Electrochemistry Chapter 20 Redox Reactions n Reduction Oxidation





















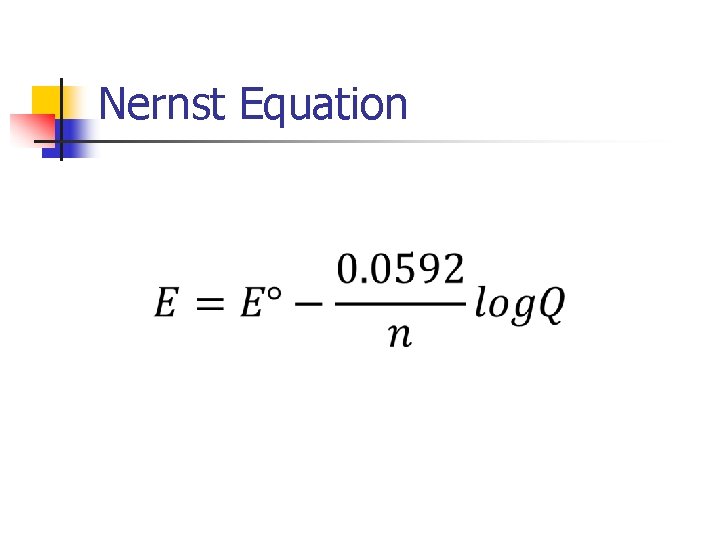
![Concentration Cells n n E°cell = 0 [reactants]<[products] Concentration Cells n n E°cell = 0 [reactants]<[products]](https://slidetodoc.com/presentation_image_h2/695cd63381fe64a95a1d69a5ab056184/image-52.jpg)



- Slides: 57

Electrochemistry Chapter 20

Redox Reactions n Reduction – Oxidation, or redox, involves the transfer of electrons n Reduction – gain of electrons n Oxidation – loss of electrons

Redox Reactions n LEO the lion goes GER n Lose Electrons Oxidation n Gain Electrons Reduction

Redox Reactions n OIL RIG n Oxidation Is Losing (OIL) n Reduction Is Gaining (RIG)

Oxidation Numbers (States) n Positive, negative or neutral values assigned to an atom to keep track of the number of electrons lost or gained. n Charge

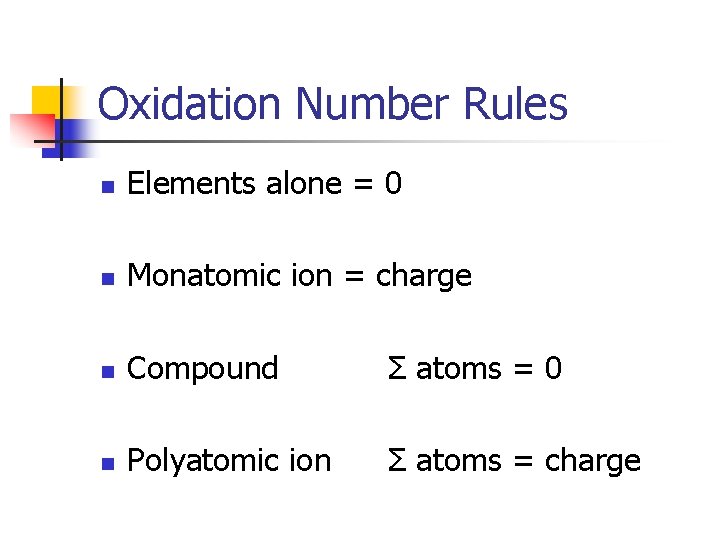
Oxidation Number Rules n Elements alone = 0 n Monatomic ion = charge n Compound Σ atoms = 0 n Polyatomic ion Σ atoms = charge

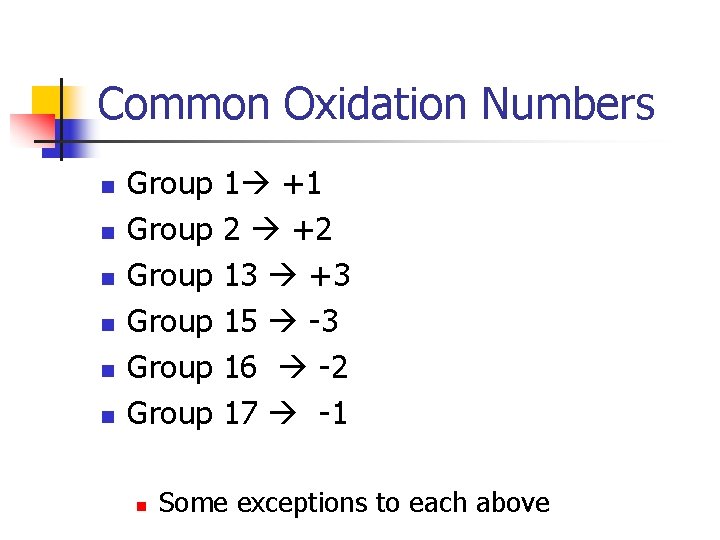
Common Oxidation Numbers n n n Group Group n 1 +1 2 +2 13 +3 15 -3 16 -2 17 -1 Some exceptions to each above

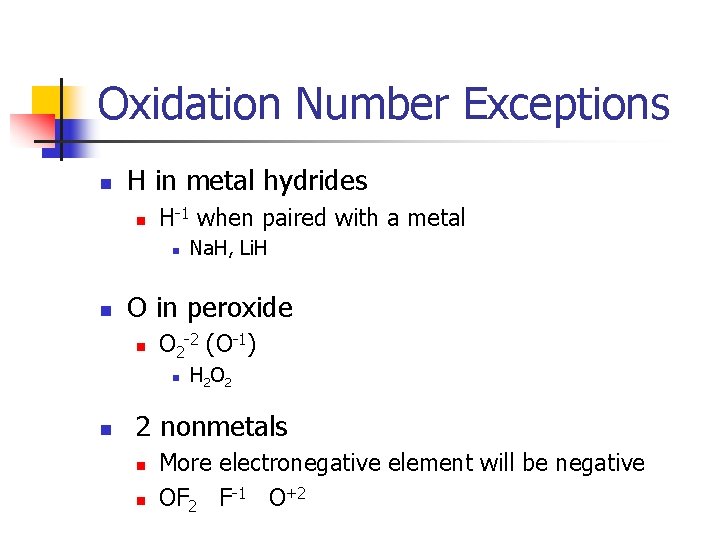
Oxidation Number Exceptions n H in metal hydrides n H-1 when paired with a metal n n O in peroxide n O 2 -2 (O-1) n n Na. H, Li. H H 2 O 2 2 nonmetals n n More electronegative element will be negative OF 2 F-1 O+2

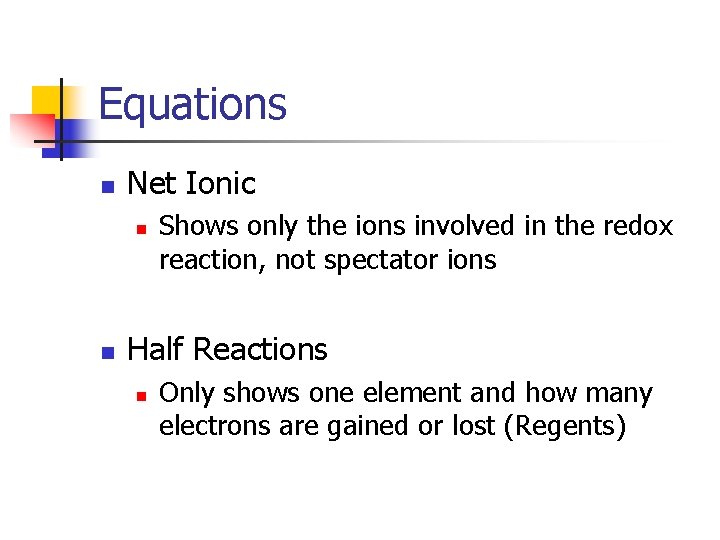
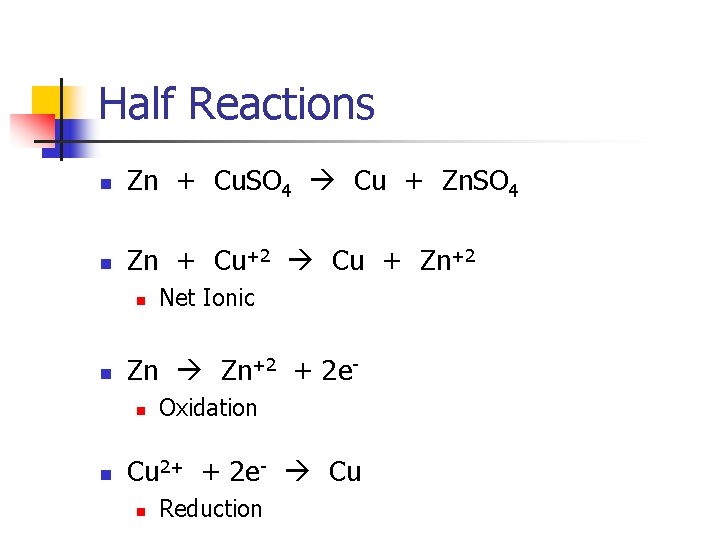
Equations n Net Ionic n n Shows only the ions involved in the redox reaction, not spectator ions Half Reactions n Only shows one element and how many electrons are gained or lost (Regents)

Half Reactions n Zn + Cu. SO 4 Cu + Zn. SO 4 n Zn + Cu+2 Cu + Zn+2 n n Zn Zn+2 + 2 en n Net Ionic Oxidation Cu 2+ + 2 e- Cu n Reduction

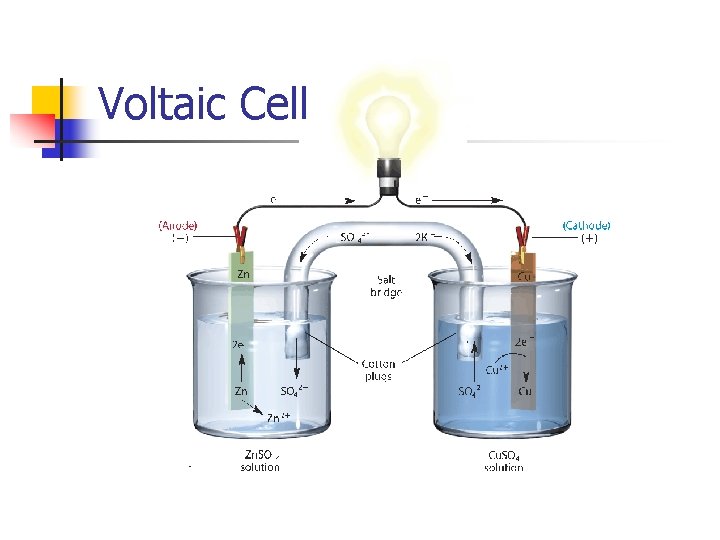
Electrochemical Cells n Voltaic (Chemical) n n Spontaneous reaction based on potential difference between metals Electrolytic n Non-spontaneous reaction using an outside power source

Electrodes n Cathode – electrode where reduction takes place n n Red Cat Anode – electrode where oxidation takes place n An Ox

Homework n n Read Chapter 20 Sections 1 and 2 (20. 1 -20. 2)


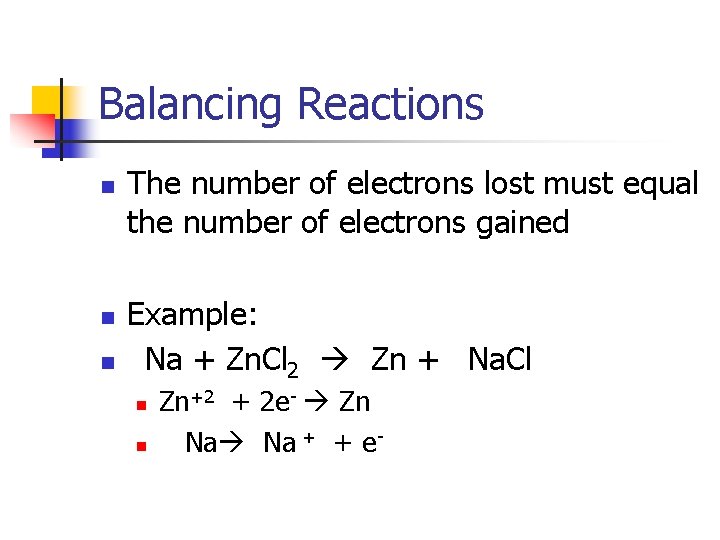
Balancing Reactions n n n The number of electrons lost must equal the number of electrons gained Example: 2 Na + Zn. Cl 2 Zn + 2 Na. Cl n n Zn+2 + 2 e- Zn 2(Na Na + + e- )

Balancing Redox Reactions in Acidic solution n n n Balance all elements except H, O Balance O by adding H 2 O Balance H by adding H+ Balance charge by adding e. Multiply half reactions to balance electrons lost/gained Combine & cancel like species

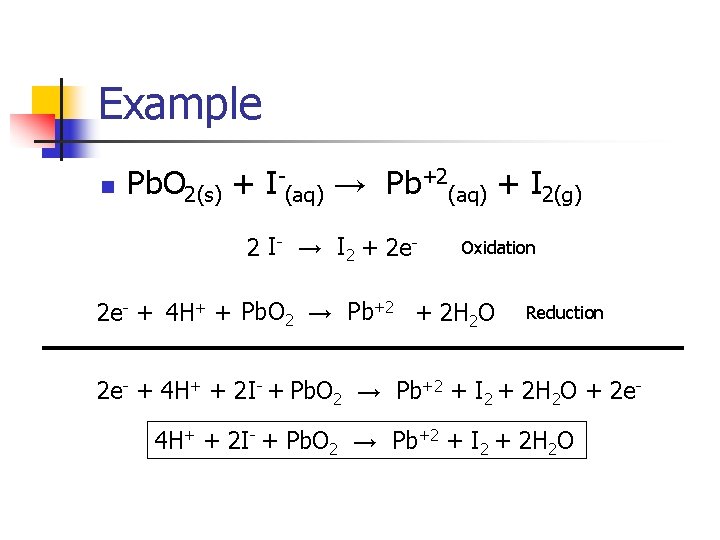
Example n Pb. O 2(s) + I-(aq) → Pb+2(aq) + I 2(g) 2 I- → I 2 + 2 e- Oxidation 2 e- + 4 H+ + Pb. O 2 → Pb+2 + 2 H 2 O Reduction 2 e- + 4 H+ + 2 I- + Pb. O 2 → Pb+2 + I 2 + 2 H 2 O + 2 e 4 H+ + 2 I- + Pb. O 2 → Pb+2 + I 2 + 2 H 2 O

Balancing Redox Reactions in Basic solution n n Follow balancing for acidic solution Add OH- to both sides equally to balance out H+ Combine H+ and OH- to make H 2 O Combine and cancel like species

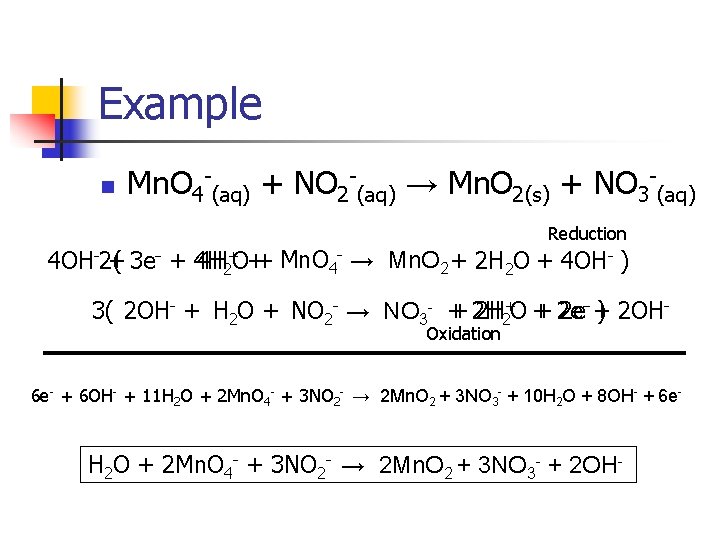
Example n Mn. O 4 -(aq) + NO 2 -(aq) → Mn. O 2(s) + NO 3 -(aq) Reduction 4 OH-2( + 3 e- + 4 H 4 H 2+O++ Mn. O 4 - → Mn. O 2 + 2 H 2 O + 4 OH- ) 3( 2 OH- + H 2 O + NO 2 - → NO 3 - + + 2 H 2 H 2+O + + 2 e 2 e-- + ) 2 OHOxidation 6 e- + 6 OH- + 11 H 2 O + 2 Mn. O 4 - + 3 NO 2 - → 2 Mn. O 2 + 3 NO 3 - + 10 H 2 O + 8 OH- + 6 e- H 2 O + 2 Mn. O 4 - + 3 NO 2 - → 2 Mn. O 2 + 3 NO 3 - + 2 OH-

Homework n (Re)Read Chapter 20. 2 Answer questions 20, 22 n Read Chapter 20. 3 -20. 4 n


Electrochemical Cells n Voltaic (Galvanic) n n Spontaneous reaction based on potential difference between metals Electrolytic n Non-spontaneous reaction using an outside power source

Electrodes n Cathode – electrode where reduction takes place n n Red Cat Anode – electrode where oxidation takes place n An Ox

Spontaneous Reaction n Some metals are more likely to ionize than another metals n n n Lose electrons (oxidize) Activity Series from Regents When 2 metals are paired together, one will lose and one will gain electrons

Voltaic Cell



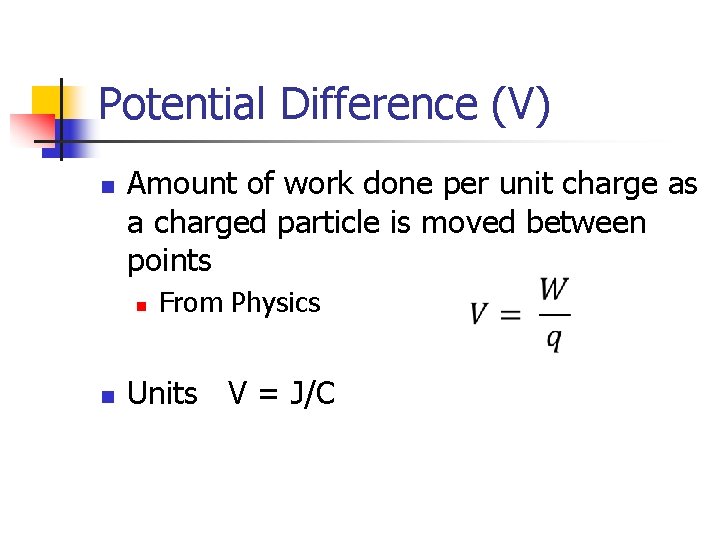
Potential Difference (V) n Amount of work done per unit charge as a charged particle is moved between points n n From Physics Units V = J/C

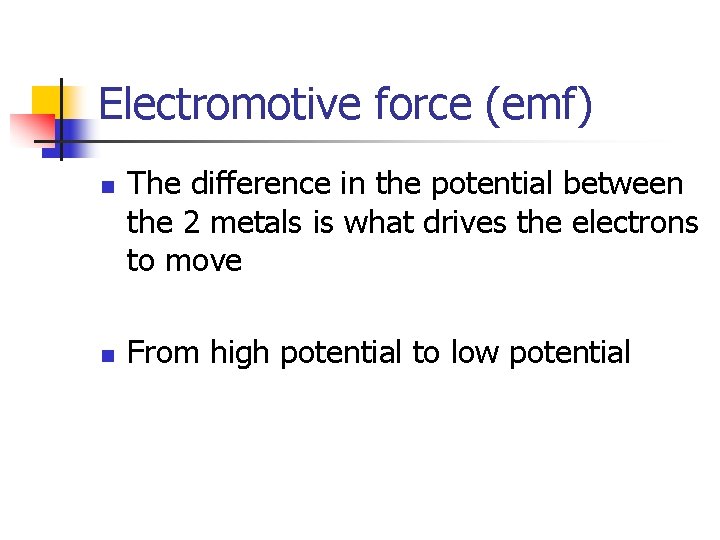
Electromotive force (emf) n n The difference in the potential between the 2 metals is what drives the electrons to move From high potential to low potential

Half Reaction Potential n n Measured with a standard hydrogen electrode to determine the potential of each half reaction Reported as Reduction Potentials n n Table 20. 1 Appendix E


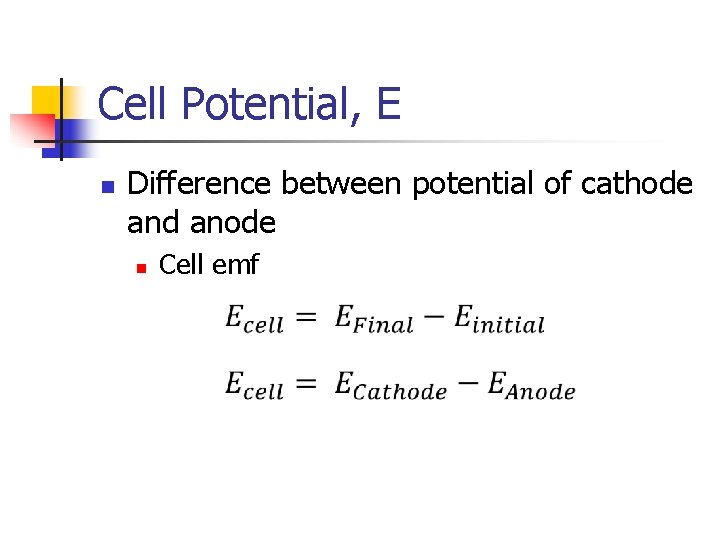
Cell Potential, E n Difference between potential of cathode and anode n Cell emf

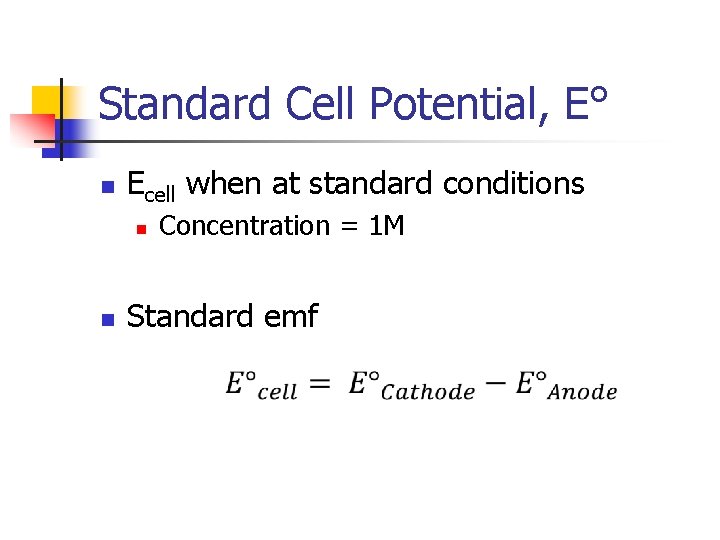
Standard Cell Potential, E° n Ecell when at standard conditions n n Concentration = 1 M Standard emf

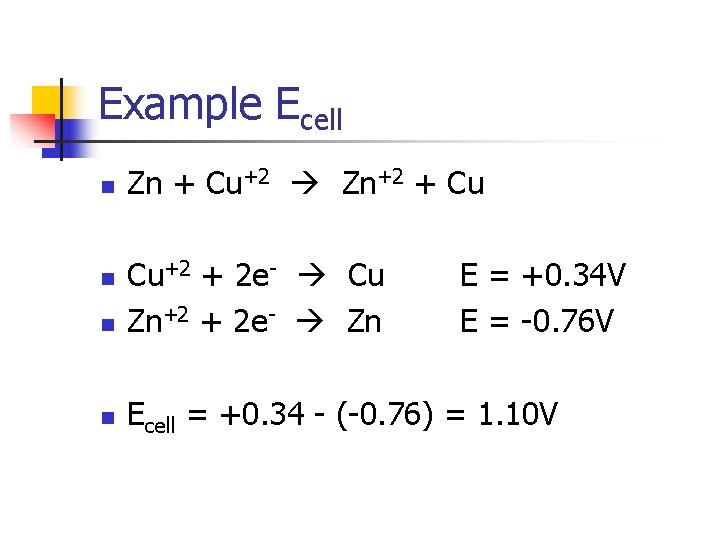
Example Ecell n Zn + Cu+2 Zn+2 + Cu n Cu+2 + 2 e- Cu Zn+2 + 2 e- Zn n Ecell = +0. 34 - (-0. 76) = 1. 10 V n E = +0. 34 V E = -0. 76 V

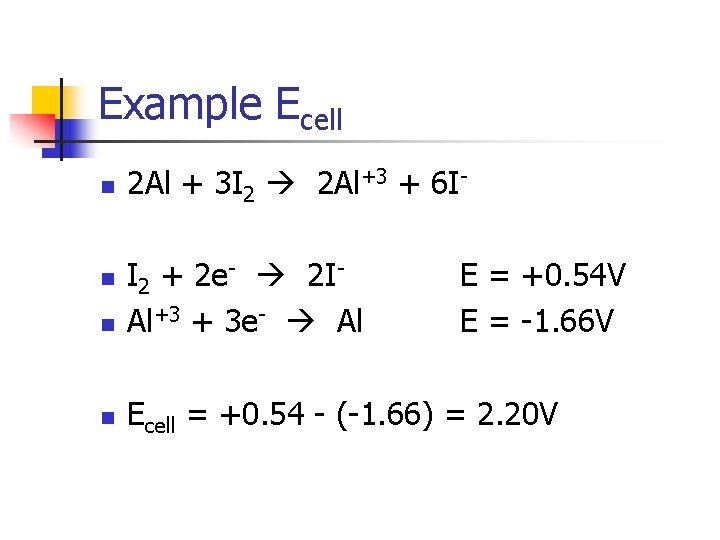
Example Ecell n 2 Al + 3 I 2 2 Al+3 + 6 I- n I 2 + 2 e- 2 IAl+3 + 3 e- Al n Ecell = +0. 54 - (-1. 66) = 2. 20 V n E = +0. 54 V E = -1. 66 V

Homework n (Re)Read Chapter 20. 3 -20. 4 Answer questions 6, 34, 36, 38 n Read Chapter 20. 5 n


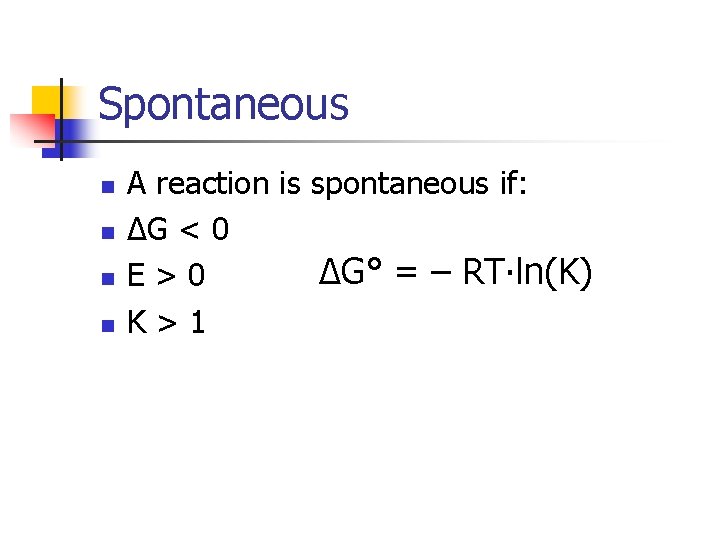
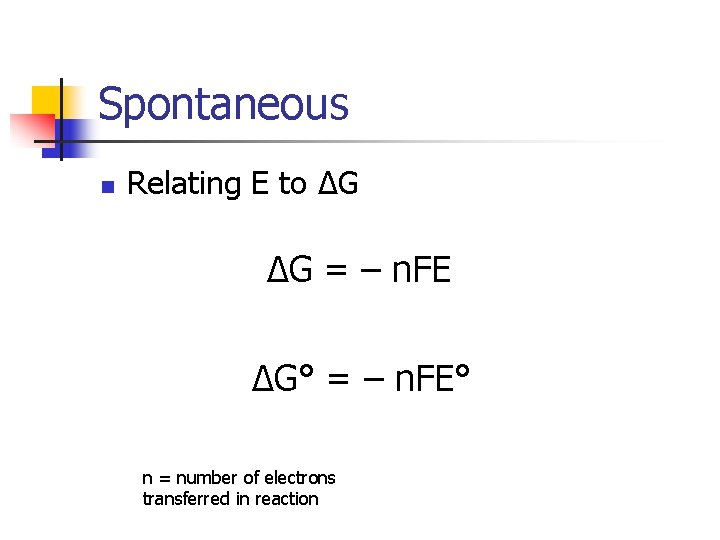
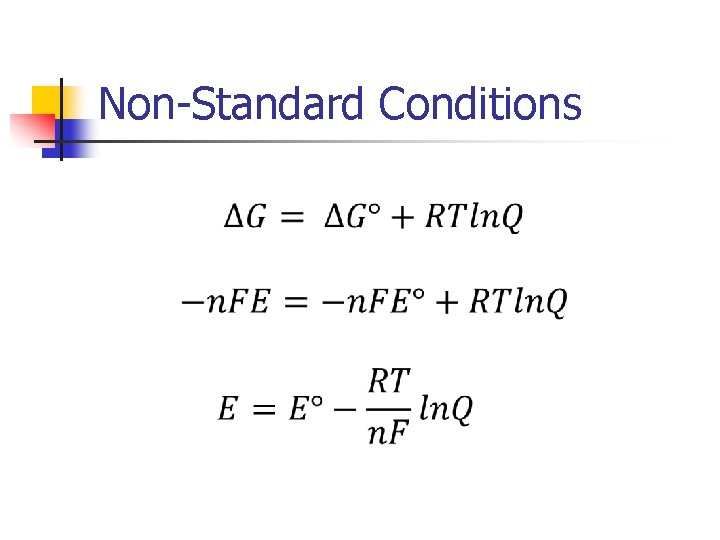
Spontaneous n n A reaction is spontaneous if: ΔG < 0 ΔG° = – RT∙ln(K) E>0 K>1

Spontaneous n Relating E to ΔG ΔG = – n. FE ΔG° = – n. FE° n = number of electrons transferred in reaction

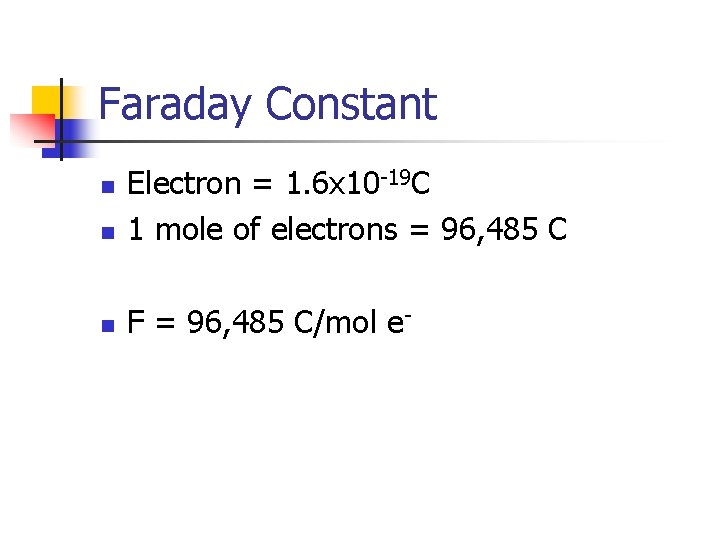
Faraday Constant n Electron = 1. 6 x 10 -19 C 1 mole of electrons = 96, 485 C n F = 96, 485 C/mol e- n

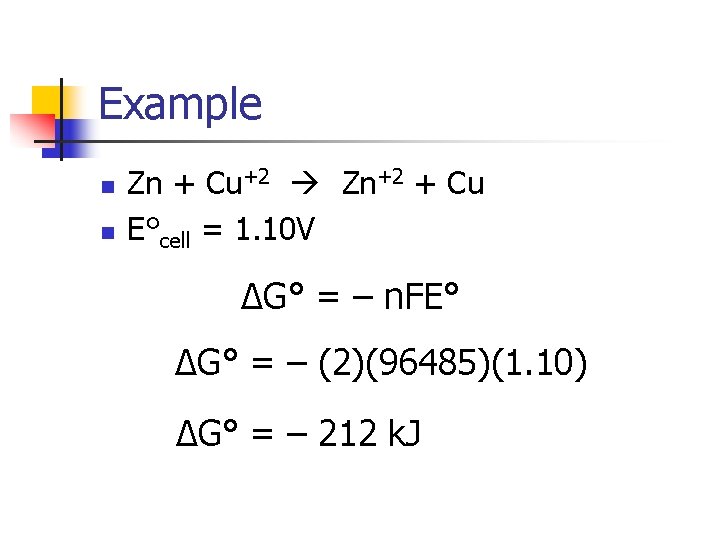
Example n n Zn + Cu+2 Zn+2 + Cu E°cell = 1. 10 V ΔG° = – n. FE° ΔG° = – (2)(96485)(1. 10) ΔG° = – 212 k. J

Example n n 2 Al + 3 I 2 2 Al+3 + 6 IE°cell = 2. 20 V ΔG° = – n. FE° ΔG° = – (6)(96485)(2. 20) ΔG° = – 1274 k. J

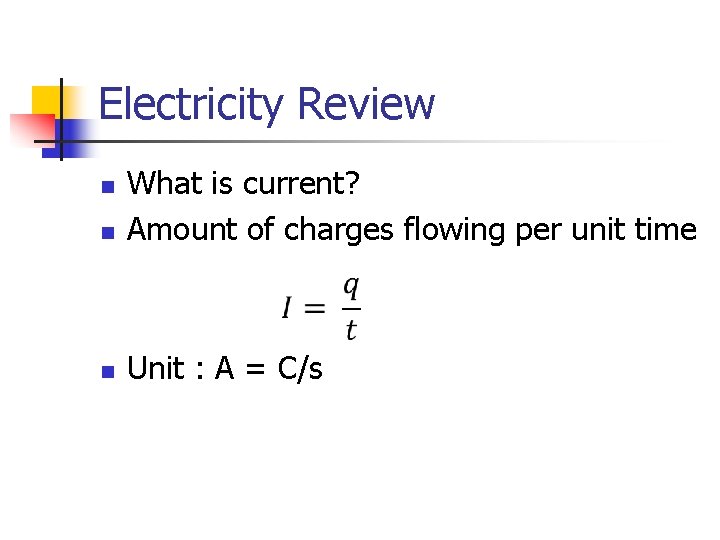
Electricity Review n What is current? Amount of charges flowing per unit time n Unit : A = C/s n

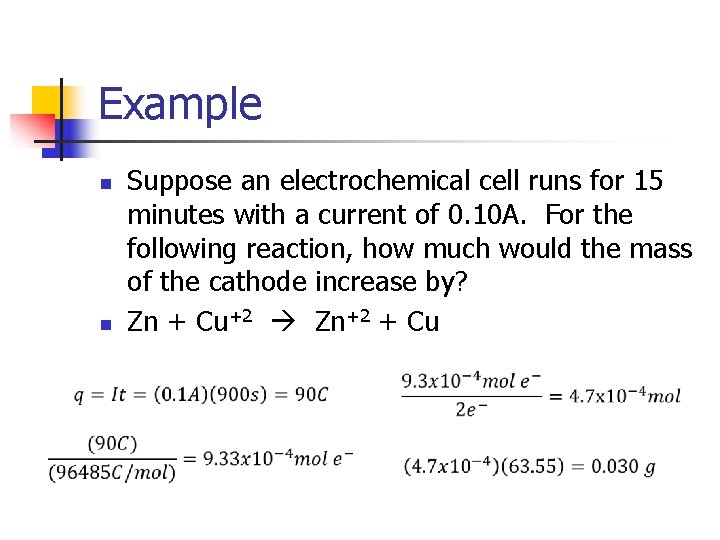
Example n n Suppose an electrochemical cell runs for 15 minutes with a current of 0. 10 A. For the following reaction, how much would the mass of the cathode increase by? Zn + Cu+2 Zn+2 + Cu

Homework n (Re)Read Chapter 20. 5 Answer questions #50, 52, 54, 56 n Quiz Wed n


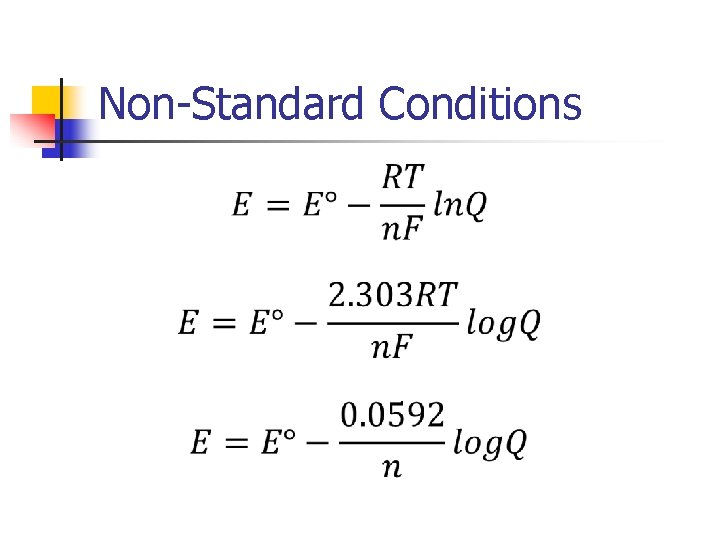
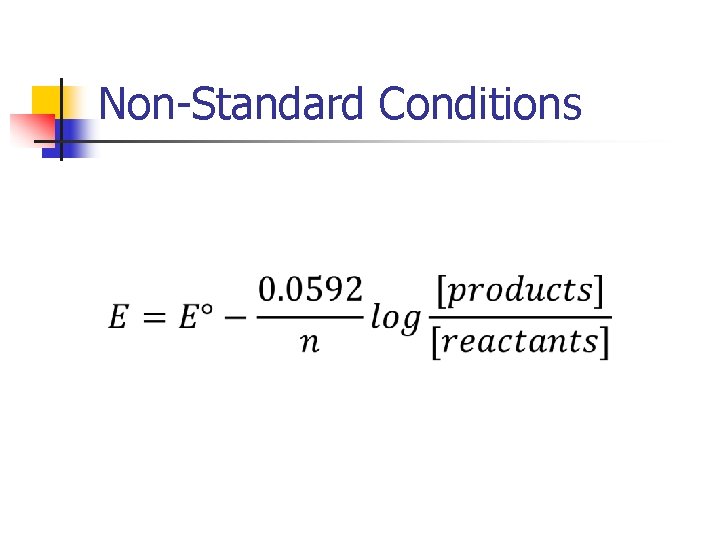
Non-Standard Conditions

Non-Standard Conditions
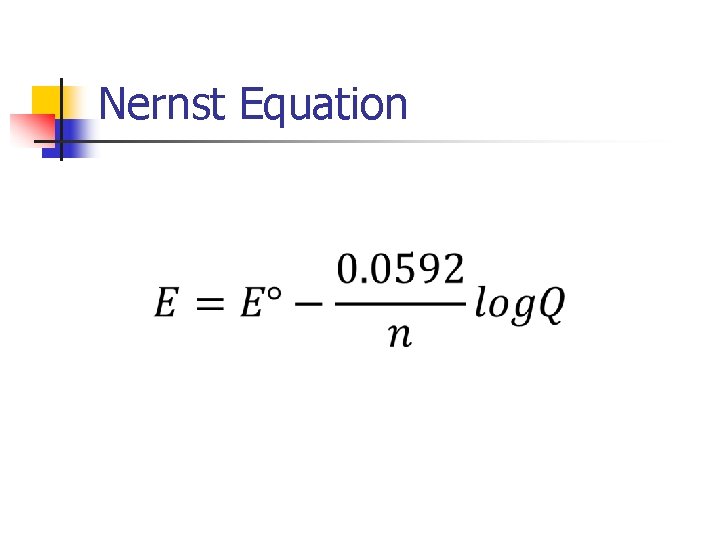
Nernst Equation

Non-Standard Conditions

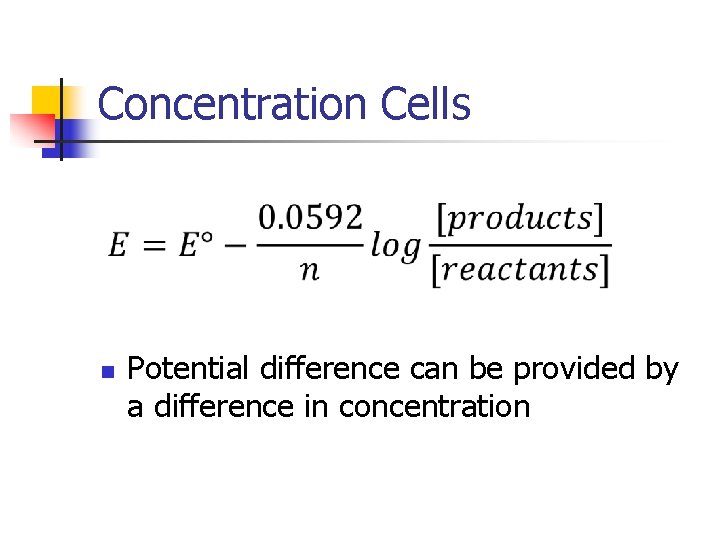
Concentration Cells n Potential difference can be provided by a difference in concentration
![Concentration Cells n n Ecell 0 reactantsproducts Concentration Cells n n E°cell = 0 [reactants]<[products]](https://slidetodoc.com/presentation_image_h2/695cd63381fe64a95a1d69a5ab056184/image-52.jpg)
Concentration Cells n n E°cell = 0 [reactants]<[products]


Electrolysis n Process of using electrical current to drive a non-spontaneous reaction n Electrolytic Cell n n n Oxidation at anode Reduction at cathode E (–) n n Electroplating Electropolishing

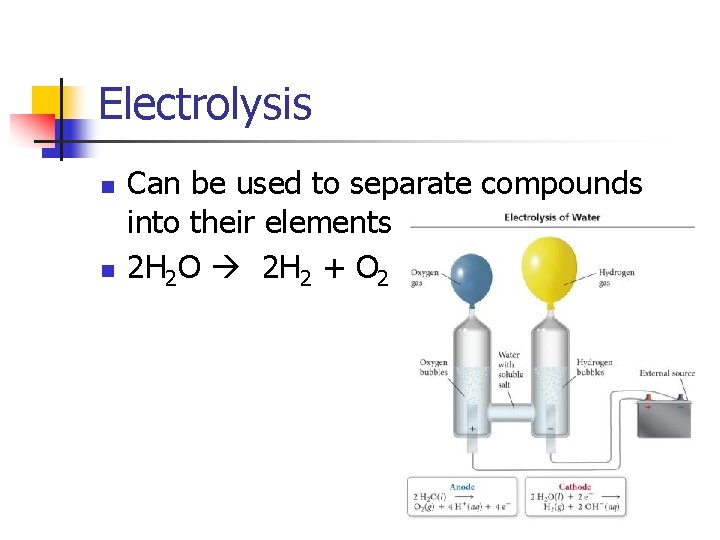
Electrolysis n n Can be used to separate compounds into their elements 2 H 2 O 2 H 2 + O 2

