Chapter Twentyfour Organic Chemistry Chapter TwentyFour Organic Chemistry

- Slides: 42

Chapter Twenty-four Organic Chemistry

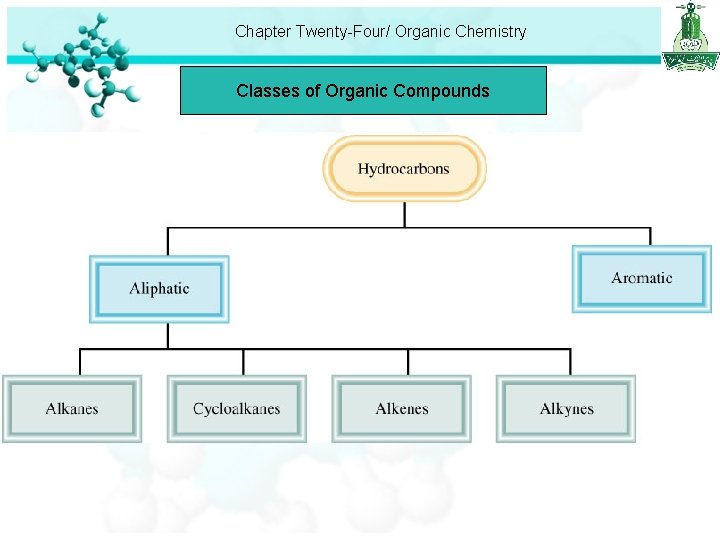
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds • The branch of chemistry that deals with carbon compounds is organic chemistry. • Classes of organic compounds can be distinguished according to functional groups they contain. • A functional group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. • Most organic compounds are derived from a group of compounds known as hydrocarbons because they are made up of only hydrogen and carbon. • Carbon has the ability to form long chains and ring structure.

Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds

Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds • Aliphatic hydrocarbons divided into: – Alkanes: Only single covalent bonds are present, general formula Cn. H 2 n+2, n = 1, 2, …. – Cycloalkanes: alkanes whose carbon atoms are joined in rings, general formula Cn. H 2 n, n = 3, 4, …. – Alkenes: contain at least one carbon-carbon double bond, general formula Cn. H 2 n, n = 2, 3 …. – Alkynes: contain at least one carbon-carbon triple bond, general formula Cn. H 2 n-2, n = 2, 3 ….

Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Example: C 10 H 22 is the formula of an: a-alkane. b-alkene. c-alkyne. d-aromatic hydrocarbon.

Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes • Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • only single covalent bonds • saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH 4 methane C 2 H 6 ethane C 3 H 8 propane • Alkane Nomenclature: • IUPAC: International Union of Pure and Applied Chemistry • The first four alkanes (methane, propane, and butane) have nonsystematic names.

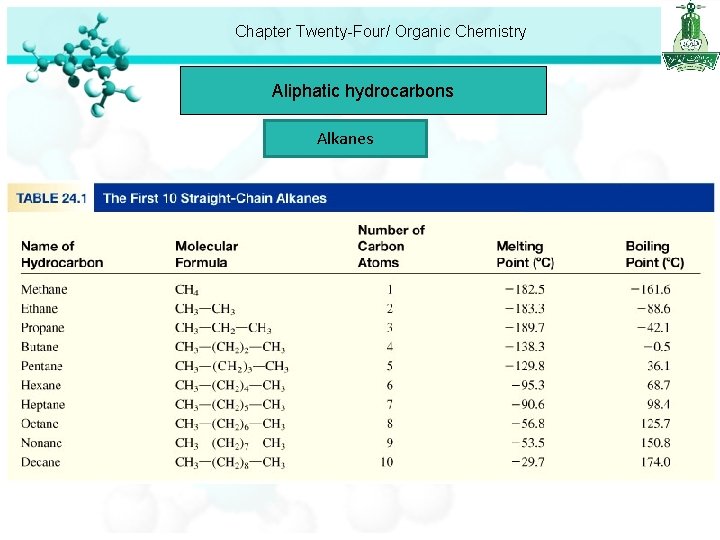
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes

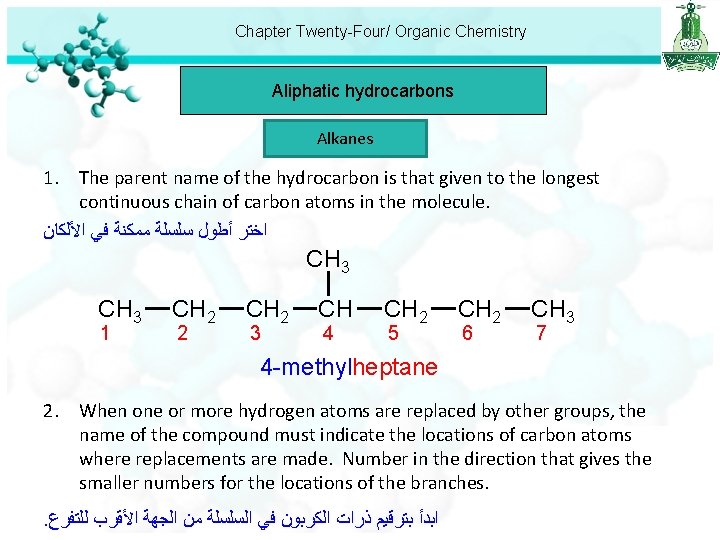
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes 1. The parent name of the hydrocarbon is that given to the longest continuous chain of carbon atoms in the molecule. ﺍﺧﺘﺮ ﺃﻄﻮﻝ ﺳﻠﺴﻠﺔ ﻣﻤﻜﻨﺔ ﻓﻲ ﺍﻷﻠﻜﺎﻥ CH 3 1 CH 2 2 CH 2 3 CH 4 CH 2 5 CH 2 6 CH 3 7 4 -methylheptane 2. When one or more hydrogen atoms are replaced by other groups, the name of the compound must indicate the locations of carbon atoms where replacements are made. Number in the direction that gives the smaller numbers for the locations of the branches. . ﺍﺑﺪﺃ ﺑﺘﺮﻗﻴﻢ ﺫﺭﺍﺕ ﺍﻟﻜﺮﺑﻮﻥ ﻓﻲ ﺍﻟﺴﻠﺴﻠﺔ ﻣﻦ ﺍﻟﺠﻬﺔ ﺍﻷﻘﺮﺏ ﻟﻠﺘﻔﺮﻉ

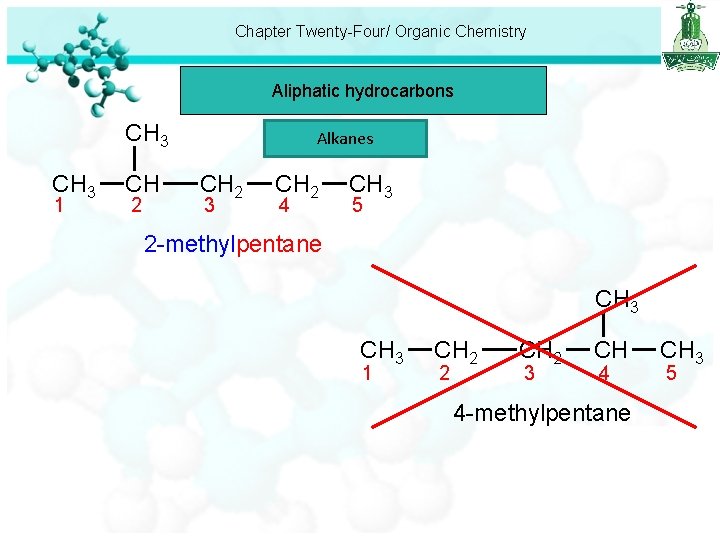
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons CH 3 1 CH 2 Alkanes CH 2 3 CH 2 4 CH 3 5 2 -methylpentane CH 3 1 CH 2 2 CH 2 3 CH 4 4 -methylpentane CH 3 5

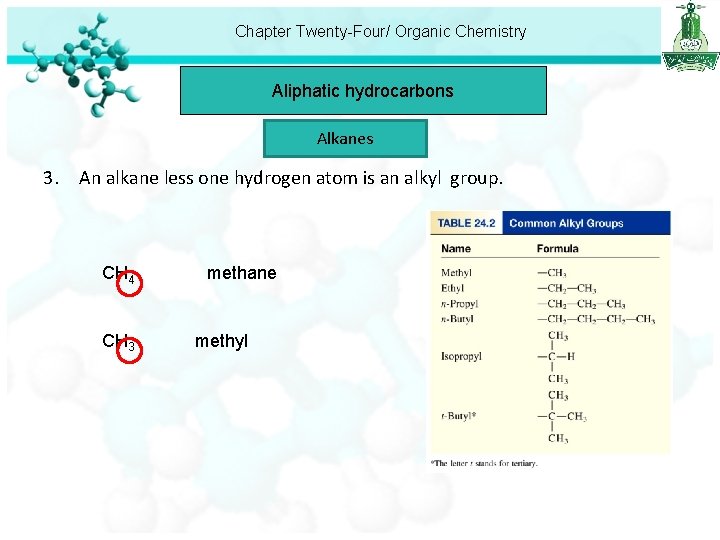
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes 3. An alkane less one hydrogen atom is an alkyl group. CH 4 CH 3 methane methyl

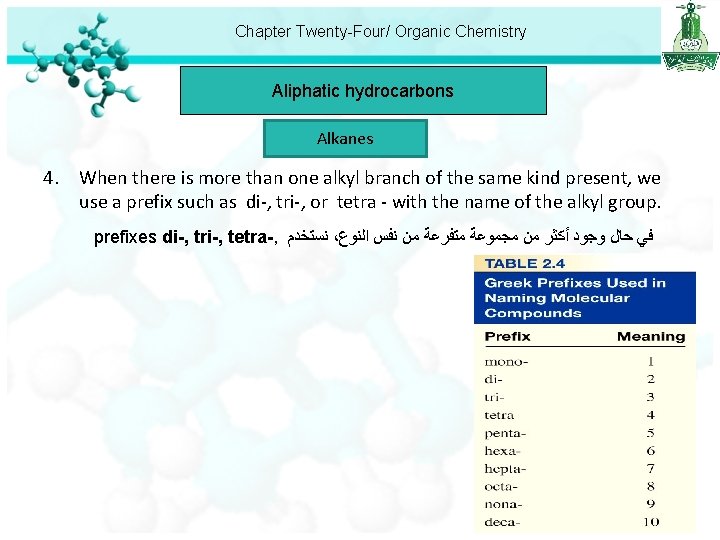
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes 4. When there is more than one alkyl branch of the same kind present, we use a prefix such as di-, tri-, or tetra - with the name of the alkyl group. prefixes di-, tri-, tetra-, ﻧﺴﺘﺨﺪﻡ ، ﻓﻲ ﺣﺎﻝ ﻭﺟﻮﺩ ﺃﻜﺜﺮ ﻣﻦ ﻣﺠﻤﻮﻋﺔ ﻣﺘﻔﺮﻋﺔ ﻣﻦ ﻧﻔﺲ ﺍﻟﻨﻮﻉ

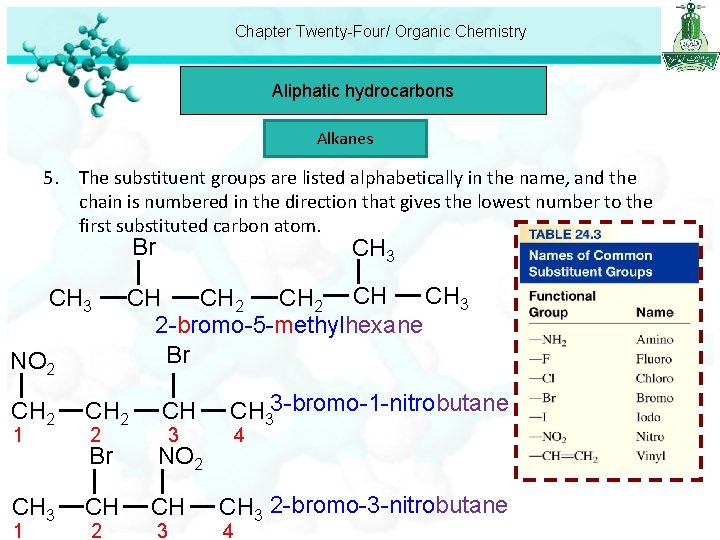
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes 5. The substituent groups are listed alphabetically in the name, and the chain is numbered in the direction that gives the lowest number to the first substituted carbon atom. Br CH 3 NO 2 CH 2 1 CH 3 CH CH 2 -bromo-5 -methylhexane Br CH 2 CH Br NO 2 CH CH 2 2 3 3 CH 33 -bromo-1 -nitrobutane 4 CH 3 2 -bromo-3 -nitrobutane 4

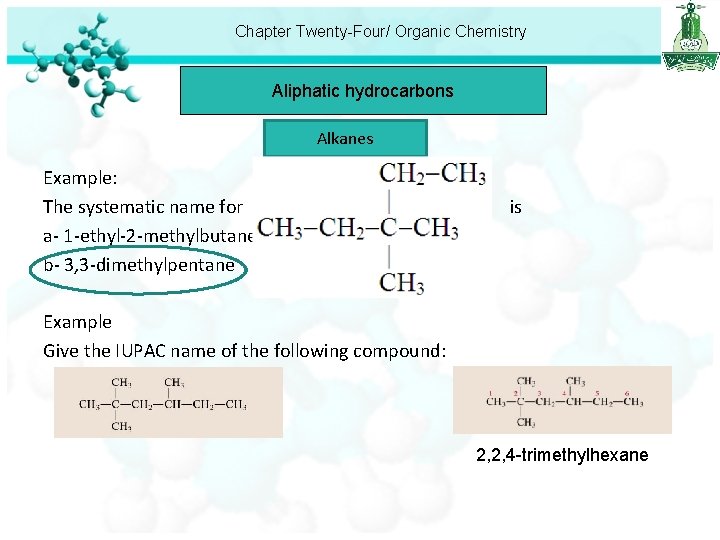
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes Example: The systematic name for is a- 1 -ethyl-2 -methylbutane. b- 3, 3 -dimethylpentane Example Give the IUPAC name of the following compound: 2, 2, 4 -trimethylhexane

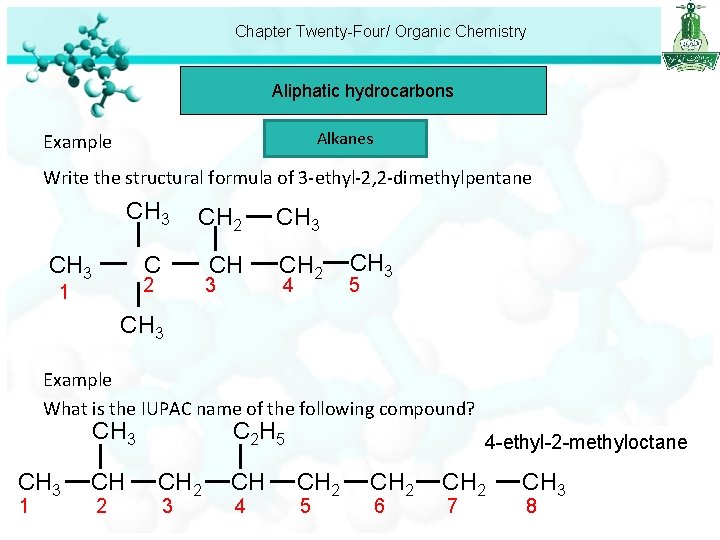
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes Example Write the structural formula of 3 -ethyl-2, 2 -dimethylpentane CH 3 CH 2 CH 3 C CH CH 2 CH 3 3 2 1 4 CH 3 5 CH 3 Example What is the IUPAC name of the following compound? CH 3 1 CH 2 C 2 H 5 CH 2 3 CH 4 4 -ethyl-2 -methyloctane CH 2 5 CH 2 6 CH 2 7 CH 3 8

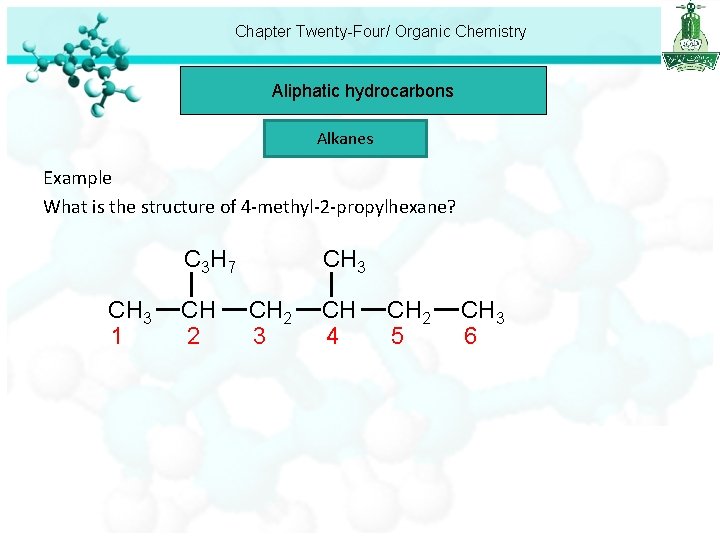
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes Example What is the structure of 4 -methyl-2 -propylhexane? C 3 H 7 CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 CH 3 6

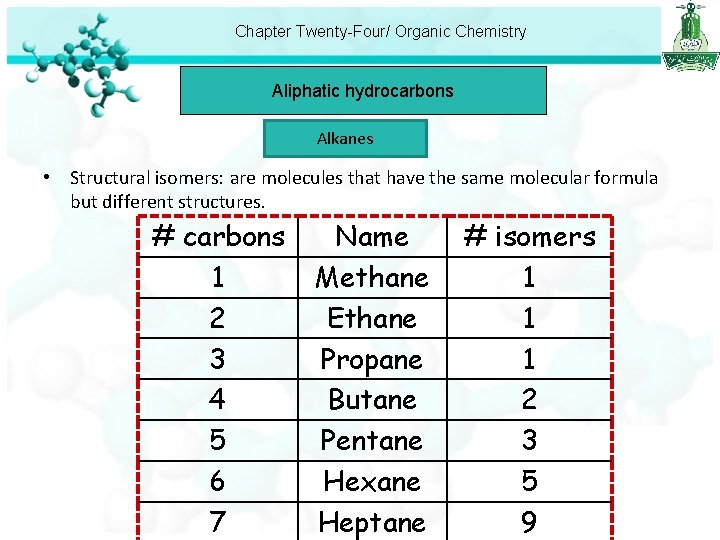
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes • Structural isomers: are molecules that have the same molecular formula but different structures. # carbons 1 2 3 4 5 6 7 Name Methane Ethane Propane Butane Pentane Hexane Heptane # isomers 1 1 1 2 3 5 9

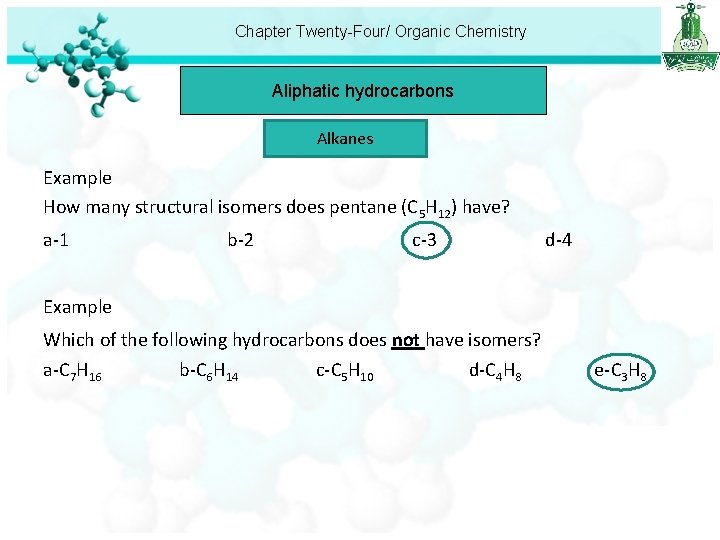
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkanes Example How many structural isomers does pentane (C 5 H 12) have? a-1 b-2 c-3 d-4 Example Which of the following hydrocarbons does not have isomers? a-C 7 H 16 b-C 6 H 14 c-C 5 H 10 d-C 4 H 8 e-C 3 H 8

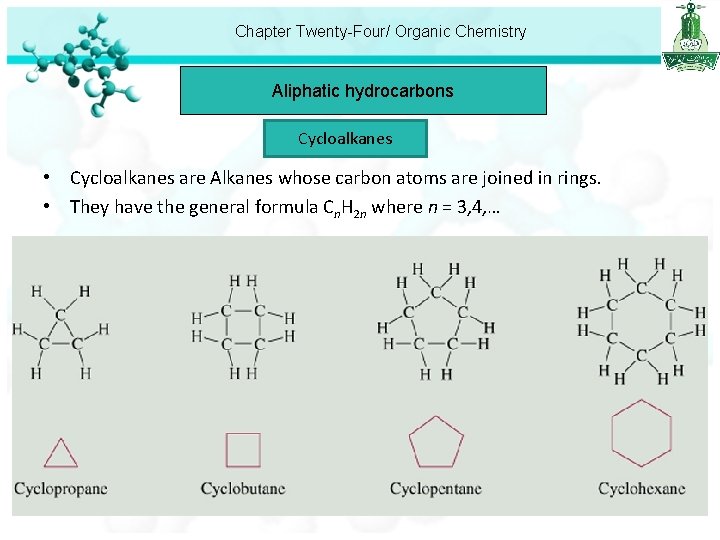
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Cycloalkanes • Cycloalkanes are Alkanes whose carbon atoms are joined in rings. • They have the general formula Cn. H 2 n where n = 3, 4, …

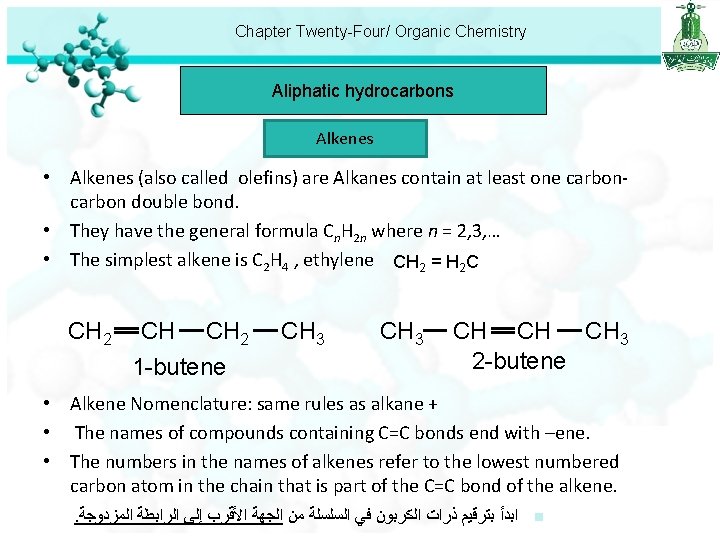
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkenes • Alkenes (also called olefins) are Alkanes contain at least one carbon double bond. • They have the general formula Cn. H 2 n where n = 2, 3, … • The simplest alkene is C 2 H 4 , ethylene CH 2 = H 2 C CH 2 CH CH 2 1 -butene CH 3 CH CH CH 3 2 -butene • Alkene Nomenclature: same rules as alkane + • The names of compounds containing C=C bonds end with –ene. • The numbers in the names of alkenes refer to the lowest numbered carbon atom in the chain that is part of the C=C bond of the alkene. . ﺍﺑﺪﺃ ﺑﺘﺮﻗﻴﻢ ﺫﺭﺍﺕ ﺍﻟﻜﺮﺑﻮﻥ ﻓﻲ ﺍﻟﺴﻠﺴﻠﺔ ﻣﻦ ﺍﻟﺠﻬﺔ ﺍﻷﻘﺮﺏ ﺇﻟﻰ ﺍﻟﺮﺍﺑﻄﺔ ﺍﻟﻤﺰﺩﻭﺟﺔ n

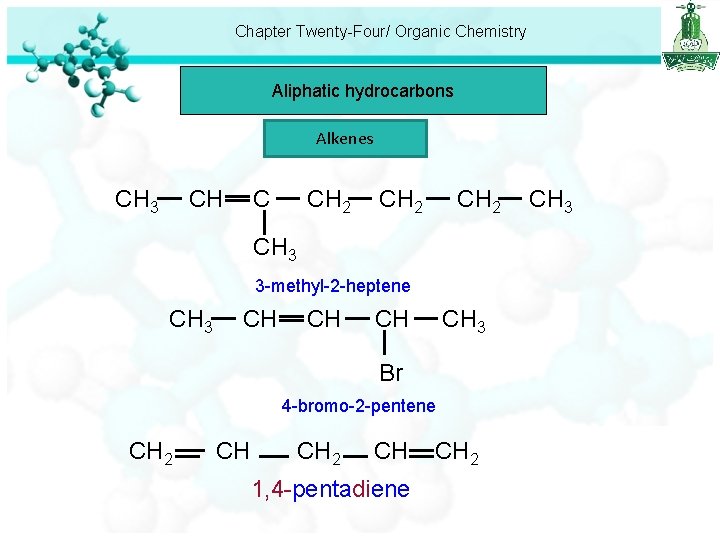
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkenes CH CH 3 CH 2 CH 3 3 -methyl-2 -heptene CH 3 CH CH 3 Br 4 -bromo-2 -pentene CH 2 CH 1, 4 -pentadiene CH 2 CH 3

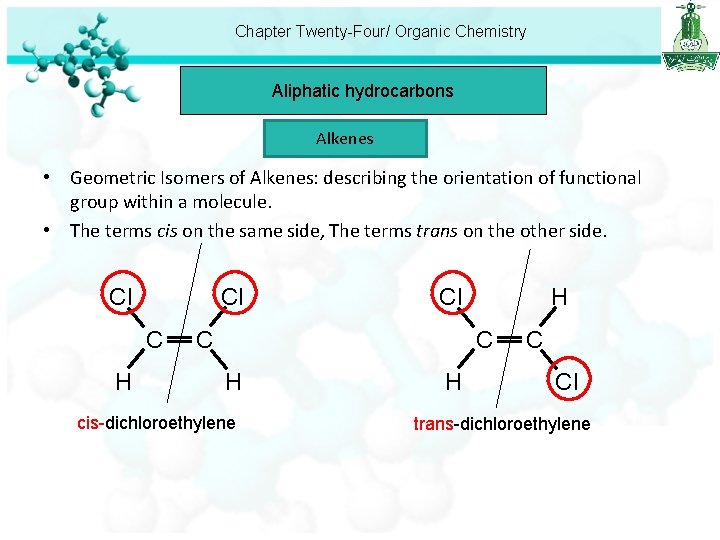
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkenes • Geometric Isomers of Alkenes: describing the orientation of functional group within a molecule. • The terms cis on the same side, The terms trans on the other side. Cl Cl C H H Cl C C H cis-dichloroethylene H C Cl trans-dichloroethylene

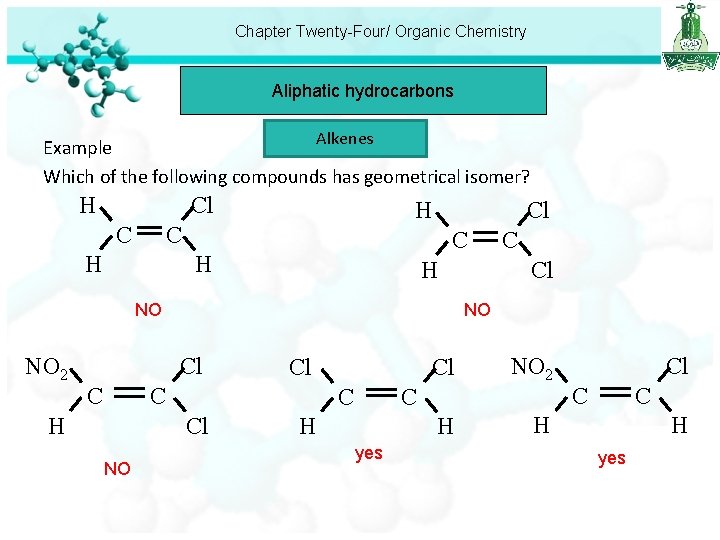
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkenes Example Which of the following compounds has geometrical isomer? H Cl C H H NO NO 2 Cl C H Cl C C Cl NO Cl C C H H yes NO 2 Cl C C H H yes

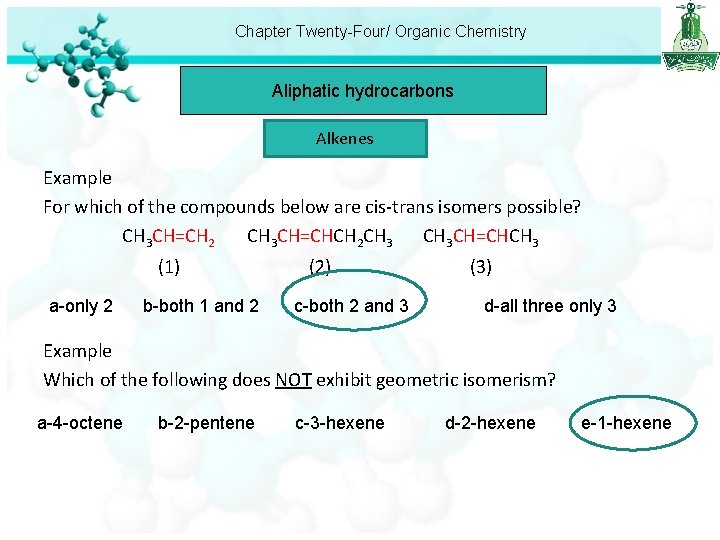
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkenes Example For which of the compounds below are cis-trans isomers possible? CH 3 CH=CH 2 CH 3 CH=CHCH 2 CH 3 CH=CHCH 3 (1) a-only 2 b-both 1 and 2 (2) c-both 2 and 3 (3) d-all three only 3 Example Which of the following does NOT exhibit geometric isomerism? a-4 -octene b-2 -pentene c-3 -hexene d-2 -hexene e-1 -hexene

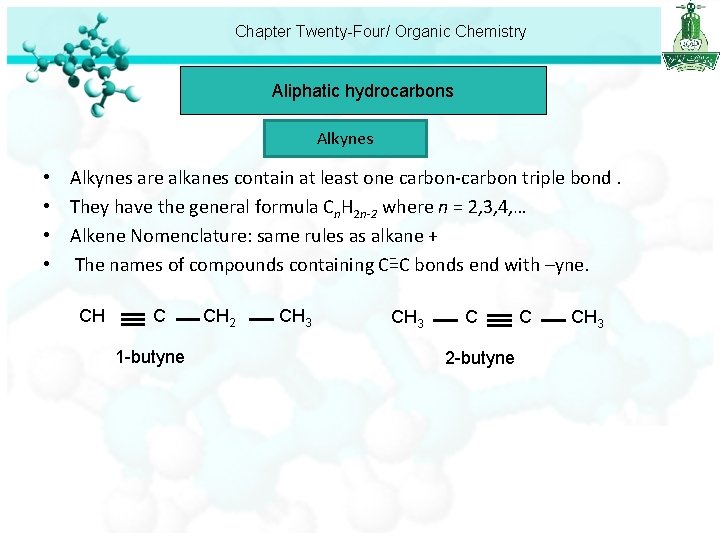
Chapter Twenty-Four/ Organic Chemistry Aliphatic hydrocarbons Alkynes • • Alkynes are alkanes contain at least one carbon-carbon triple bond. They have the general formula Cn. H 2 n-2 where n = 2, 3, 4, … Alkene Nomenclature: same rules as alkane + The names of compounds containing C=C bonds end with –yne. CH C 1 -butyne CH 2 CH 3 C 2 -butyne C CH 3

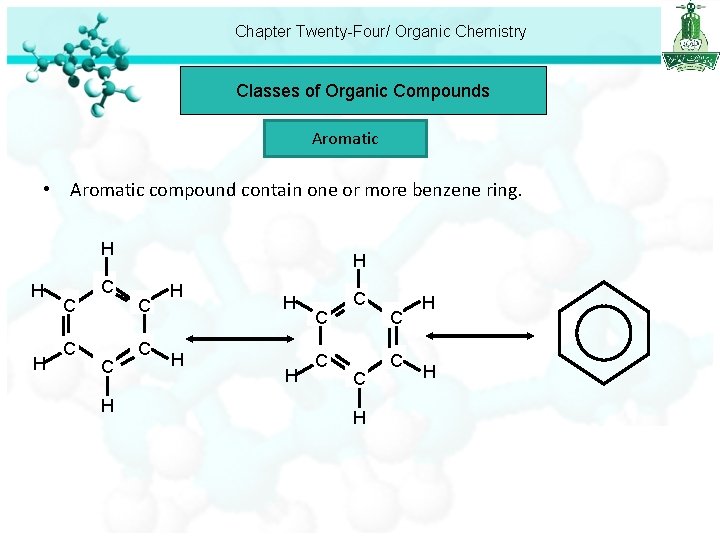
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic • Aromatic compound contain one or more benzene ring. H H H C C H H C C H H

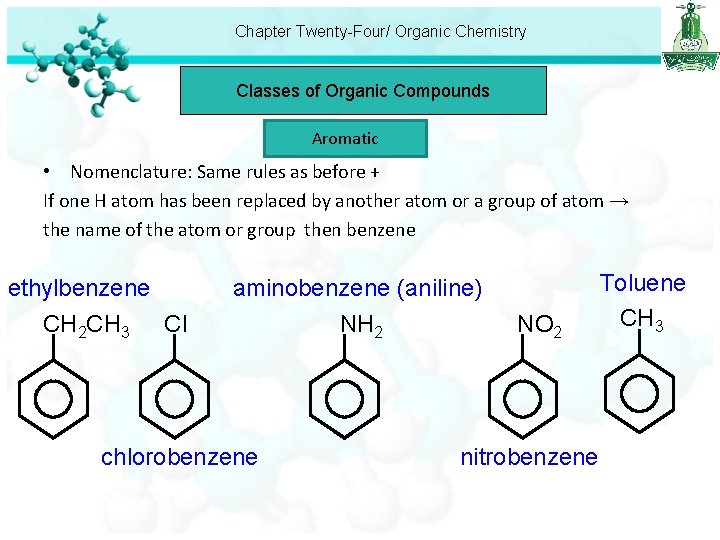
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic • Nomenclature: Same rules as before + If one H atom has been replaced by another atom or a group of atom → the name of the atom or group then benzene ethylbenzene CH 2 CH 3 Cl aminobenzene (aniline) NH 2 chlorobenzene NO 2 nitrobenzene Toluene CH 3

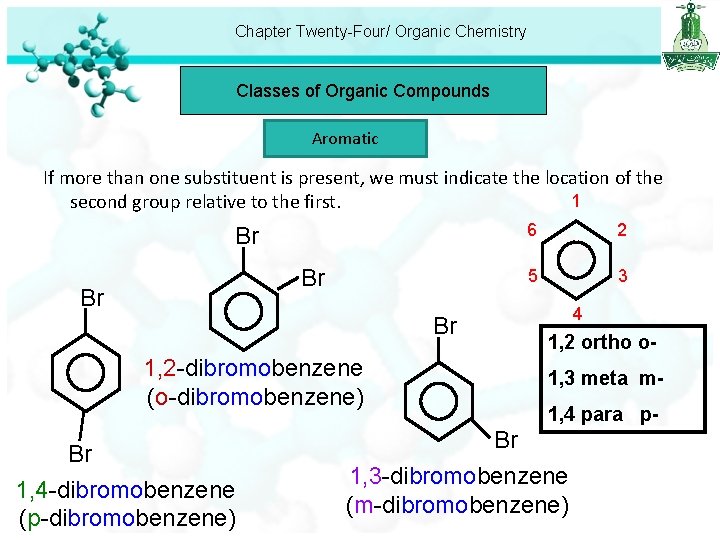
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic If more than one substituent is present, we must indicate the location of the 1 second group relative to the first. Br Br 1, 2 -dibromobenzene (o-dibromobenzene) Br 1, 4 -dibromobenzene (p-dibromobenzene) 6 2 5 3 4 1, 2 ortho o 1, 3 meta m 1, 4 para p- Br 1, 3 -dibromobenzene (m-dibromobenzene)

Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic

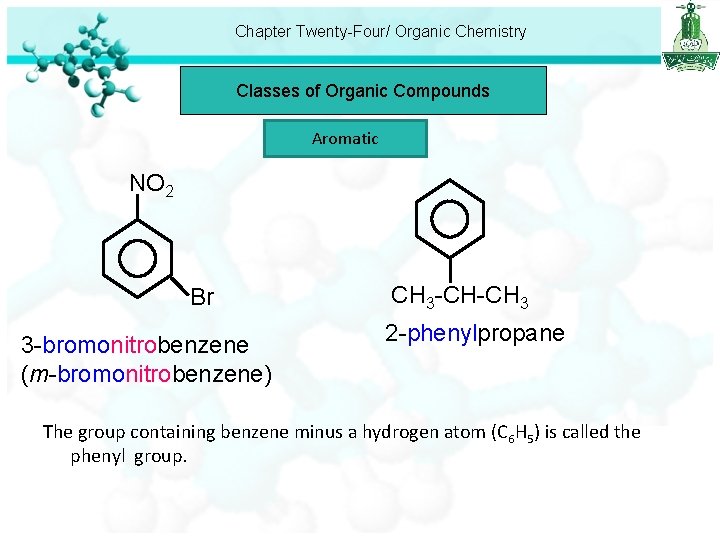
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic NO 2 Br 3 -bromonitrobenzene (m-bromonitrobenzene) CH 3 -CH-CH 3 2 -phenylpropane The group containing benzene minus a hydrogen atom (C 6 H 5) is called the phenyl group.

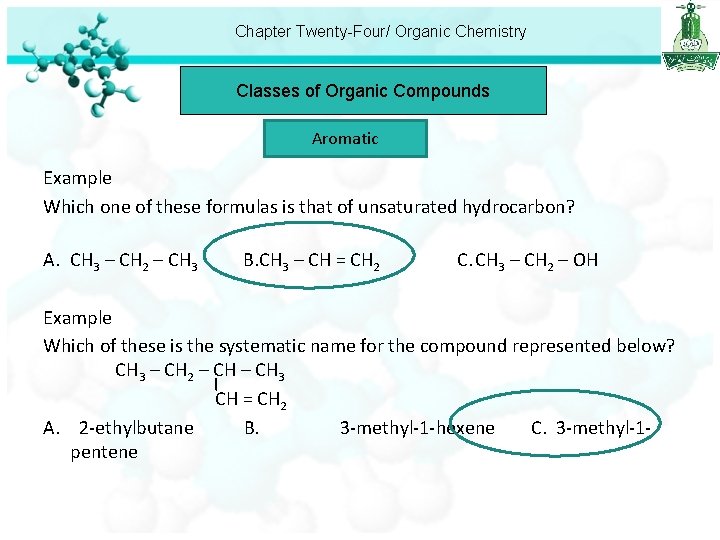
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Aromatic Example Which one of these formulas is that of unsaturated hydrocarbon? A. CH 3 – CH 2 – CH 3 B. CH 3 – CH = CH 2 C. CH 3 – CH 2 – OH Example Which of these is the systematic name for the compound represented below? CH 3 – CH 2 – CH 3 CH = CH 2 A. 2 -ethylbutane B. 3 -methyl-1 -hexene C. 3 -methyl-1 pentene

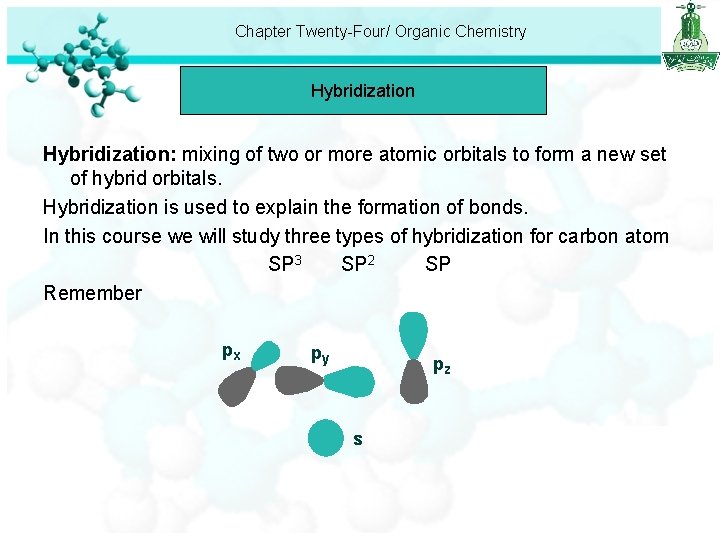
Chapter Twenty-Four/ Organic Chemistry Hybridization: mixing of two or more atomic orbitals to form a new set of hybrid orbitals. Hybridization is used to explain the formation of bonds. In this course we will study three types of hybridization for carbon atom SP 3 SP 2 SP Remember px py pz s

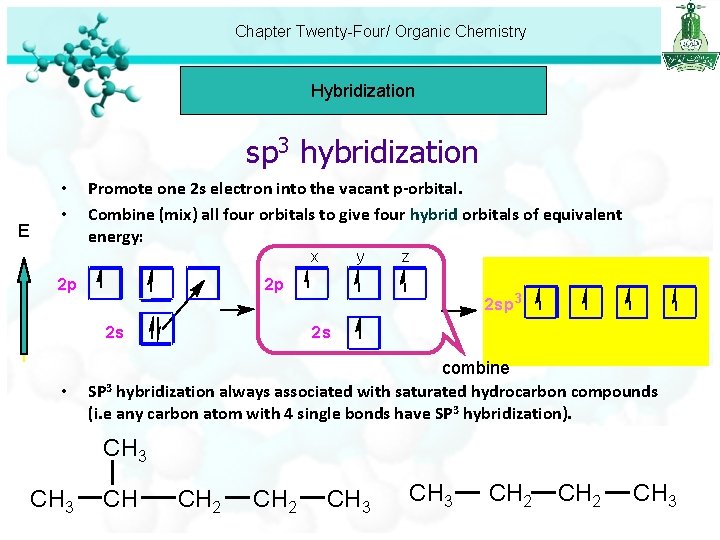
Chapter Twenty-Four/ Organic Chemistry Hybridization sp 3 hybridization E • • Promote one 2 s electron into the vacant p-orbital. Combine (mix) all four orbitals to give four hybrid orbitals of equivalent energy: x 2 p z 2 p 2 s • y 2 sp 3 2 s combine SP 3 hybridization always associated with saturated hydrocarbon compounds (i. e any carbon atom with 4 single bonds have SP 3 hybridization). CH 3 CH CH 2 CH 3

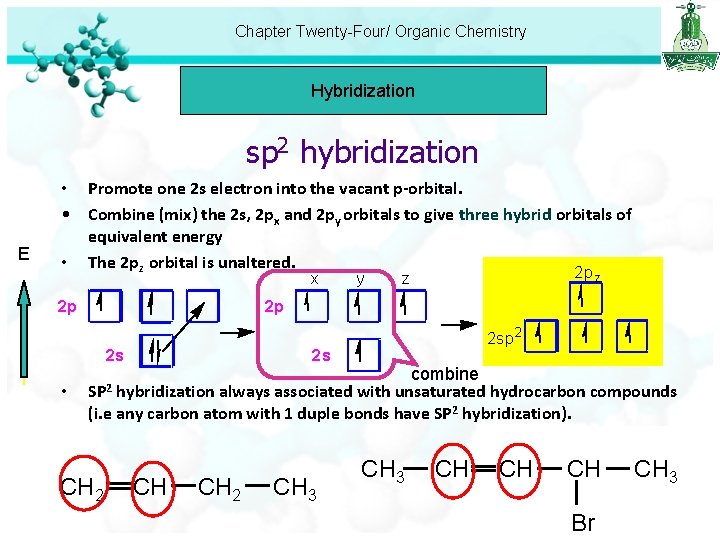
Chapter Twenty-Four/ Organic Chemistry Hybridization sp 2 hybridization E • Promote one 2 s electron into the vacant p-orbital. • Combine (mix) the 2 s, 2 px and 2 py orbitals to give three hybrid orbitals of equivalent energy • The 2 pz orbital is unaltered. x 2 p 2 p z z 2 p 2 s • y 2 sp 2 2 s combine hybridization always associated with unsaturated hydrocarbon compounds (i. e any carbon atom with 1 duple bonds have SP 2 hybridization). SP 2 CH CH 2 CH 3 CH CH CH Br CH 3

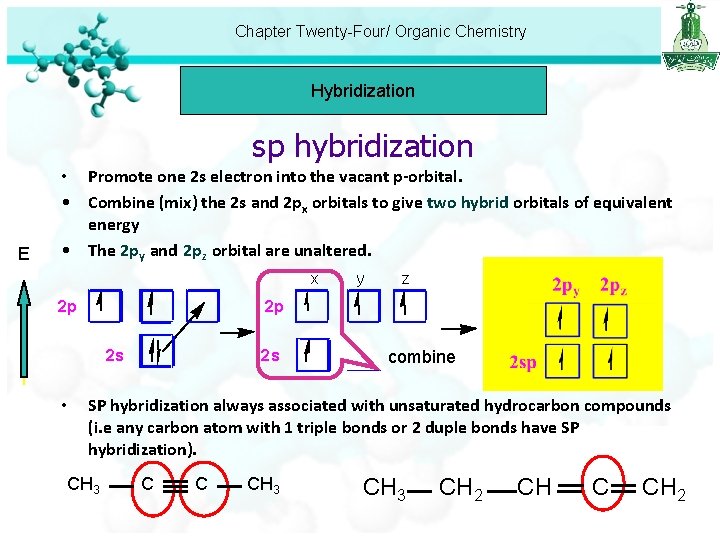
Chapter Twenty-Four/ Organic Chemistry Hybridization sp hybridization E • Promote one 2 s electron into the vacant p-orbital. • Combine (mix) the 2 s and 2 px orbitals to give two hybrid orbitals of equivalent energy • The 2 py and 2 pz orbital are unaltered. x 2 p z 2 p 2 s • y 2 s combine SP hybridization always associated with unsaturated hydrocarbon compounds (i. e any carbon atom with 1 triple bonds or 2 duple bonds have SP hybridization). CH 3 C C CH 3 CH 2 CH C CH 2

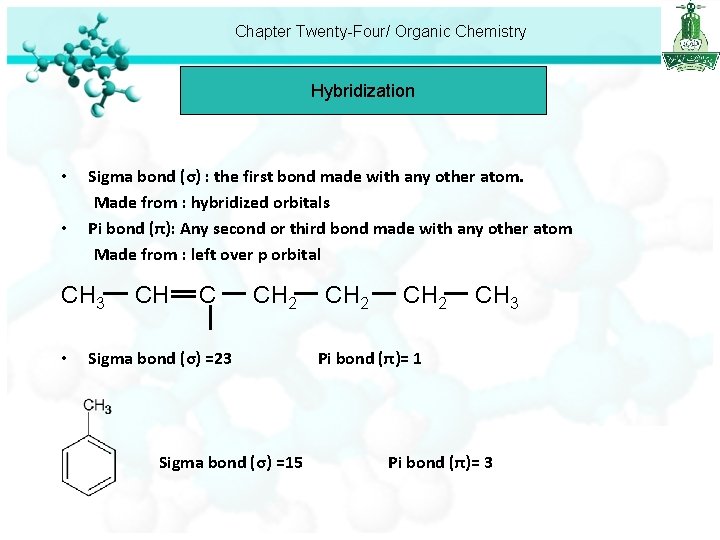
Chapter Twenty-Four/ Organic Chemistry Hybridization • • Sigma bond (σ) : the first bond made with any other atom. Made from : hybridized orbitals Pi bond (π): Any second or third bond made with any other atom Made from : left over p orbital CH 3 • CH C CH 2 Sigma bond (σ) =23 Sigma bond (σ) =15 CH 2 CH 3 Pi bond (π)= 1 Pi bond (π)= 3

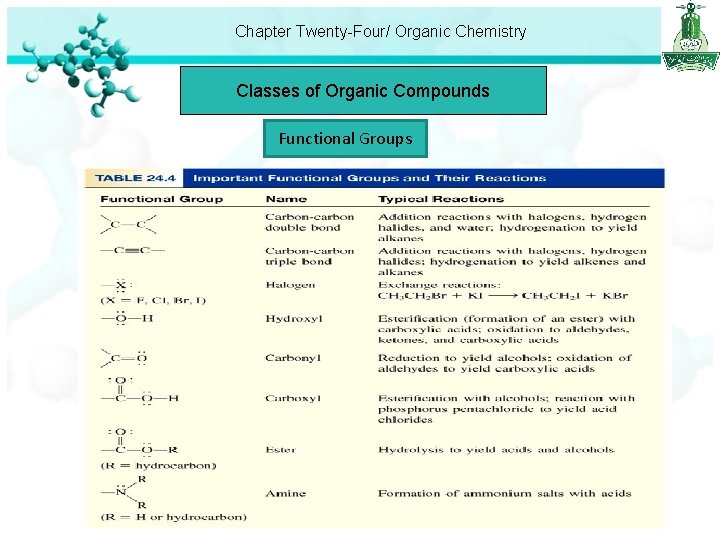
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups Functional groups are responsible for most of the reactions of the parent compounds. Functional groups are: Alcohol , Ethers , Aldhyde , Ketones , Carboxylic acid , Esters , Amines , Aminoacid

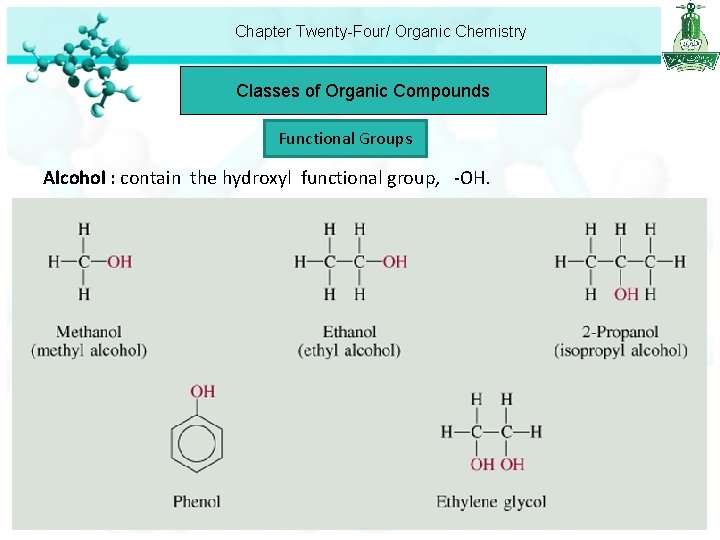
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups Alcohol : contain the hydroxyl functional group, -OH.

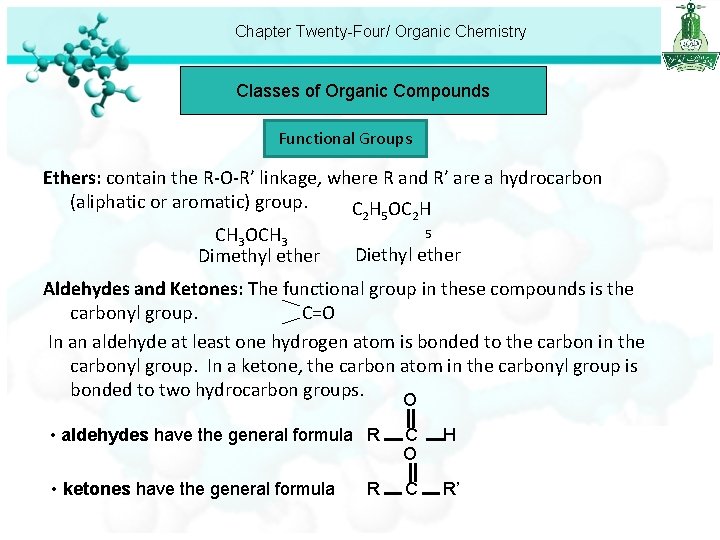
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups Ethers: contain the R-O-R’ linkage, where R and R’ are a hydrocarbon (aliphatic or aromatic) group. C H OC H CH 3 OCH 3 Dimethyl ether 2 5 Diethyl ether Aldehydes and Ketones: The functional group in these compounds is the carbonyl group. C=O In an aldehyde at least one hydrogen atom is bonded to the carbon in the carbonyl group. In a ketone, the carbon atom in the carbonyl group is bonded to two hydrocarbon groups. O • aldehydes have the general formula R C O H • ketones have the general formula C R’ R

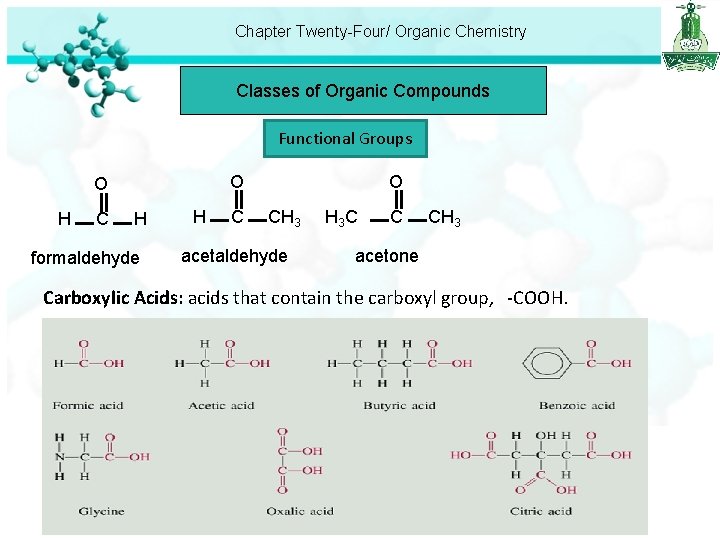
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups H C O O O H formaldehyde H C CH 3 acetaldehyde H 3 C C CH 3 acetone Carboxylic Acids: acids that contain the carboxyl group, -COOH.

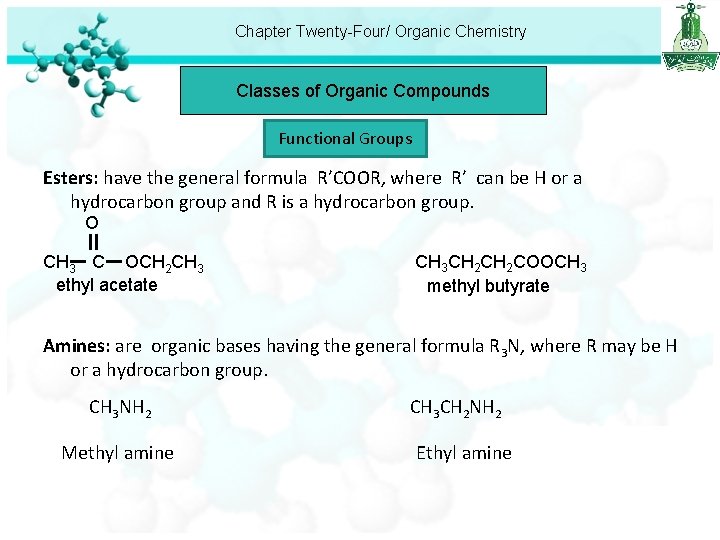
Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups Esters: have the general formula R’COOR, where R’ can be H or a hydrocarbon group and R is a hydrocarbon group. O CH 3 C OCH 2 CH 3 ethyl acetate CH 3 CH 2 COOCH 3 methyl butyrate Amines: are organic bases having the general formula R 3 N, where R may be H or a hydrocarbon group. CH 3 NH 2 Methyl amine CH 3 CH 2 NH 2 Ethyl amine

Chapter Twenty-Four/ Organic Chemistry Classes of Organic Compounds Functional Groups

 Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s What is organic chemistry
What is organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Nonene
Nonene Analytical chemistry chapter 1
Analytical chemistry chapter 1 Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Father of organic chemistry
Father of organic chemistry Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Formula of alkene
Formula of alkene Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Nomenclature of ethers
Nomenclature of ethers Is alkane an organic compound
Is alkane an organic compound Ario practice problems
Ario practice problems Functional groups priority
Functional groups priority Objective lab report example
Objective lab report example Organic chemistry conversion chart
Organic chemistry conversion chart Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry Organic chemistry wade
Organic chemistry wade Meth eth prop but pent table
Meth eth prop but pent table Cracking organic chemistry
Cracking organic chemistry Meth eth prop but
Meth eth prop but Organic chemistry myanmar
Organic chemistry myanmar Electrophilic addition hbr
Electrophilic addition hbr Mass spec of chlorine
Mass spec of chlorine Hono organic chemistry
Hono organic chemistry Geminal and vicinal
Geminal and vicinal Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry reaction pathways
Organic chemistry reaction pathways Alkene alcohol naming
Alkene alcohol naming What is organic chemistry like
What is organic chemistry like Ch4o organic or inorganic
Ch4o organic or inorganic Organic chemistry vocabulary
Organic chemistry vocabulary Separation scheme of caffeine from vivarin tablets
Separation scheme of caffeine from vivarin tablets A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis































































