Unit 16 Electrochemistry Oxidation Reduction Oxidation verses Reduction







- Slides: 23

Unit 16 Electrochemistry Oxidation & Reduction

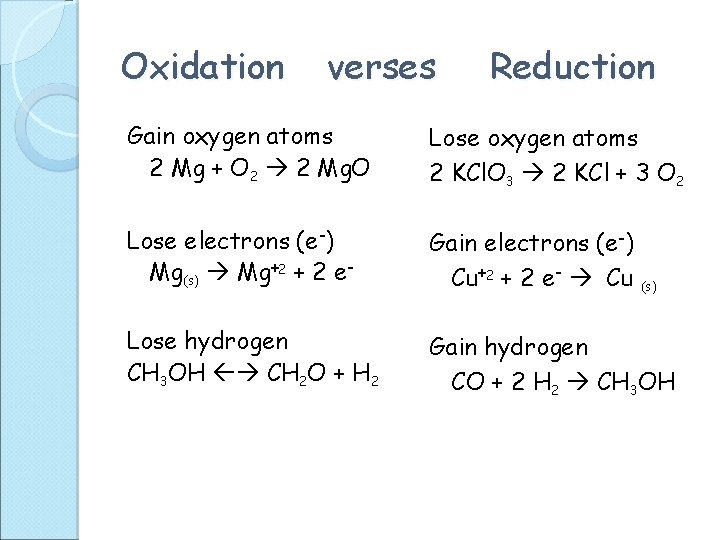
Oxidation verses Reduction Gain oxygen atoms 2 Mg + O 2 2 Mg. O Lose oxygen atoms 2 KCl. O 3 2 KCl + 3 O 2 Lose electrons (e-) Mg(s) Mg+2 + 2 e- Gain electrons (e-) Cu+2 + 2 e- Cu (s) Lose hydrogen CH 3 OH CH 2 O + H 2 Gain hydrogen CO + 2 H 2 CH 3 OH

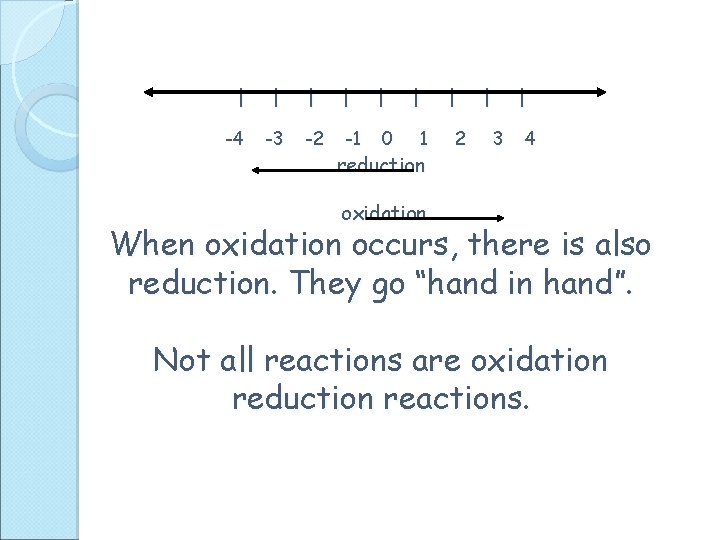
l l l -4 -3 -2 -1 0 l 1 reduction l 2 l l 3 4 oxidation When oxidation occurs, there is also reduction. They go “hand in hand”. Not all reactions are oxidation reduction reactions.

Using Appendix 6 chart: To determine the strength as an oxidizing agent, look at the left side of appendix 6. The chemical that is higher on the list (has a greater magnitude + voltage) is the better oxidizing agent. Which is better? Br 2 or Sn+2 To determine the strength as a reducing agent, look at the right side of appendix 6. The chemical that is lower on the list (has the greater magnitude – voltage) is the better reducing agent. Which is better? Pb(s) or H 2 O 2

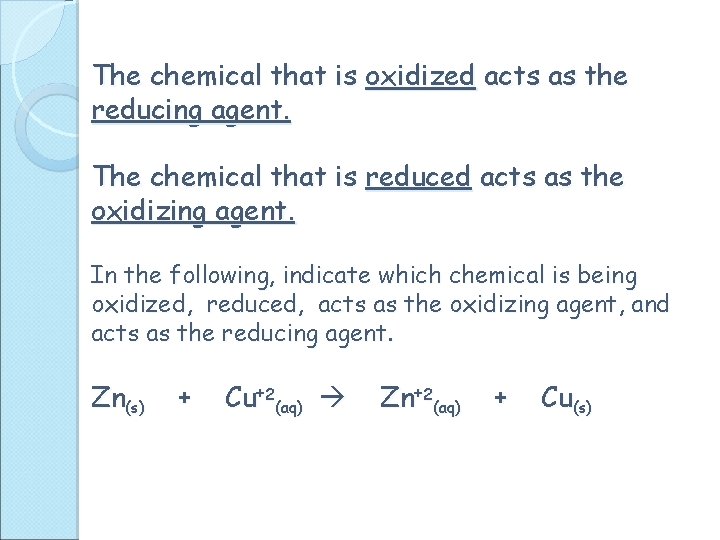
The chemical that is oxidized acts as the reducing agent. The chemical that is reduced acts as the oxidizing agent. In the following, indicate which chemical is being oxidized, reduced, acts as the oxidizing agent, and acts as the reducing agent. Zn(s) + Cu+2(aq) Zn+2(aq) + Cu(s)

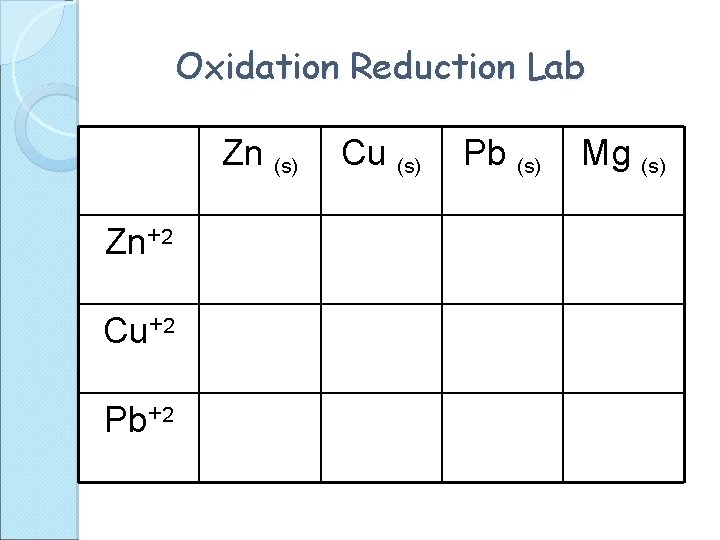
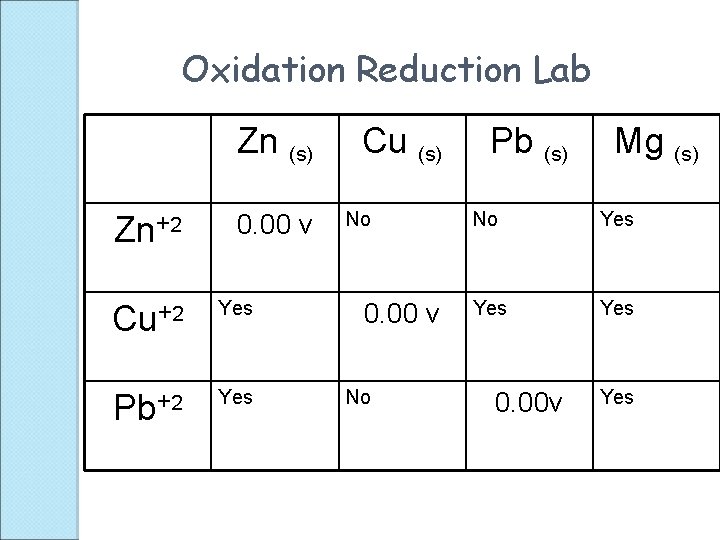
Oxidation Reduction Lab Zn (s) Zn+2 Cu+2 Pb+2 Cu (s) Pb (s) Mg (s)

Electricity is the movement of electrons to provide energy. Two factors affect the amount of energy produced. 1. Voltage is the push behind the e-. Voltage is measured in volts 2. Current which is how many e- pass a point each second. Current is measured in Amperes. 1 Amp = 6. 0 x 1018 e-/s An electrochemical Cell (Galvanic or voltaic cell) is a chemical device to make electricity from chemical reactions. It employs oxidation and reduction. It will have a positive (+) voltage while it works.

Cell Potential (Eocell) or Electromotive force (emf) is the pull or “driving force” on the e-. It is measured in volts (Eo ) Standard reduction potential is the potential for the half – reaction at 1 M the standard being measured off 2 H+ + 2 e- H 2 , Pt electrode = 0. 00 v Remember that these are listed as Reduction Potentials. When there is reduction, there is also oxidation. One of the two half – reactions will need to be reversed (so that it is written as an oxidation)

To calculate the voltage of an electrochemical cell: 1. Obtain the two half – reactions from the reduction potential chart. 2. Determine which half – reaction is the oxidation, reverse the reaction. When the reaction is reversed, the sign on the voltage is changed. 3. Balance the e- in the two half – reactions by multiplying the coefficients by some whole number. Remember that voltage is a property of the kind of chemical, not the amount. Do not multiply the voltage. 4. Add the two half – reactions to determine the net voltage for the cell.

Oxidation Reduction Lab Zn (s) Zn+2 0. 00 v Cu+2 Yes Pb+2 Yes Cu (s) No 0. 00 v No Pb (s) Mg (s) No Yes Yes 0. 00 v Yes

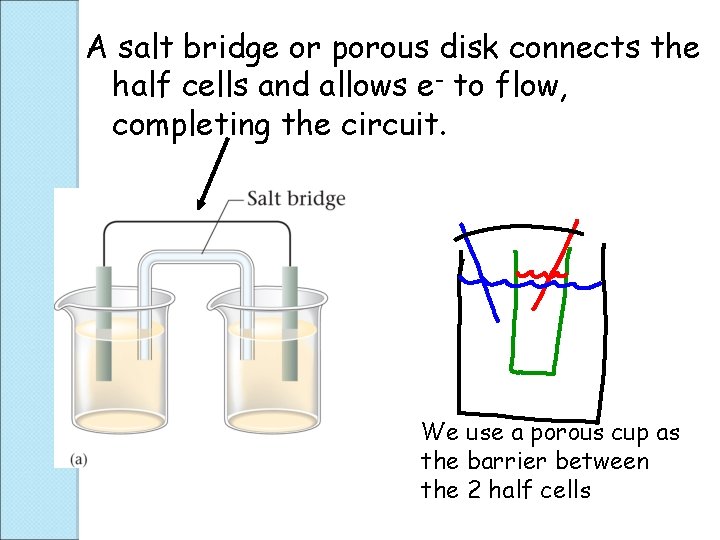
A salt bridge or porous disk connects the half cells and allows e- to flow, completing the circuit. We use a porous cup as the barrier between the 2 half cells

Calculate the voltage of a Galvanic cell made with Mg and Cu used to run the clock. Mg+2 + 2 e- Mg(s) Cu+2 + 2 e- Cu(s) volts

Line Notation : A way to denote a galvanic cell Anode: the oxidation is written first Cathode: the reduction is written second One line separates the phases of a half cell Two lines separate the anode and cathode Mg l Mg+2 ll Cu+2 l Cu

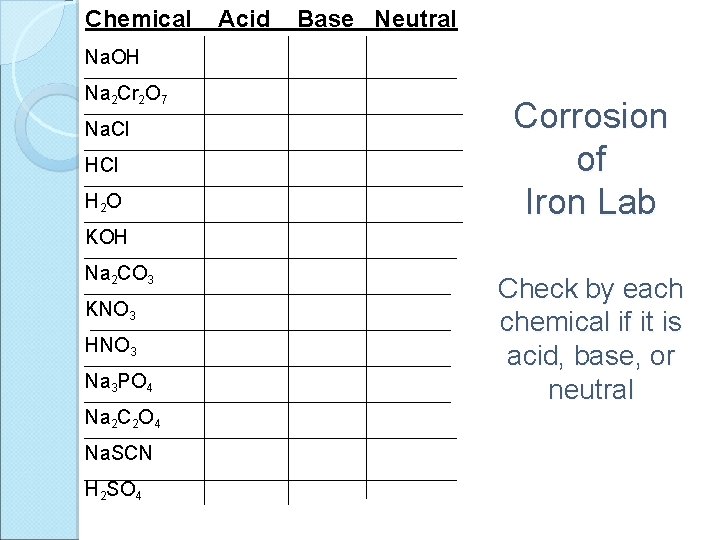
Chemical Acid Base Neutral Na. OH Na 2 Cr 2 O 7 Na. Cl H 2 O Corrosion of Iron Lab KOH Na 2 CO 3 KNO 3 HNO 3 Na 3 PO 4 Na 2 C 2 O 4 Na. SCN H 2 SO 4 Check by each chemical if it is acid, base, or neutral

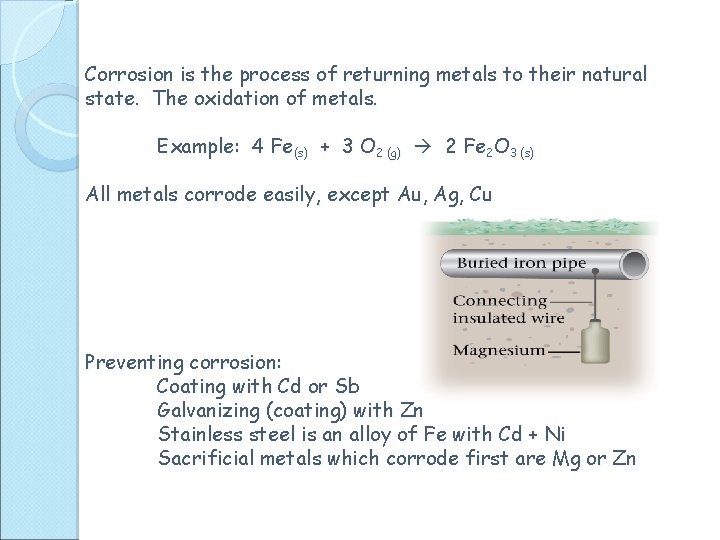
Corrosion is the process of returning metals to their natural state. The oxidation of metals. Example: 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O 3 (s) All metals corrode easily, except Au, Ag, Cu Preventing corrosion: Coating with Cd or Sb Galvanizing (coating) with Zn Stainless steel is an alloy of Fe with Cd + Ni Sacrificial metals which corrode first are Mg or Zn

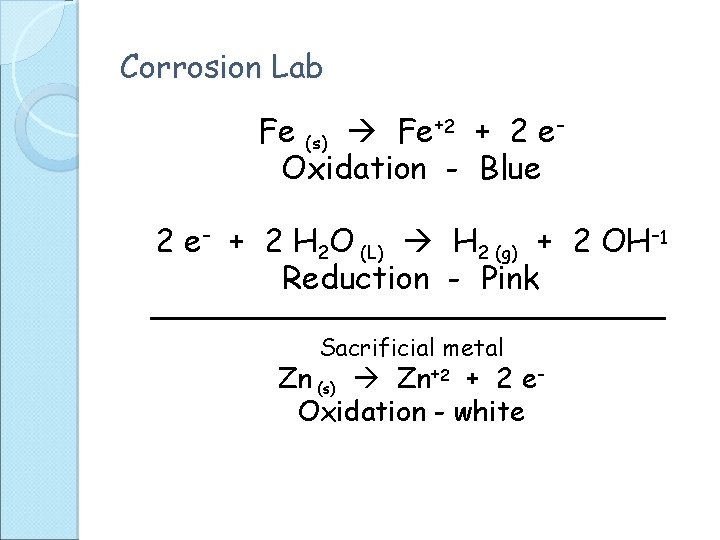
Corrosion Lab Fe (s) Fe+2 + 2 e. Oxidation - Blue 2 e- + 2 H 2 O (L) H 2 (g) + 2 OH-1 Reduction - Pink Sacrificial metal Zn (s) Zn+2 + 2 e. Oxidation - white

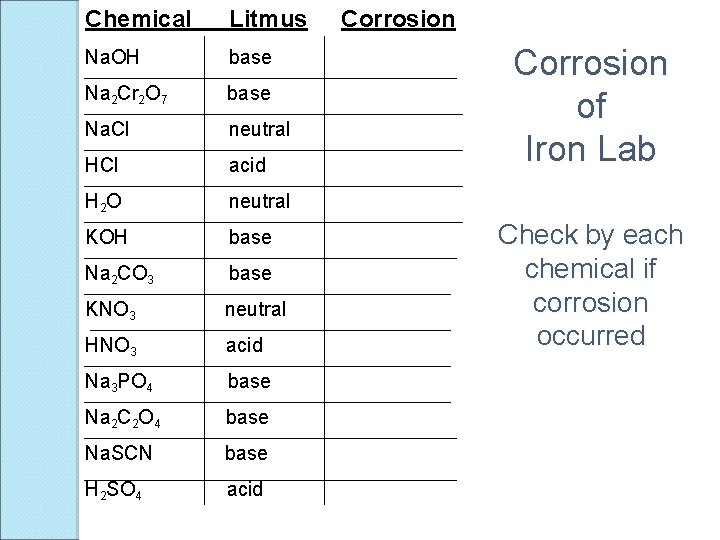
Chemical Litmus Na. OH base Na 2 Cr 2 O 7 base Na. Cl neutral HCl acid H 2 O neutral KOH base Na 2 CO 3 base KNO 3 neutral HNO 3 acid Na 3 PO 4 base Na 2 C 2 O 4 base Na. SCN base H 2 SO 4 acid Corrosion of Iron Lab Check by each chemical if corrosion occurred

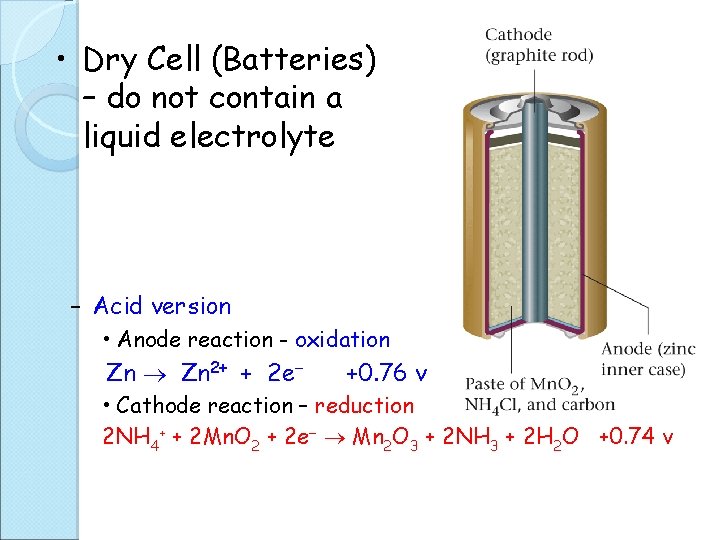
A battery is a group of galvanic cells connected in series. (add potentials Eocell) Wet cell – contain fluid medium for e- flow Dry cell - contain a paste medium for e- flow Primary cell – one time use, not rechargeable Secondary cell - rechargeable

• Dry Cell (Batteries) – do not contain a liquid electrolyte – Acid version • Anode reaction - oxidation Zn 2+ + 2 e +0. 76 v • Cathode reaction – reduction 2 NH 4+ + 2 Mn. O 2 + 2 e Mn 2 O 3 + 2 NH 3 + 2 H 2 O +0. 74 v

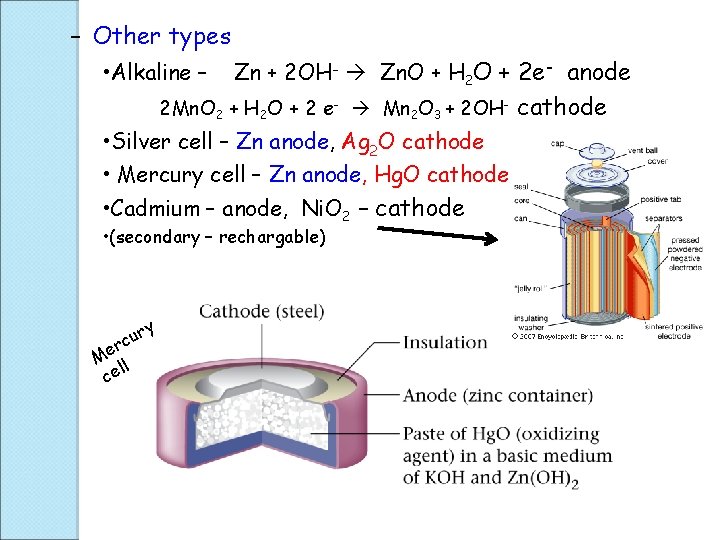
– Other types • Alkaline – Zn + 2 OH- Zn. O + H 2 O + 2 e- anode 2 Mn. O 2 + H 2 O + 2 e- Mn 2 O 3 + 2 OH- • Silver cell – Zn anode, Ag 2 O cathode • Mercury cell – Zn anode, Hg. O cathode • Cadmium – anode, Ni. O 2 – cathode • (secondary – rechargable) y r u c r Me l l ce cathode

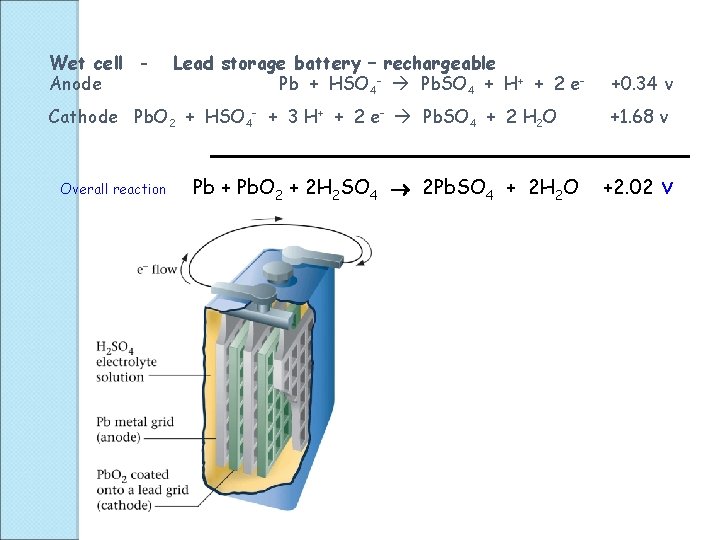
Wet cell Anode Lead storage battery – rechargeable Pb + HSO 4 - Pb. SO 4 + H+ + 2 e- Cathode Pb. O 2 + HSO 4 - + 3 H+ + 2 e- Pb. SO 4 + 2 H 2 O Overall reaction Pb + Pb. O 2 + 2 H 2 SO 4 2 Pb. SO 4 + 2 H 2 O +0. 34 v +1. 68 v +2. 02 v

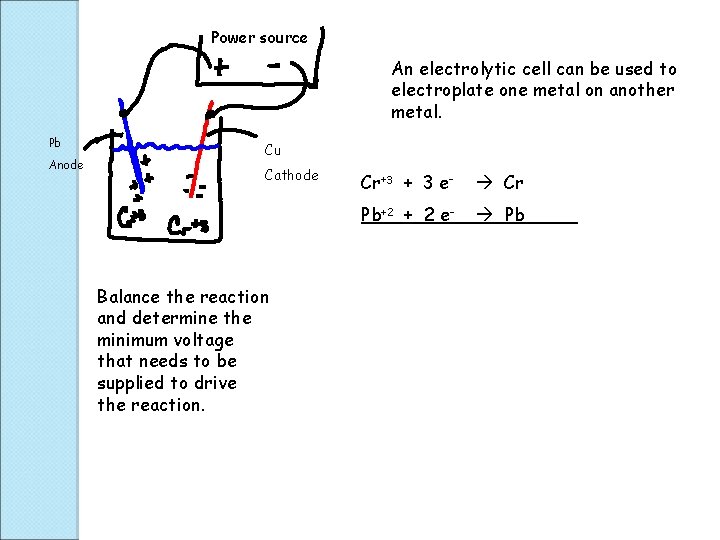
An Electrolytic cell is a cell with a negative voltage, so there is no reaction. An outside source of energy is used as a way to drive the reaction. This type of cell is often used as a controlled way to deposit thin layers of metals, electroplating, on another metal.

Power source An electrolytic cell can be used to electroplate one metal on another metal. Pb Anode Cu Cathode Balance the reaction and determine the minimum voltage that needs to be supplied to drive the reaction. Cr+3 + 3 e- Cr Pb+2 + 2 e- Pb