REDOX Reactions Oxidation Reduction Oxidation Reduction Reaction These





- Slides: 8

REDOX Reactions Oxidation – Reduction

Oxidation – Reduction Reaction These reactions involve a transfer of electrons Half reactions MUST occur simultaneously Oxidation – loss of electrons Oxidizing agent – a substance that has the potential to cause another substance to be oxidized

Oxidation – Reduction Reaction Reduction – involves the gain of electrons Reducing agent – a substance that has the potential to cause another substance to be reduced (gain electrons)

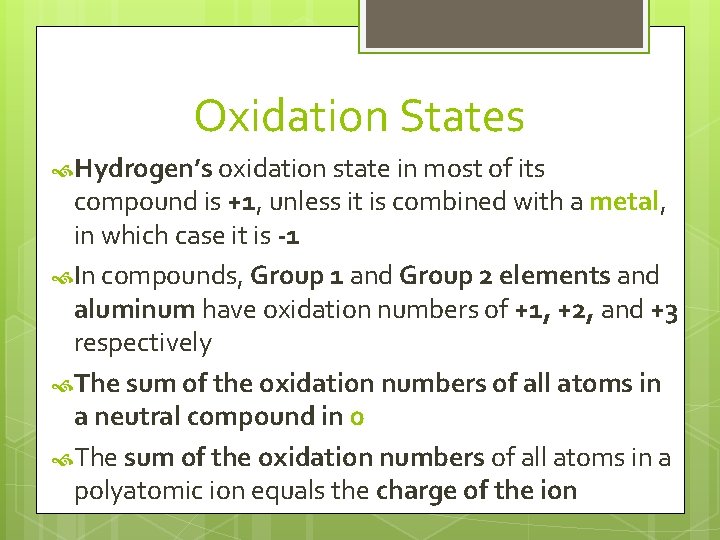
Oxidation States The oxidation number of a pure element is 0 The oxidation number of a monatomic ion equals the charge on the ion The more electronegative element in a binary compound is assigned the number equal to the charge it would have it were an ion The oxidation number of fluorine in a compound is always -1 Oxygen has an oxidation number of -2 unless it is combined with Fluorine, in which it is +1 or +2, or it is in a peroxide, in which it is -1

Oxidation States Hydrogen’s oxidation state in most of its compound is +1, unless it is combined with a metal, in which case it is -1 In compounds, Group 1 and Group 2 elements and aluminum have oxidation numbers of +1, +2, and +3 respectively The sum of the oxidation numbers of all atoms in a neutral compound in 0 The sum of the oxidation numbers of all atoms in a polyatomic ion equals the charge of the ion

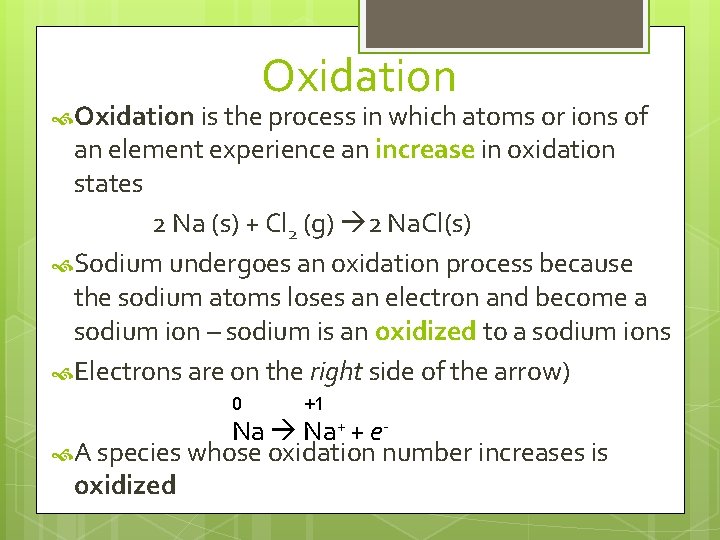
Oxidation is the process in which atoms or ions of an element experience an increase in oxidation states 2 Na (s) + Cl 2 (g) 2 Na. Cl(s) Sodium undergoes an oxidation process because the sodium atoms loses an electron and become a sodium ion – sodium is an oxidized to a sodium ions Electrons are on the right side of the arrow) 0 +1 Na Na+ + e A species whose oxidation number increases is oxidized

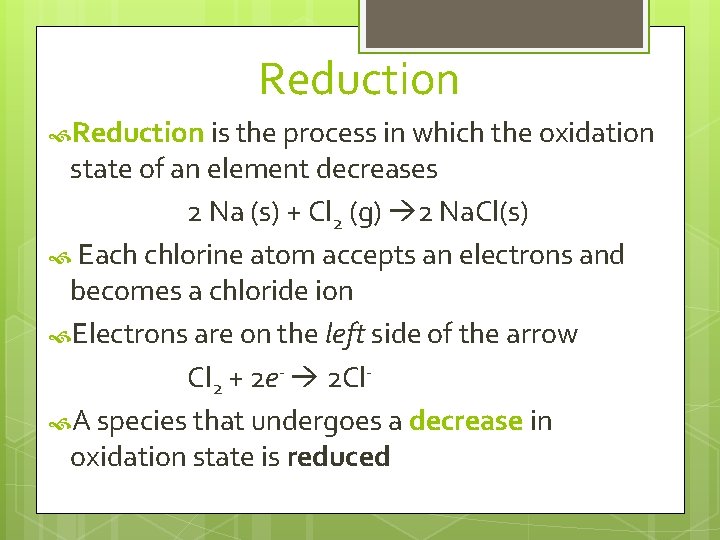
Reduction is the process in which the oxidation state of an element decreases 2 Na (s) + Cl 2 (g) 2 Na. Cl(s) Each chlorine atom accepts an electrons and becomes a chloride ion Electrons are on the left side of the arrow Cl 2 + 2 e- 2 Cl A species that undergoes a decrease in oxidation state is reduced

Oxidation – Reduction Reaction Lose Electrons Oxidation “Leo” the lion says “Ger” Gain Electrons Reduction