The Atomic Nucleus and Radioactivity Or Youre so







- Slides: 10

The Atomic Nucleus and Radioactivity Or You’re so hot you’re Glowing

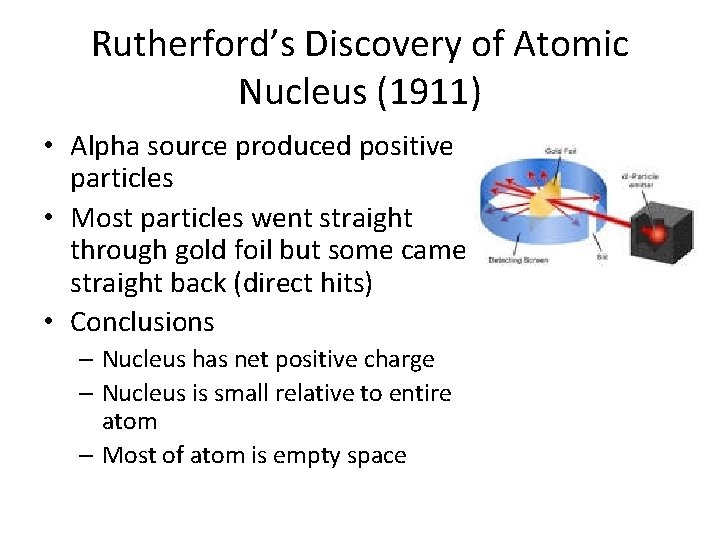
Rutherford’s Discovery of Atomic Nucleus (1911) • Alpha source produced positive particles • Most particles went straight through gold foil but some came straight back (direct hits) • Conclusions – Nucleus has net positive charge – Nucleus is small relative to entire atom – Most of atom is empty space

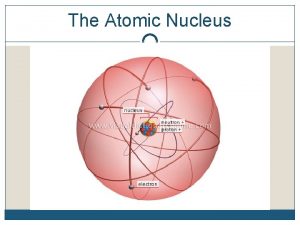
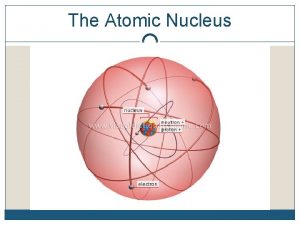
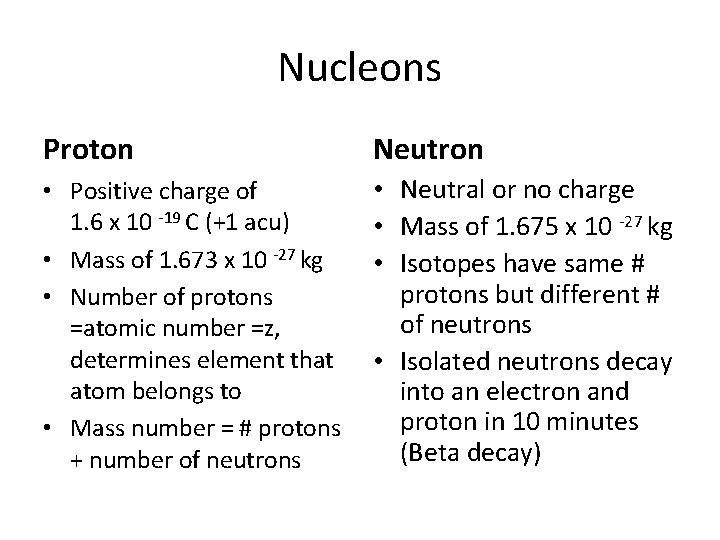
Nucleons Proton Neutron • Positive charge of 1. 6 x 10 -19 C (+1 acu) • Mass of 1. 673 x 10 -27 kg • Number of protons =atomic number =z, determines element that atom belongs to • Mass number = # protons + number of neutrons • Neutral or no charge • Mass of 1. 675 x 10 -27 kg • Isotopes have same # protons but different # of neutrons • Isolated neutrons decay into an electron and proton in 10 minutes (Beta decay)

Strong Nuclear Force • The strong nuclear force holds the atomic nucleus together (opposes electric force) • Attractive force between neutron and proton only. Thus bigger atoms need more neutrons. • Only acts at distances the same size as one proton or neutron • Strongest of all action at a distance or field forces

Radioactive Decay • The breaking up of an atomic nucleus because there is not enough strong force to hold it together – Occurs for light element isotopes w/ a lot of neutrons – Occurs for all elements over atomic number 83 • Result of radioactive decay or transmutation is a new element. – Charge is conserved – Number nucleons is conserved

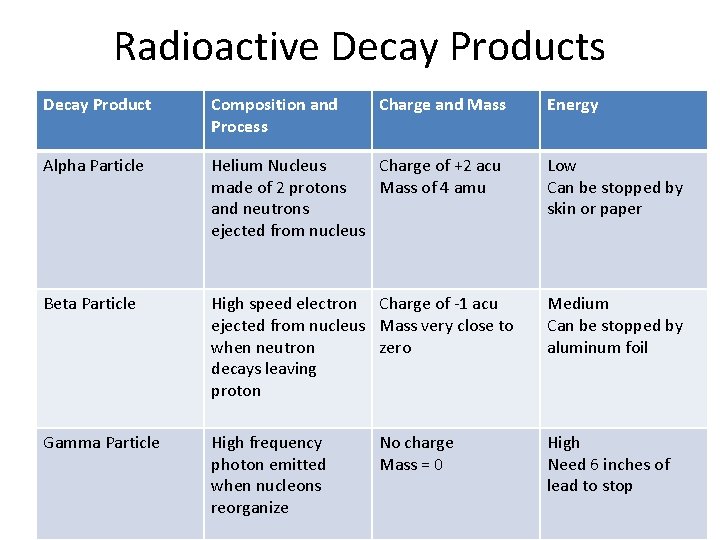
Radioactive Decay Products • Alpha Decay – Helium nucleus is ejected from nucleus – 2 protons and 2 neutrons give it a mass of 4 amu and a charge of +2 – Low energy, stopped by skin or paper • Beta Decay – Electron is ejected when neutron decays leaving proton. Charge is -1 – Medium energy, stopped by foil • Gamma Decay – EM photon released when nucleus reorganizes, zero charge – High energy (stopped by 6 inches of lead)

The Three Cookie Problem • As a final cruel physics test Mr. De. Voe gives you three radioactive cookies. One each with alpha, beta and gamma radiation. • Which cookie do you eat? • Which cookie do you smash on your hand? • Which cookie do you put in your pocket?

Sources and Uses of Radioactivity • Yearly dose of radio activity includes – – 56% from Earth and Cosmic Rays 42% from medical and dental x-rays 2. 9% weapons test fall out 0. 1% Nuclear Power Plant Operation • Radioactivity used to trace materials inside and outside human body • Cancer cell DNA found to be more susceptible to radiation than healthy cells => radiation therapy • Rate of decay unaffected by temp. , pressure, physical or chemical changes. Can be used to see how old material is if know how much of radioactive element existed originally.

Radioactive Decay Products Decay Product Composition and Process Charge and Mass Alpha Particle Helium Nucleus Charge of +2 acu made of 2 protons Mass of 4 amu and neutrons ejected from nucleus Low Can be stopped by skin or paper Beta Particle High speed electron Charge of -1 acu ejected from nucleus Mass very close to when neutron zero decays leaving proton Medium Can be stopped by aluminum foil Gamma Particle High frequency photon emitted when nucleons reorganize High Need 6 inches of lead to stop No charge Mass = 0 Energy

Radioactive Dating • Half Life = time for half of radioactive element to decay into its daughter elements – 1 half life, 50% parent, 50% daughter, 1: 1 ratio – 2 half lives, 25% parent, 75% daughter 1: 3 ratio – 3 half lives, 12. 5% parent, 87. 5% daughter 1: 7 ratio • Common radio element half lives – Carbon 14 5780 years – Uranium 238 4. 6 Billion Years
 Electric forces within an atomic nucleus tend to
Electric forces within an atomic nucleus tend to Two protons in an atomic nucleus are typically
Two protons in an atomic nucleus are typically Relative atomic mass of beryllium
Relative atomic mass of beryllium How to calculate abundance in isotopes
How to calculate abundance in isotopes Difference between atomic mass and atomic number
Difference between atomic mass and atomic number Natural and artificial radioactivity
Natural and artificial radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Natural and artificial radioactivity
Natural and artificial radioactivity Datación radiométrica
Datación radiométrica Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions How to write email writing
How to write email writing