2 protons, so the atomic number of the element is lowered by 2

Transmutation of the Elements CHECK YOUR NEIGHBOR When an element ejects an alpha particle and a beta particle, the atomic number of that element A. B. C. D. reduces by 1. increases by 1. reduces by 2. increases by 2.

Transmutation of the Elements CHECK YOUR ANSWER When an element ejects an alpha particle and a beta particle, the atomic number of that element A. B. C. D. reduces by 1. increases by 1. reduces by 2. increases by 2. Explanation: Alpha emission reduces atomic number by 2, and beta emission increases atomic number by 1, so net result is 1.

Exercise: Transmutation of the Elements Write the nuclear reactions from Uranium-238 to lead-206 through a series of alpha and beta decays

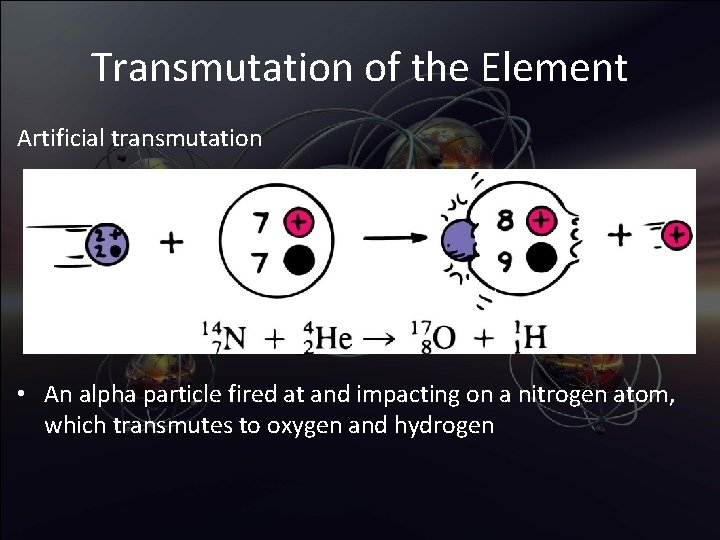
Transmutation of the Element Artificial transmutation • An alpha particle fired at and impacting on a nitrogen atom, which transmutes to oxygen and hydrogen

Transmutation of the Elements CHECK YOUR NEIGHBOR Atoms can transmute into completely different atoms in A. B. C. D. nature. laboratories. Both A and B. Neither A nor B.

Transmutation of the Elements CHECK YOUR ANSWER Atoms can transmute into completely different atoms in A. B. C. D. nature. laboratories. Both A and B. Neither A nor B. Explanation: Atomic transmutation occurs in nature, in laboratories, and as far as we know, throughout the cosmos

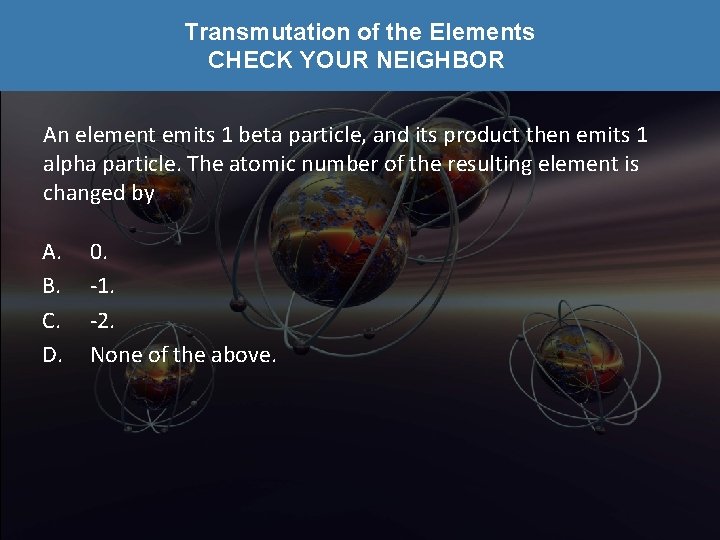
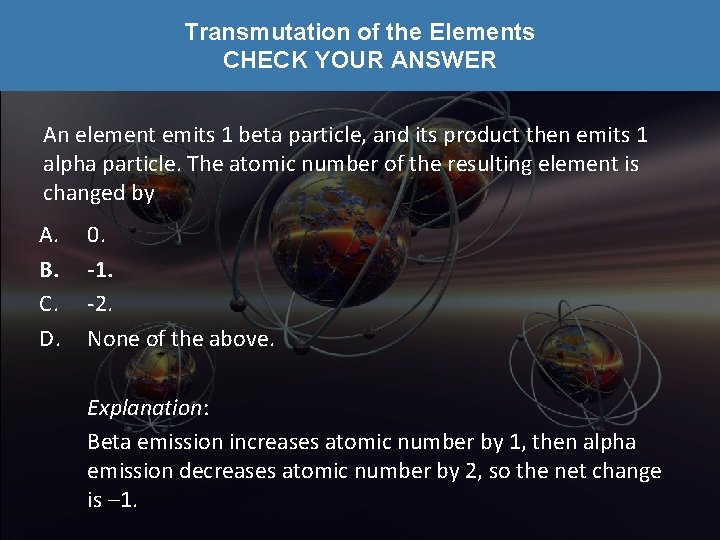
Transmutation of the Elements CHECK YOUR NEIGHBOR An element emits 1 beta particle, and its product then emits 1 alpha particle. The atomic number of the resulting element is changed by A. B. C. D. 0. -1. -2. None of the above.

Transmutation of the Elements CHECK YOUR ANSWER An element emits 1 beta particle, and its product then emits 1 alpha particle. The atomic number of the resulting element is changed by A. B. C. D. 0. -1. -2. None of the above. Explanation: Beta emission increases atomic number by 1, then alpha emission decreases atomic number by 2, so the net change is – 1.

Radiation Detectors • Geiger counter detects incoming radiation by a short pulse of current triggered when radiation ionizes a gas in the tube

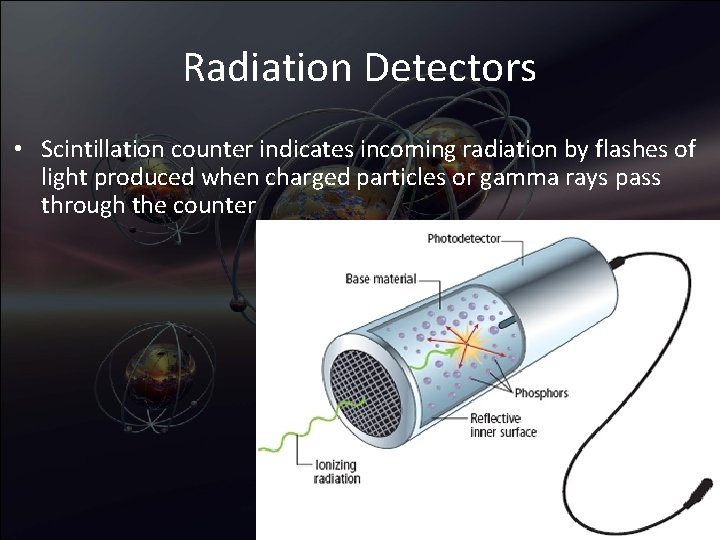
Radiation Detectors • Scintillation counter indicates incoming radiation by flashes of light produced when charged particles or gamma rays pass through the counter

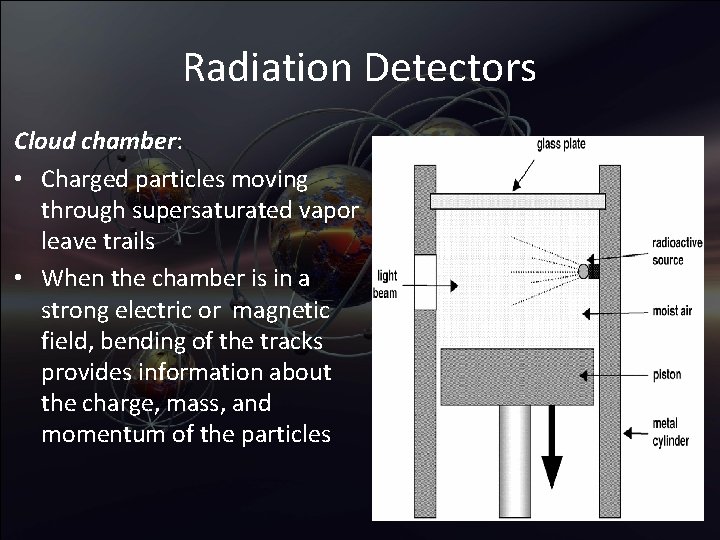
Radiation Detectors Cloud chamber: • Charged particles moving through supersaturated vapor leave trails • When the chamber is in a strong electric or magnetic field, bending of the tracks provides information about the charge, mass, and momentum of the particles

Radiation Detectors Bubble chamber: • Liquid hydrogen is heated under pressure in a glass and stainless steel chamber to a point just short of boiling • If the pressure in the chamber is suddenly released at the moment an ion-producing particle enters, a thin trail of bubbles is left along the particle’s path

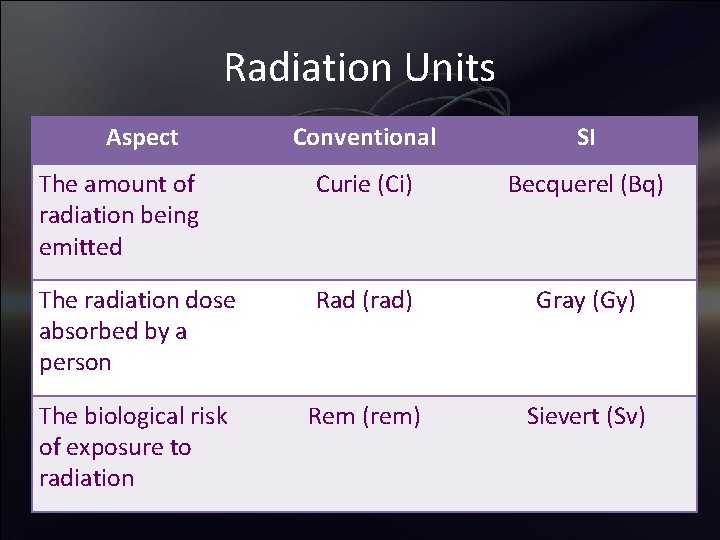
Radiation Units • Different units of measure are used depending on what aspect of radiation is being measured – The amount of radiation being emitted – The radiation dose absorbed by a person – The biological risk of exposure to radiation

Radiation Units Aspect Conventional SI The amount of radiation being emitted Curie (Ci) Becquerel (Bq) The radiation dose absorbed by a person Rad (rad) Gray (Gy) The biological risk of exposure to radiation Rem (rem) Sievert (Sv)

Radiation Units – Ci and Bq • The amount of radiation being emitted, by a radioactive material is measured using the conventional unit curie (Ci), or the SI unit becquerel (Bq) • of radioactive atoms in a radioactive material over a period of time • 1 Bq = 1 disintegration per second • 1 Ci = 37 X 109 Bq • Ci or Bq may be used to refer to the amount of radioactive materials released into the environment

Radiation Units – rad and Gy • The radiation dose absorbed by a person (that is, the amount of energy deposited per unit of weight of human tissue by radiation) is measured using the conventional unit rad or the SI unit gray (Gy) • The rad, which stands for radiation absorbed dose, was the conventional unit of measurement • 1 Gy = 100 rad

Radiation Units – rem and Sv • The biological risk of exposure to radiation is measured using the conventional unit rem or the SI unit sievert (Sv) • Scientists have assigned a number, Quality Factor (Q), to each type of ionizing radiation (alpha and beta particles, gamma rays, and x-rays) depending on that type's ability to transfer energy to the cells of the body • To estimate a person's biological risk in rems rem = rad X Q • 1 Sv = 100 rem

Environmental Radiation • Units of radiation Particle Radiation Quality Factor alpha 1 rad 10 beta 10 rad 1 Health effect = 10 rems • Doses of radiation – Lethal doses of radiation begin at 500 rems

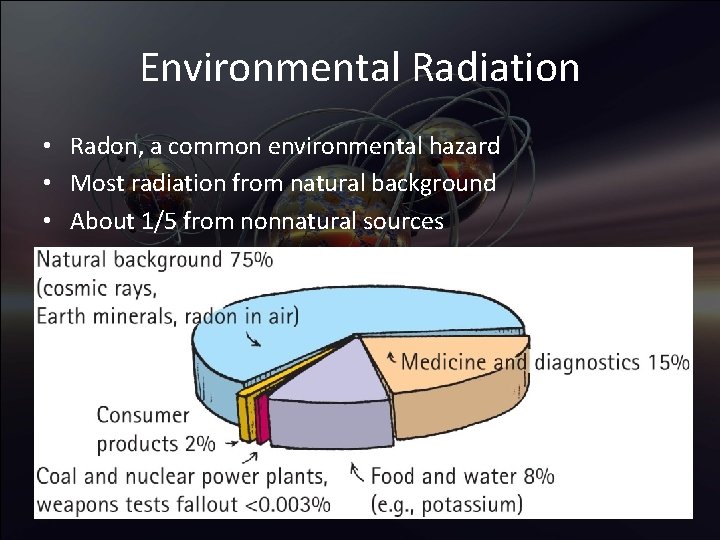
Environmental Radiation • Source received annually Natural origin Typical dose (mrem) Cosmic radiation 26 Ground 33 Air (Radon-222) Human tissues (K-40; Ra-226) 198 35

Environmental Radiation • Radon, a common environmental hazard • Most radiation from natural background • About 1/5 from nonnatural sources

Environmental Radiation • Doses of radiation Human origin Typical dose (mrem) Medical procedures Diagnostic X-rays 40 Nuclear diagnostics 15 TV tubes, other consumer products 11 Weapons-test fallout 1 Commercial fossil-fuel power plants <1 Commercial nuclear power plants << 1

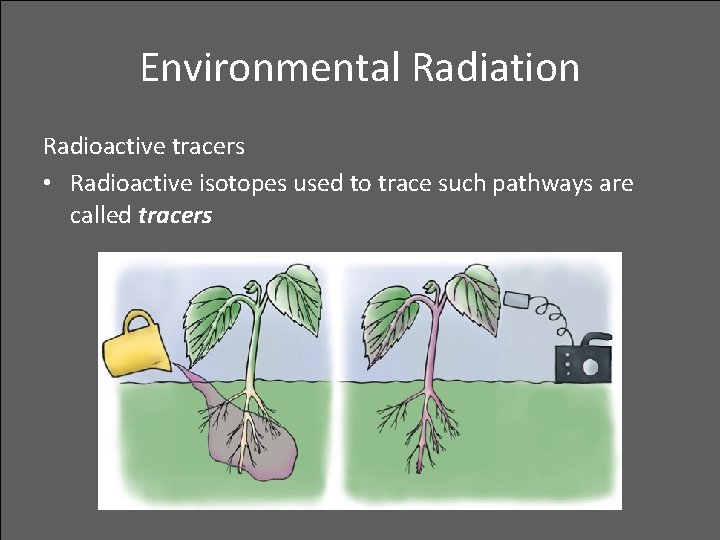
Environmental Radiation Radioactive tracers • Radioactive isotopes used to trace such pathways are called tracers

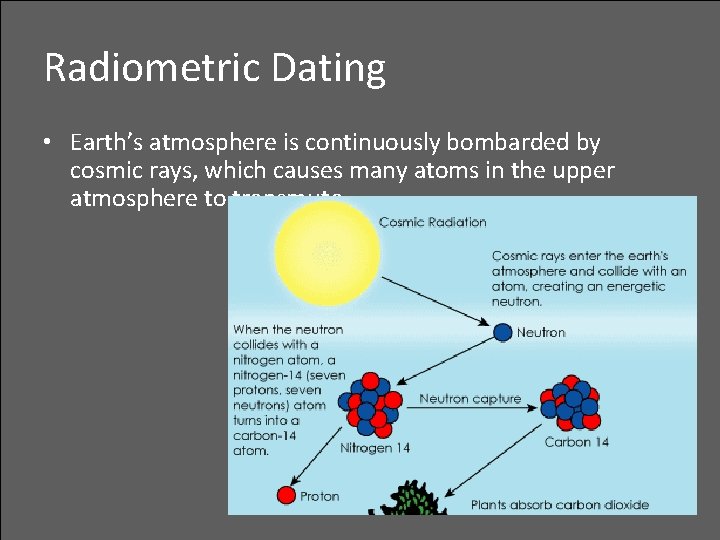
Radiometric Dating • Earth’s atmosphere is continuously bombarded by cosmic rays, which causes many atoms in the upper atmosphere to transmute

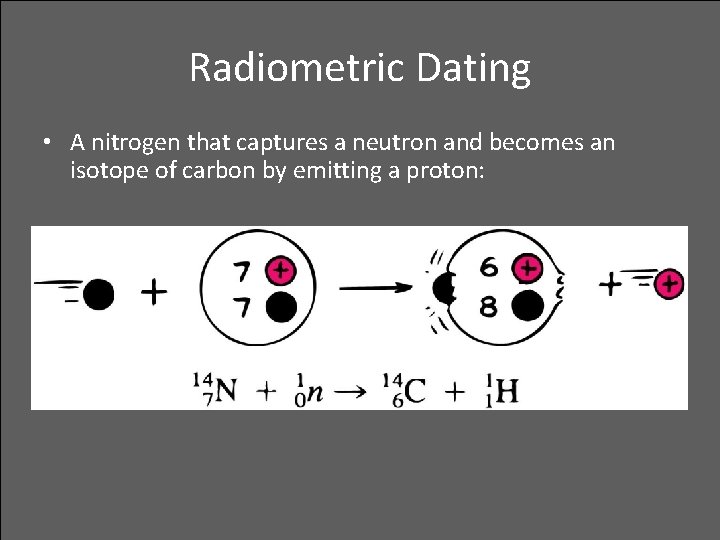
Radiometric Dating • A nitrogen that captures a neutron and becomes an isotope of carbon by emitting a proton:

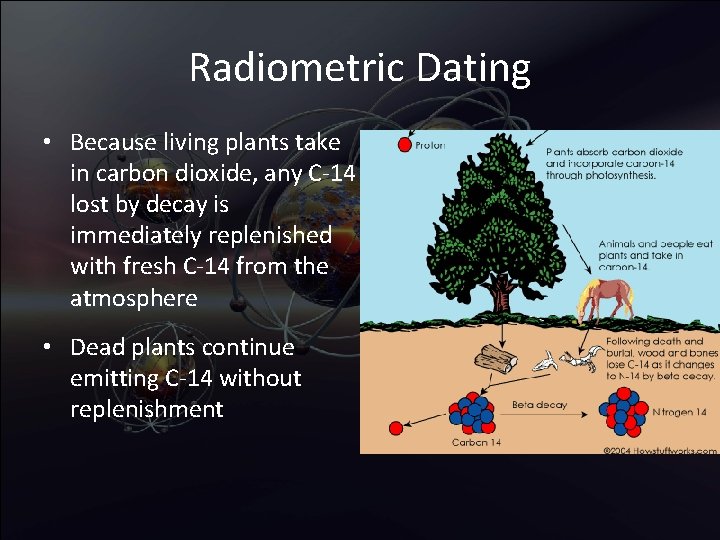
Radiometric Dating • Because living plants take in carbon dioxide, any C-14 lost by decay is immediately replenished with fresh C-14 from the atmosphere • Dead plants continue emitting C-14 without replenishment

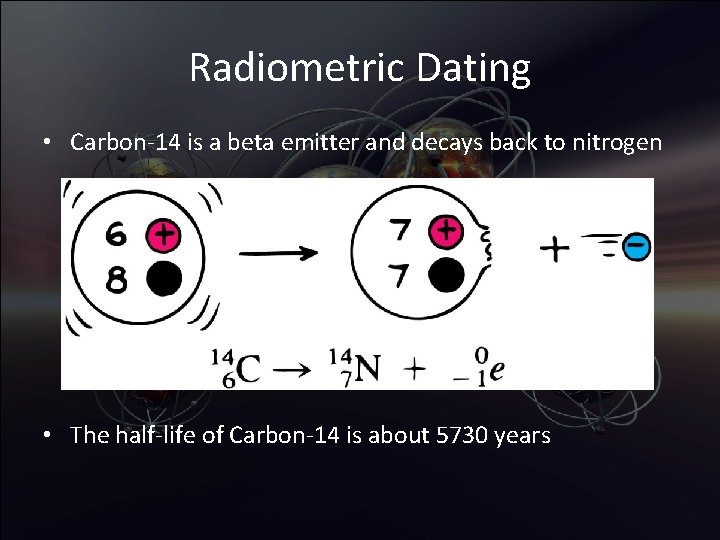
Radiometric Dating • Carbon-14 is a beta emitter and decays back to nitrogen • The half-life of Carbon-14 is about 5730 years

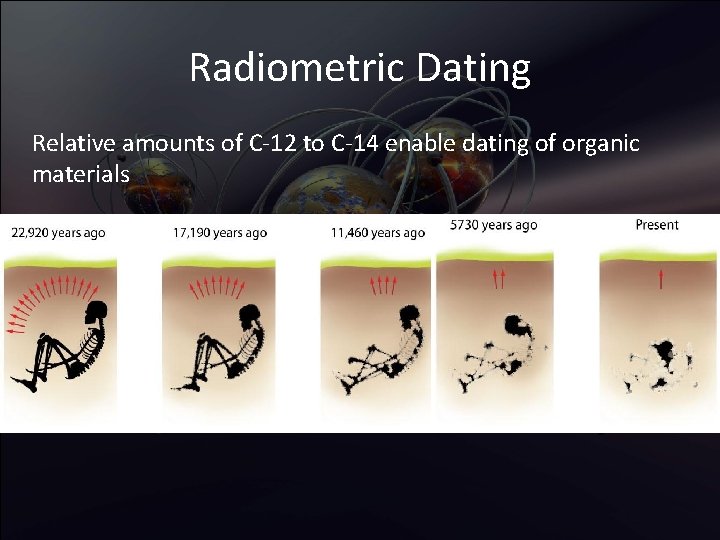
Radiometric Dating Relative amounts of C-12 to C-14 enable dating of organic materials

Radiometric Dating CHECK YOUR NEIGHBOR The half-life of carbon-14 is about 5730 years, which means that the present amount in your bones will reduce to zero A. B. C. D. when you die. in about 5730 years. in about twice 5730 years. None of the above.

Radiometric Dating CHECK YOUR ANSWER The half-life of carbon-14 is about 5730 years, which means that the present amount in your bones will reduce to zero A. B. C. D. when you die. in about 5730 years. in about twice 5730 years. None of the above. Explanation: In theory, the amount never reaches zero. In eons to come, trace amounts of the carbon-14 in your bones, even if completely dissolved, will still exist.

Q&A

The End
 Eleanor roosevelt high school
Eleanor roosevelt high school Electric forces within an atomic nucleus tend to
Electric forces within an atomic nucleus tend to Two protons in an atomic nucleus are typically
Two protons in an atomic nucleus are typically Relative formula mass of hcl
Relative formula mass of hcl Atomic mass of oxygen
Atomic mass of oxygen Atomic
Atomic Guided reading activity lesson 2 roosevelt and taft
Guided reading activity lesson 2 roosevelt and taft Natural and artificial radioactivity
Natural and artificial radioactivity Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Natural and artificial radioactivity
Natural and artificial radioactivity Datación radiométrica
Datación radiométrica Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions периодический закон
периодический закон Atomic raduis
Atomic raduis Atomic number vs atomic radius
Atomic number vs atomic radius Who discovered radioactivity
Who discovered radioactivity Who discovered radioactivity
Who discovered radioactivity Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Natural vs artificial radioactivity
Natural vs artificial radioactivity Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Mta
Mta Radioactivity
Radioactivity The great unconformity
The great unconformity Natural radioactivity
Natural radioactivity Environmental radioactivity
Environmental radioactivity Natural radioactivity
Natural radioactivity Radioactivity phenomenon
Radioactivity phenomenon Units of radioactivity
Units of radioactivity Radioactive decay equation
Radioactive decay equation Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Defination of radioactivity
Defination of radioactivity Beta decay of carbon
Beta decay of carbon Books poem by eleanor farjeon questions and answers
Books poem by eleanor farjeon questions and answers Amelia and eleanor go for a ride read aloud
Amelia and eleanor go for a ride read aloud Eleanor of aquitaine family tree
Eleanor of aquitaine family tree Eleanor rigby album
Eleanor rigby album Eleanor birrell
Eleanor birrell Imagine theres no lonely people
Imagine theres no lonely people Figurative language in eleanor rigby
Figurative language in eleanor rigby Ichabod crane (colonel)
Ichabod crane (colonel) Eleanor joyce
Eleanor joyce The great schism
The great schism Education as human capital
Education as human capital Semantic memory
Semantic memory Eleanor
Eleanor While he the house his friend called
While he the house his friend called Eleanor margo
Eleanor margo Eleanor wynn
Eleanor wynn Eleanor marx
Eleanor marx Eleanor womack
Eleanor womack Zusammengesetze
Zusammengesetze Compare and contrast fdr and hoover
Compare and contrast fdr and hoover Lesson 11 atomic pudding models of the atom
Lesson 11 atomic pudding models of the atom Atomic alchemy
Atomic alchemy Herbert stoddard
Herbert stoddard Roosevelt wilson and taft venn diagram
Roosevelt wilson and taft venn diagram Chapter 33 franklin d roosevelt and the shadow of war
Chapter 33 franklin d roosevelt and the shadow of war Progressivism and the republican roosevelt
Progressivism and the republican roosevelt Roosevelt corollary
Roosevelt corollary Theodore roosevelt college and career academy
Theodore roosevelt college and career academy Teddy roosevelt and the square deal
Teddy roosevelt and the square deal Intermediate mass
Intermediate mass Language
Language What are minimal pairs in linguistics
What are minimal pairs in linguistics Onset coda and nucleus
Onset coda and nucleus Building vocabulary: the nucleus, dna, and chromosomes
Building vocabulary: the nucleus, dna, and chromosomes Nuclear membrane
Nuclear membrane Teddy roosevelt death
Teddy roosevelt death Teddy roosevelt death
Teddy roosevelt death Explain the roosevelt corollary
Explain the roosevelt corollary Explain the roosevelt corollary
Explain the roosevelt corollary Explain the roosevelt corollary
Explain the roosevelt corollary Teddy roosevelt imperialist
Teddy roosevelt imperialist Explain the roosevelt corollary
Explain the roosevelt corollary Duke ellington teddy roosevelt
Duke ellington teddy roosevelt Explain the roosevelt corollary
Explain the roosevelt corollary Metonimia en a roosevelt
Metonimia en a roosevelt A roosevelt recursos literarios
A roosevelt recursos literarios Roosevelt corollary
Roosevelt corollary What steps did roosevelt take to solve each problem?
What steps did roosevelt take to solve each problem? Roosevelt corollary political cartoon
Roosevelt corollary political cartoon Theodore roosevelt
Theodore roosevelt Chapter 17 section 3 teddy roosevelt's square deal
Chapter 17 section 3 teddy roosevelt's square deal Chapter 9 section 3 teddy roosevelts square deal
Chapter 9 section 3 teddy roosevelts square deal Roosevelt corollary drawing
Roosevelt corollary drawing Teddy roosevelt's square deal worksheet
Teddy roosevelt's square deal worksheet Bertillonage
Bertillonage Roosevelt corollary cartoon
Roosevelt corollary cartoon Roosevelt corollary
Roosevelt corollary New deal
New deal Theodore roosevelt on immigrants
Theodore roosevelt on immigrants Teddy roosevelt imperialist
Teddy roosevelt imperialist Wilson vs roosevelt
Wilson vs roosevelt Roosevelt corollary cartoon
Roosevelt corollary cartoon